Abstract
Background
Surgical procedures involving the hip, knee, or spine represent a majority of orthopaedic procedures performed electively in the health care system. Postoperative care is a key aspect of surgery and mobilisation without injury is the primary objective. Recent advances in wearable technologies allow objective evaluation of walking metrics to inform and guide postoperative care following orthopaedic surgery.
Purpose
The aim of this scoping review is to explore current applications of wearable devices, objective data capture and gait analysis in monitoring postoperative recovery following commonly performed elective orthopaedic procedures of the hip, knee and spine.
Methods
A search against pre-defined criteria was performed on the following scientific databases from date of inception to February 28th, 2021: Medline (via OvidSP), Embase (via OvidSP) and Cochrane Library (via CENTRAL). Data were collected according to a predetermined checklist including study participants, surgery, wearable device (model), sensor location, and monitoring parameters such as mobility metrics, monitoring timepoints and monitoring duration for each study included in our review. Quality was assessed independently using the Newcastle Ottawa Scale (NOS).
Conclusions
To our knowledge, this is the first review of wearable monitoring (of postoperative recovery) following hip, knee and spine surgery. Patients undergoing elective orthopaedic procedures may benefit from wearable monitoring of their walking health and mobility metrics.
Similar content being viewed by others
Background
Musculoskeletal conditions account for more disability and more costs to the United States health care system than any other condition [1]. When conservative treatment options fail, these diseases may be managed surgically. Over 200 000 total hip arthroplasty and 600 000 total knee arthroplasty procedures are performed per year in the USA [2, 3]. Similar high volumes of 3.1 million total hip arthroplasties and 2.5 million total knee arthroplasties are performed every year in Europe, as well as over 95,000 joint replacements performed every year in Australia [4,5,6]. Total Medicare reimbursements for lumbar surgery alone in the USA exceeds $1 billion per year [7]. Together, surgical procedures involving the hip, knee, or spine represent a majority of orthopaedic procedures performed electively in the health care system and form a significant proportion of all surgical procedures performed by a typical hospital by both sheer case numbers and expenses.
Postoperative care is a key aspect of surgery and involves facilitating safe recovery. In the context of orthopaedic procedures especially, mobilisation without injury is the primary objective during postoperative rehabilitation [8]. Other objectives may include the early detection of any postoperative complications. Typically, patients receive immediate postoperative care as an inpatient until discharge followed by outpatient clinic visits of diminishing frequency [8]. Further, assessment of postoperative outcomes may be obtained at arbitrarily fixed timepoints, via patient reported outcome measures (PROM) such as the Owestry Disability Index, Oxford Knee Score and the Hip disability and Osteoarthritis Outcome Score. Although these questionnaire-based clinical tools offer meaningful insight into a patient’s functional outcomes such as extent of disability and impact on activities of daily living, they are limited by subjectivity due to patients’ reporting bias and mode of administration. [9, 10]
Walking is an essential activity of daily living, and is directly related to the function and health of mechanical, musculoskeletal and neurological systems [11]. Commonly dubbed as “ the sixth vital sign”, walking metrics such as gait velocity and step count are important indicators of not only general health status but also decline and recovery [12]. Moreover, these walking metrics provide an objective alternate measure of functional outcomes and disability to the inherently subjective patient reported outcome measures.
Recent advances in wearable technologies allow objective evaluation of these walking metrics to inform and guide postoperative care following elective orthopaedic surgery. ‘Wearable devices’ (wearables) containing various microelectromechanical sensors (MEMS) such as accelerometers and/or gyroscopes have recently emerged as a method of objectively measuring walking metrics. These devices can accurately capture a range of metrics including simple mobility metrics such as step count and physical activity levels to complex walking parameters, such as gait velocity, cadence, and stride length [13]. Advantageously, they are small, cheap, and marry the convenience of at-home postoperative monitoring with accuracy and objectivity. They can be worn at a single point on the body or multiple points, can function on their own, or be incorporated into various devices, such as watches, phones, jewellery, pendants, or insoles [14]. Most notably, wearable monitoring offers objective and continuous data capture of these ‘walking metrics’ to monitor patient recovery. Unlike PROMS, which offer a “snapshot” into a patient’s functional status at a particular point in their ongoing recovery, wearable devices enable continuous data capture of their mobility data to more holistically detail patients’ recovery.
The objective of this scoping review is to explore current applications of wearable devices, objective data capture and gait analysis in monitoring postoperative recovery following elective orthopaedic procedures. Procedures of the hip, knee and spine are amongst the most common. Therefore, eight commonly undertaken elective orthopaedic procedures were considered for inclusion: total hip replacement, total knee replacement, arthroscopic anterior cruciate ligament reconstruction, arthroscopic meniscal repair of the knee, arthroscopic partial meniscectomy of the knee, lumbar spine decompression and lumbar spine fusion.
Methods
Eligibility criteria
The focus of this scoping review was on published original articles written in English and published between 1980 and August 2021, including all study designs such as case reports, short series, cohort studies, randomised trials, or other study designs. The PRISMA statement guidelines were followed in identifying, screening, and selecting studies for inclusion, and extracting data.
Inclusion criteria
-
1.
Articles involving wearable devices for the purpose of continuous and objective monitoring of postoperative recovery.
-
2.
The wearable device is capable of measuring gait or mobility metrics.
-
3.
Postoperative monitoring after the following orthopaedic procedures:
-
a.
total hip replacement,
-
b.
total knee replacement,
-
c.
arthroscopic anterior cruciate ligament reconstruction,
-
d.
arthroscopic meniscal repair of the knee,
-
e.
arthroscopic partial meniscectomy of the knee,
-
f.
lumbar spine decompression
-
g.
lumbar spine fusion
-
a.
-
4.
Articles written in English.
-
5.
Articles published between 1980 – August 2021.
Exclusion criteria
-
1.
Wearable technology studies involving non-mobility or gait data capture.
-
2.
Studies assessing patient function during a single walking bout (non-continuous)
-
3.
Studies involving robotic ‘feedback’ wearables, exoskeletons or smartphones.
-
4.
Studies of artificial intelligence algorithms or predictive modelling of patient outcomes
-
5.
Systematic Reviews
-
6.
Conference Abstracts
Search strategy
Relevant studies were identified through a systematic search for published papers in the following scientific databases from date of inception to February 28th, 2021: Medline (via OvidSP), Embase (via OvidSP) and Cochrane Library (via CENTRAL). The search ‘concepts’ were wearable (gait-tracking) devices and elective orthopaedic procedures (see Table 1).
Study selection
The literature search was completed by two authors (PN and RDF). Titles and abstracts of all studies identified were screened for relevance. Studies which were not relevant based on the title and abstract screen were excluded from the review. The full text of the record was reviewed if relevance was uncertain, and third reviewer consulted (RJM) if necessary until consensus agreement was reached regarding inclusion/exclusion. The full text of all selected relevant records was reviewed, and eligibility was determined using the eligibility criteria defined above. The quality of each included record was assessed by two authors (PN and RDF), and relevant information extracted.
Data collection
Following the selection of articles, data was collated by two reviewers (PN and RDF). Data were collected according to a predetermined checklist including: study participants, surgery, wearable device (model), sensor location, and monitoring parameters such as mobility metrics, monitoring timepoints and monitoring duration for each study included in our review. Each study was also appraised independently for bias using the Newcastle Ottawa Scale by two reviewers (PN and RDF) and a third senior reviewer consulted for discrepancies (MM) [15]. Quality assessments from the Newcastle–Ottawa scale was converted to summary categories of good, fair, and poor quality according to the Agency for Healthcare Research and Quality (AHRQ) standards.
Results
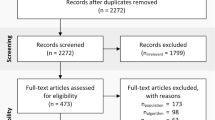
Following database searches for each orthopaedic procedure we identified a total of 640 relevant records (see Table 2). After removal of duplicates, 527 studies remained. Four hundred forty-nine references were excluded on title and abstract screen. A further 53 articles were excluded upon full-text review, leaving a final 26 studies to be included in qualitative synthesis. A flowchart of this process is shown in Fig. 1.
The 26 included studies comprised the procedures of ACL reconstruction (1 study) [16], Lumbar Decompression (2 studies) [17, 17], Lumbar Fusion (5 studies) [18,19,20,21,22], TKR (9 studies) [23,24,25,26,27,28,29,30,31] and THR procedures (9 studies) [23,24,25,26,27,28,29,30,31] as seen in Table 3. No studies related to arthroscopic partial meniscectomy and meniscal repairs of the knee were retrieved from the literature search. Sample sizes ranged from 12 participants [32] to as high as 242 participants [33]. A detailed summary of these studies is included in Table 4. Due differences in study design, wearable monitoring protocols and surgical cohorts between studies it was not possible to meta-analyse findings from included studies.
Commonly employed devices for continuous wearable monitoring were ActivPAL (PAL Technologies Ltd., Glasgow, United Kingdom) [18, 26, 31, 34], ActiGraph GT1M (ActiGraph LLC, Fort Walton Beach, FL, USA) [17, 19, 36, 37], Lifecorder EX (Suzuken Co. Ltd, Nagoya, Japan) [28, 35] and MiBand2 (Xiaomi, China) [17, 22]. Some studies also employed consumer fitness wearable devices such as Fitbit (Fitbit Inc., San Francisco, California, United States) [20, 21, 27] and Withings (Withings Inc, France) [33]. Other studies permitted use of patients’ own device and/or involved the use of their smartphone’s step counter functionality. [17, 29]
Most common sensor placement locations employed included wrist [17, 20,21,22, 27, 33, 34, 36, 37], waist [19, 23,24,25, 28, 32, 35,36,37] and thigh [18, 23, 26, 31, 34]. Although majority involved single-point wearables, a select few studies employed more than one wearable device [23, 38]. Captured data typically comprised of physical activity intensities (for example light, moderate or vigorous) and physical activity durations (for example sitting, standing, with few studies also collecting basic spatial and temporal gait metrics such as step count [16,17,18, 20,21,22, 25,26,27,28,29,30, 33,34,35,36,37], gait cycles [23] and stride frequency [38]. Some studies additionally collected caloric/energy expenditures [20, 24, 32]. Studies typically monitored patient mobility at specific timepoints of recovery such as several weeks, 3 months, and 6 months postoperatively (as seen in Table 4). However few studies monitored the entire recovery period from operative timepoints to 6 months postoperatively [16, 17, 20, 21, 30, 33], with majority monitoring “recovery windows” at perioperative and/or post-recovery timepoints.
In terms of quality of included studies, most were of good quality according to the AHRQ standards. Of the 26 included studies, 22 studies [16, 17, 17,18,19,20, 22,23,24,25,26,27,28,29,30,31, 33, 34, 36, 36,37,38] were of good quality, with 3 studies [21, 32, 35] of fair quality and 1 study of poor quality, as seen in Table 5. However, 7 had short follow-up durations (< 3 months) [17, 20, 29, 33, 34, 36, 37] with 12 studies reporting follow-up data on less than 80% of recruited participants. [16,17,18,19, 21, 23, 25, 26, 32, 36, 37, 37]
Discussion
The findings of the present review demonstrate thus far supportive data for clinical applications of wearable monitoring of patient recovery following common elective orthopaedic surgeries. However, higher quality evidence with large-volume studies is needed, with applications following some surgeries such as arthroscopic meniscal repair of the knee, and arthroscopic partial meniscectomy of the knee, yet to be validated. Moreover, current studies are limited to basic mobility metrics such as step count and activity profiles. Future studies may incorporate other quantitative gait metrics (beyond step count) such as gait velocity, step or stride length, gait asymmetry and gait variability. Most studies are single-centre clinical series with small to moderate sample sizes. Notably prevalent are patient compliance issues, with included studies typically reporting follow-up data on less than 80% of recruited participants, as seen in Table 5.
Included studies demonstrated a wide variety of uses and benefits for wearable monitoring. Benefits of wearable devices in facilitating remote patient monitoring has been reported by numerous authors with Ramkumar et al. [29] suggesting the possibility of real-time collection of other data such as range of motion, patient reported outcome measures, opioid consumption, and home exercise compliance. Gamification and remote monitoring was reported as a means of improving recovery outcomes following knee and hip arthroplasty in Mehta et al. (2020)’s randomised clinical trial [33]. Although wearable monitoring was found to offer no direct effect as an intervention (in improving mobility levels), the rate of rehospitalisation was found to be significantly reduced (3.4% versus 12.2%, p = 0.01) suggesting overall benefits to recovery outcomes. By contrast, a multi-model wearable monitoring program coupled with physical therapy counselling by Li et al. [27] resulted in mean improvements in (moderate-vigorous) physical activity levels of 13.1 min per day (95% CI 1.6 to 24.5). Despite these discrepancies in which outcomes are improved, it is likely wearable monitoring offers some sort of benefit to postoperative care and recovery.
Other uses of wearable monitoring that are yet to be explored in larger orthopaedic surgery cohorts includes the screening and early detection of complications in the peri- and postoperative period. The detection of recurrent disc herniation following microdiscectomy has previously been detailed by Mobbs et al.’s case report in 2018 [39], suggesting such wearable monitoring for postoperative complications may be clinically feasible.
Another application of wearable monitoring may be the tracking of postoperative recovery against “normalised” trajectories to guide mobility interventions. For example, Carmichael et al. (2019) proposes clearly defined normal recovery trajectories (differing with both admission and operation type) in 210 patients following both minimally invasive and open abdominal and thoracic surgery [40]. Through wearable monitoring postoperative recovery “trajectories” can be quantified and continuously tracked to inform timely intervention and counselling to improve postoperative mobility.
However, most studies tended to employ a “snapshot” capture of activity levels over a set time (for instance 24 h or 7 days) preoperatively which was compared to similar postoperative data capture after a set recovery duration (for instance 6 months) [32, 37]. A limitation of this “snapshot” approach is the lack of continuous data capture over the duration of postoperative recovery which may not reflect how recovery outcomes may improve and decrease over a unique recovery trajectory [40]. Such snapshots, for example Thewlis et al.’s (2019) report of no significant difference between preoperative and postoperative activity profiles in terms of sedentary duration (620 ± 143 min/day preoperatively versus 641 ± 133 min/day, respectively) may not reflect fluctuations over the course of recovery [37]. Additionally, arbitrary study period of 3 months or 6 months may not necessarily be sufficient duration for these recovery trajectories that differ with operation type, admission and patient characteristics [40]. As such, Matsunaga-Myoji et al. (2020) reports improving (moderate-vigorous) physical activity levels (58.3 versus 72.3 min/week, p = 0.008) between 1 and 3 years postoperatively, following total knee replacement. [35]
Findings from the few studies which have undertaken continuous and objective activity tracking, for example by Steinen et al. (2020) and Scheer et al. (2017), suggest patient recovery following spinal surgeries may also follow these defined trajectories [21, 22]. A challenge facing continuous recovery trajectory monitoring remains patient compliance, with only 68% of Carmichael et al.’s participants completing follow-up at four weeks postoperatively [40]. These issues are not consistent, with some included studies also reporting follow-up data for > 90% of recruited participants [20, 29, 30]. Future studies may explore methods of participant retainment and compliance, such as incentives, gamification and/or counselling.
The continuous stream of objective data regarding patient performance provided by wearable monitoring may also be used to predict recovery outcomes. Regression analysis by Taniguchi et al. (2016) demonstrated postoperative physical activity in the first month predicted activity levels up to 6 months postoperatively (following total knee arthroplasty) to be predicted [30]. Pre-operative mobility characteristics were used for similar predictive modelling of recovery outcomes following total hip and knee arthroplasty by Lebleu et al. (2021) [36]. Existing risk-prediction models based on patient-reported and/or functional outcome measures [41], may benefit from such objective data capture from wearable monitoring.
Strengths and limitations
To our knowledge, this is the first review of wearable monitoring (of postoperative recovery) following hip, knee, and spine surgery. Other strengths include systematic search of literature from date of inception to February 28th, 2021, across 3 unique databases as well as standardised quality assessments of included studies via the Newcastle Ottawa Scale. However, limitations include restriction of search to only hip, knee, and spine surgeries – despite these encompassing the majority. Future studies may explore surgery for other gait altering pathologies – such as deep-brain stimulation for Parkinson’s, vascular claudication as well as ankle surgery.
Conclusion
Elective orthopaedic procedures are likely a very suitable patient population to benefit from wearable monitoring with their recovery and rehabilitation directly related to their walking health and mobility. Wearable monitoring may also enable timely postoperative care and intervention during recovery providing benefits to patients, healthcare providers and insurance providers alike since orthopaedic surgeries comprise a significant proportion of health care. Predictive modelling of post-recovery outcomes, and development of recovery trajectories from common orthopaedic procedures may enable timely mobility interventions to assist postoperative rehabilitation.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Haralson RH, Zuckerman JD. Prevalence, health care expenditures, and orthopedic surgery workforce for musculoskeletal conditions. JAMA. 2009;302:1586–7.
Liu SS, Della Valle AG, Besculides MC, et al. Trends in mortality, complications, and demographics for primary hip arthroplasty in the United States. Int Orthop. 2009;33:643–51.
Inacio M, Paxton E, Graves S, et al. Projected increase in total knee arthroplasty in the United States–an alternative projection model. Osteoarthritis Cartilage. 2017;25:1797–803.
Inacio MC, Graves SE, Pratt NL, et al. Increase in total joint arthroplasty projected from 2014 to 2046 in Australia: a conservative local model with international implications. Clin Orthopaedics Related Res. 2017;475:2130–7.
Kurtz SM, Ong KL, Lau E, et al. International survey of primary and revision total knee replacement. Int Orthop. 2011;35:1783–9.
Lübbeke A, Silman A, Barea C, et al. Mapping existing hip and knee replacement registries in Europe. Health Policy. 2018;122:548–57.
Weinstein JN, Lurie JD, Olson P, et al. United States trends and regional variations in lumbar spine surgery: 1992–2003. Spine. 2006;31:2707.
Lo B, Atallah L, Aziz O, et al. Real-time pervasive monitoring for postoperative care. In: 4th international workshop on wearable and implantable body sensor networks (BSN 2007) 2007, Springer, pp.122–127
Gagnier JJ, Johnston BC. Poor quality patient reported outcome measures bias effect estimates in orthopaedic randomized studies. J Clin Epidemiol. 2019;116:36–8.
Acosta J, Tang P, Regal S, et al. Investigating the bias in orthopaedic patient-reported outcome measures by mode of administration: a meta-analysis. JAAOS Global Res Rev. 2020;4:e2000194.
Mobbs RJ. Gait velocity (walking speed) is an indicator of spine health, and objective measure of pre and post intervention recovery for spine care providers. J Spine Surg. 2020;6:353.
Fritz S, Lusardi M. White paper:“walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32:46–9.
Valenti G, Bonomi AG, Westerterp KR. Walking as a contributor to physical activity in healthy older adults: 2 week longitudinal study using accelerometry and the doubly labeled water method. JMIR Mhealth Uhealth. 2016;4: e5445.
Lee I-M, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. The lancet. 2012;380:219–29.
Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000.
Schliemann B, Glasbrenner J, Rosenbaum D, et al. Changes in gait pattern and early functional results after ACL repair are comparable to those of ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26:374–80.
Mobbs RJ, Mobbs RR, Choy WJ. Proposed objective scoring algorithm for assessment and intervention recovery following surgery for lumbar spinal stenosis based on relevant gait metrics from wearable devices: the Gait Posture index (GPi). J Spine Surg. 2019;5:300–9. https://doi.org/10.21037/jss.2019.09.06.
Smuck M, Muaremi A, Zheng P, et al. Objective measurement of function following lumbar spinal stenosis decompression reveals improved functional capacity with stagnant real-life physical activity. Spine J. 2018;18:15–21. https://doi.org/10.1016/j.spinee.2017.08.262.
Gilmore SJ, Hahne AJ, Davidson M, et al. Physical activity patterns of patients immediately after lumbar surgery. Disabil Rehabil. 2020;42:3793–9. https://doi.org/10.1080/09638288.2019.1610512.
Inoue M, Orita S, Inage K, et al. Objective evaluation of postoperative changes in real-life activity levels in the postoperative course of lumbar spinal surgery using wearable trackers. BMC Musculoskelet Disord. 2020;21:72. https://doi.org/10.1186/s12891-020-3102-2.
Mobbs RJ, Phan K, Maharaj M, et al. Physical activity measured with accelerometer and self-rated disability in lumbar spine surgery: a prospective study. Global Spine Journal. 2016;6:459–64. https://doi.org/10.1055/s-0035-1565259.
Scheer JK, Bakhsheshian J, Keefe MK, et al. Initial experience with real-time continuous physical activity monitoring in patients undergoing spine surgery. Clinical Spine Surgery. 2017;30:E1434–43. https://doi.org/10.1097/BSD.0000000000000521.
Stienen MN, Rezaii PG, Ho AL, et al. Objective activity tracking in spine surgery: a prospective feasibility study with a low-cost consumer grade wearable accelerometer. Sci Rep. 2020;10:4939. https://doi.org/10.1038/s41598-020-61893-4.
Brandes M, Ringling M, Winter C, et al. Changes in physical activity and health-related quality of life during the first year after total knee arthroplasty. Arthritis Care Res (Hoboken). 2011;63:328–34. https://doi.org/10.1002/acr.20384.
Caliskan E, Igdir V, Dogan O, et al. Primary total knee replacement leads to an increase in physical activity but no changes in overall time of sedentary behaviour: a retrospective cohort study using an accelerometer. Int Orthop. 2020;44:2597–602. https://doi.org/10.1007/s00264-020-04720-9.
Frimpong E, McVeigh JA, van der Jagt D, et al. Light intensity physical activity increases and sedentary behavior decreases following total knee arthroplasty in patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2019;27:2196–205. https://doi.org/10.1007/s00167-018-4987-2.
Frimpong E, van der Jagt DR, Mokete L, et al. Improvements in objectively measured activity behaviors do not correlate with improvements in patient-reported outcome measures following total knee arthroplasty. J Arthroplasty. 2020;35:712-719.e714. https://doi.org/10.1016/j.arth.2019.10.016.
Li LC, Feehan LM, Xie H, et al. Effects of a 12-Week Multifaceted Wearable-Based Program for People With Knee Osteoarthritis: randomized Controlled Trial. JMIR Mhealth Uhealth. 2020;8:e19116. https://doi.org/10.2196/19116.
Matsunaga-Myoji Y, Fujita K, Ide S, et al. Improved levels of physical activity in patients over 75 years following total knee arthroplasty. J Orthopaedic Surg. 2019;27:2309499019873363. https://doi.org/10.1177/2309499019873363.
Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote patient monitoring using mobile health for total knee arthroplasty: validation of a wearable and machine learning-based surveillance platform. J Arthroplasty. 2019;34:2253–9. https://doi.org/10.1016/j.arth.2019.05.021.
Taniguchi M, Sawano S, Kugo M, et al. Physical activity promotes gait improvement in patients with total knee arthroplasty. J Arthroplasty. 2016;31:984–8. https://doi.org/10.1016/j.arth.2015.11.012.
Bin Sheeha B, Granat M, Williams A, et al. Does free-living physical activity improve one-year following total knee arthroplasty in patients with osteoarthritis: A prospective study. Osteoarthritis and Cartilage Open. 2020. https://doi.org/10.1016/j.ocarto.2020.100065.
Lin BA, Thomas P, Spiezia F, et al. Changes in daily physical activity before and after total hip arthroplasty A pilot study using accelerometry. Surg. 2013;11:87–91. https://doi.org/10.1016/j.surge.2012.04.006.
Mehta SJ, Hume E, Troxel AB, et al. Effect of remote monitoring on discharge to home, return to activity, and rehospitalization after hip and knee arthroplasty: a randomized clinical trial. JAMA netw. 2020;3:e2028328.
Engdal M, Foss OA, Taraldsen K, et al. Daily physical activity in total hip arthroplasty patients undergoing different surgical approaches: a cohort study. Am J Phys Med Rehabil. 2017;96:473–8. https://doi.org/10.1097/PHM.0000000000000657.
Harding P, Holland AE, Delany C, et al. Do activity levels increase after total hip and knee arthroplasty? Clin Orthop. 2014;472:1502–11. https://doi.org/10.1007/s11999-013-3427-3.
Kalisch T, Horst F, Gosheger G, et al. Everyday Physical Activity and Sedentary Behavior After Total Joint Arthroplasty: Do Patients and Partners Develop an Active Lifestyle? Clin Interv Aging. 2021;16:403–13. https://doi.org/10.2147/CIA.S295160.
Matsunaga-Myoji Y, Fujita K, Makimoto K, et al. Three-Year follow-up study of physical activity, physical function, and health-related quality of life after total hip arthroplasty. J Arthroplasty. 2020;35:198–203. https://doi.org/10.1016/j.arth.2019.08.009.
Lebleu J, Poilvache H, Mahaudens P, et al. Predicting physical activity recovery after hip and knee arthroplasty? A longitudinal cohort study. Braz J Phys Ther. 2021;25:30–9. https://doi.org/10.1016/j.bjpt.2019.12.002.
Thewlis D, Bahl JS, Fraysse F, et al. Objectively measured 24-hour activity profiles before and after total hip arthroplasty. Bone Joint J. 2019;101B:415–25. https://doi.org/10.1302/0301-620X.101B4.BJJ-2018-1240.R1.
Vissers MM, Bussmann JB, de Groot IB, et al. Walking and chair rising performed in the daily life situation before and after total hip arthroplasty. Osteoarthritis Cartilage. 2011;19:1102–7. https://doi.org/10.1016/j.joca.2011.06.004.
Acknowledgements
The authors would like to thank the staff including Devon McCarthy, Collette and Catherine Ragy from NeuroSpine clinic for assisting with conduct of the project and provision of study materials.
Funding
The authors declare that they have no funding.
Author information
Authors and Affiliations
Contributions
PN and RDF researched literature and conceived the study. RJM and MM were involved in protocol development and gaining ethical approval. PN conducted data analysis. PN and RDF wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The Author(s) declare(s) that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Natarajan, P., Fonseka, R.D., Maharaj, M.M. et al. Continuous data capture of gait and mobility metrics using wearable devices for postoperative monitoring in common elective orthopaedic procedures of the hip, knee, and spine: a scoping review. J Orthop Surg Res 18, 812 (2023). https://doi.org/10.1186/s13018-023-04303-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-04303-5