Abstract
Objective
In this study, we aimed to compare the outcomes of the two-stage induced membrane technique (IMT) and one-stage autografting in the treatment of aseptic atrophic nonunion in lower limb long bones.
Methods
From January 2014 to January 2022, we reviewed all surgically treated long bone nonunion patients, including patients aged 18 years or older with atrophic nonunion, who were either treated with the two-stage induced membrane technique (IMT) or one-stage autografting. Outcome parameters interns of clinical, quality of life and healthcare burden were recorded and retrospectively analysed between the two treatment populations. The follow-up time was at least 1 year.
Results
In total, 103 patients who met the criteria for aseptic atrophic nonunion were enrolled. Among them, 41 (39.8%) patients were treated with two-stage IMT, and 62 (60.2%) patients were treated with one-stage autologous bone grafting. The follow-up time was 12 to 68 months, with an average of 28.4 months. The bone healing rate was comparable in both groups (IMT: 92.7% vs. one-stage grafting: 91.9%, P = 0.089) at 12 months post-operation, and the bone healing Lane–Sandhu score was superior in the IMT group (mean: 8.68 vs. 7.81, P = 0.002). Meanwhile, the SF-12 scores of subjective physical component score (PCS) (mean: 21.36 vs. 49.64, P < 0.01) and mental health component score (MCS) (mean: 24.85 vs. 46.14, P < 0.01) significantly increased in the IMT group, as well as in the one-stage grafting group, and no statistically significant difference was found within groups. However, the total hospital stays (median: 8 days vs. 14 days, P < 0.01) and direct medical healthcare costs (median: ¥30,432 vs. ¥56,327, P < 0.05) were greater in the IMT group, while the complications (nonunion 8, infection 3, material failure 2, and donor site pain 6) were not significantly different between the two groups (17.1% vs. 19.4, P = 0.770).
Conclusion
The data indicate that two-stage method of IMT serves as an alternative method in treating atrophic nonunion; however, it may not be a preferred option, in comprehensive considering patient clinical outcomes and healthcare burden. More evidence-based research is needed to further guide clinical decision-making.
Similar content being viewed by others
Introduction
Despite ongoing advances in health care increasing life expectancy, trauma has occurred, and skeletal failure to heal remains a continuing problem regardless of patient age or activity level. The literature [1, 2] shows that the incidence of nonunion in long bone fractures generally varies from 5 to 10%, and it is sometimes as high as 42.7% in high-risk patients. Nonunion often presents as pain or abnormal activity at the fracture site, with serious negative effects on both patient health and quality of life [3, 4]. The current treatment principles of nonunion are mainly based on the “diamond” theory [1, 5, 6]. This focuses on improving the peripheral blood supply, osteoinductive factors, osteoblasts, bone conduction matrix and mechanical stability. The variation in choosing different treatments was mainly according to the underlying causes or clinical types of nonunion.
Among all nonunion types, atrophic nonunion [1, 7,8,9] is common in clinical practice. It usually lacks local blood supply or low osteogenic potential, thus resulting in less callus formation on imaging. Of note, although these patients do not show any signs of infection, the low-toxicity infection is also a possible cause of atrophic nonunion. Based on the "diamond theory" [1, 6], such intervention strategies for nonunion should have the main goal of improving the biological environment of the fracture site. These include the treatment of potential low-toxicity infections [9]. The traditional standard intervention is local debridement plus autologous cancellous bone grafting. In addition, the recent two-stage induced membrane technique (IMT) [10,11,12] is also a treatment option. The first phase of IMT is to insert polymethylmethacrylate (PMMA) into the nonunion gap and form a bioactive membrane (induced membrane, IM) around the PMMA. In the second stage (6–8 weeks), the bone cement is removed, and cancellous bone grafting is performed. IM has high vascularization and can secrete various growth factors [10, 13, 14]. These include transforming growth factor beta 1 (TGF-β1), fibroblast growth factor 2 (FGF-2), bone morphogenetic protein 2 (BMP-2) and vascular endothelial growth factor (VEGF); IM can also mobilize osteogenic precursor cells, thus favouring bone healing. Moreover, bone cement can be used to add antibiotics to provide local anti-infection treatment [15].
However, it is unclear whether the stage method of IMT should be a priority treatment option in clinical decision-making compared to one-stage autograft. Based on the “diamond” theory, we speculate that two-stage IMT treatment can achieve better results in the management of atrophic nonunion by further improving the local biological environment. Therefore, we performed a retrospective cohort study and aimed to compare the outcome of two-stage IMT treatment versus one-stage autograft in the management of aseptic atrophic nonunion in lower limb long bones. In addition to related clinical results, patients’ quality of life and healthcare burden were also investigated and compared between these two methods. Our results may be helpful for clinical decision-making both by surgeons and patients in the management of atrophic nonunion.
Patients and methods
This is a retrospective cohort study. From January 2014 to January 2022, we reviewed all nonunion patients surgically treated at our clinic centre. The diagnosis of nonunion was made according to the U.S. Food and Drug Administration (FDA) criteria [1, 6, 16]: patients were at least 9 months after fracture and showed no signs of further healing within three consecutive months. Atrophic nonunions [9, 17] were defined as previous fractures that exhibited a lack of healing with no callus formation on X-ray. Patients who met the following criteria were included: long bone of the lower limb; atrophic nonunion; age 18 years old or greater; and received either two-stage IMT treatment or one-stage autologous bone grafting. The exclusion criteria were as follows: patients with coinfection according to preoperative clinical or imaging signs; patients with cancer; and patients with incomplete follow-up data or a follow-up time of less than 1 year. This retrospective study was approved by the local medical ethics committee.
Surgical procedures
Before surgical treatment, the previous surgery, clinical types of bone nonunion (atrophic or hypertrophic, based on the local biological microenvironment), the possible size of the defect after debridement must be carefully assessed, as well as the possibility of current or previous infection. The alignment of the bone and the extremity must also be considered. Of note, both IMT treatment and one-stage autologous bone grafts are currently acceptable treatment methods. In clinical practice, patients voluntarily select IMT treatment or one-stage autologous bone grafting, although the final surgery is also at the discretion of the responsible surgeon.
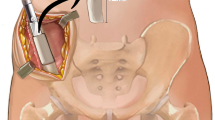
For patients treated with one-stage autologous bone grafting, the main surgical procedures included debridement and immediate bone grafting. First, the surrounding poor-quality tissue or newborn bone is extensively excised until fresh bleeding healthy tissue is left. Of note, whether a prior surgical implant was removed is determined according to whether fixation was ineffective or suspected of underlying low-toxicity infection [17]. Second, the nonunion is stabilized with suitable biomechanical implants accordingly. The definite fixation includes an intramedullary nail, osteosynthesis plate and intramedullary nail plus plate. Finally, the bone gap or defect is filled with autologous bone grafts (Fig. 1), which are obtained from the anterior superior iliac. During each procedure, multiple (often 3–5) tissue samples are collected and analysed for any type of microbial infection.
A female patient (25 years old) with bone nonunion in the right femur was treated with one-stage autologous bone grafting and had an open fracture before; no bacteria were detected after surgery. A Anteroposterior and lateral radiographs showed a clear fracture line and less callus formation at the fracture site after 12 months of initial osteosynthesis; B–E radiographs at 2, 6, 12, and 18 months after one-stage autologous bone grafting treatment. Bone union was achieved at 12 months, and complete bone consolidation was achieved at 18 months
In the two-stage method of IMT therapy, there are usually two separate surgical steps. In the first step, radical debridement of the nonhealing bone end and surrounding soft tissue is performed, and then the disconnected ends are filled with polymethylmethacrylate (PMMA) spacers. All PMMA is impregnated with antibiotics (each 40 g of PMMA powder is mixed with 4 g of vancomycin powder and 500 mg of gentamicin [18]) to treat the underlying infection. In the second step (after 6–8 weeks), the spacer is removed with careful protection of the inducing membrane, and then autologous bone grafting is performed (Fig. 2) with bone also obtained from the anterior superior iliac. In all other respects, the second step of IMT treatment is identical to one-stage autologous bone grafting.
A male patient (28 years old) with bone nonunion in the right femur was treated with the staged method of the induced membrane technique (IMT), and a low-toxicity infection (coagulase-negative Staphylococcus spp.) was detected after surgery. A Preoperative radiographs showed less callus formation at the fracture site after initial osteosynthesis. B Debridement and bone gap filled with antibiotic bone cement. C–F Radiographs at 2, 6, 12, and 18 months after IMT treatment. Bone union was achieved at 12 months, and complete bone consolidation was achieved at 18 months
Follow-up and outcome measures
To evaluate clinical bone healing outcomes and complications, both groups (two-stage IMT treatment versus one-stage autograft treatment) had regular outpatient visits at 2, 6, 9 and 12 months after surgery, and X-ray images of the involved bone were routinely performed to provide continuous observation of fracture healing. The primary outcome was fracture healing. Bone union was defined as an image showing continuous callus formation in three bone cortices. The additional Lane and Sandhu score [19, 20] was used to further compare bone healing between the two groups.
In addition, quality of life evaluated by the 12-item Health Report Brief Form (SF-12) [3, 4] was also collected pre- and postoperatively. Patient follow-up was conducted by full-time clinical research specialists, and complications were also recorded. Meanwhile, the two independent and experienced trauma surgeons were responsible for the clinical assessment of mechanical stability, weight-bearing, postoperative pain, and signs of infection. In this retrospective study, data regarding treatment burden with respect to total hospital stays and direct healthcare costs were collected in addition to information related to treatment and clinical outcome and patient demographics.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software, and the Levene test was used to determine whether measurement data were normally distributed. Continuous variables were expressed as ± s, and a t test with independent samples was used to compare two groups. Count data were expressed as a rate and were compared by the χ2 test. Data that did not follow the distribution of variance were analysed with a nonparametric test. In the two-tailed test, the differences between the two groups were considered statistically significant when the P value was < 0.05.
Results
Demographic characteristics
According to the study criteria, 103 patients with atrophic nonunion were enrolled, including 70 femurs and 33 tibias. All these patients completed at least a 1-year postoperative follow-up ranging from 12 to 68 months, with a mean of 28.4 months. According to the surgical methods, 41 patients were treated with two-stage IMT, and 62 patients were treated with one-stage autologous bone grafting. Among them, patients treated with IMT included 24 femurs and 17 tibias, with a mean age of 39.42 ± 11.90 years. On the other hand, the one-stage autologous grafting group included 46 femurs and 16 tibias with a mean age of 39.44 ± 10.39 years. The basic demographic characteristics of gender, age, smoking and diabetes mellitus, prior trauma and osteosynthesis are presented in Table 1, showing no significant difference between the two groups.
Treatment parameters and clinical outcome
Of all 103 patients, 39 (37.9%) chose an intramedullary nail for definite fixation, 26 (25.2%) chose an osteosynthesis plate alone, and 38 (36.9%) chose an intramedullary nail plus plate. Moreover, a mean bone defect gap of 2.00 ± 0.46 occurred in all patients after debridement. Intraoperative culture and PCR tests also demonstrated that a proportion of atrophic nonunion patients (n = 17, 16.5%) had low toxicity infections, among which coagulase-negative Staphylococcus spp. (n = 8, 7.77%) were the most common bacteria. The fixation methods (P = 0.603), bone defect gaps (P = 0.077) and detected species (P = 0.790) were not significantly different between the patients with two-stage IMT treatment and the patients with one-stage autologous grafts (Tables 2, 3).
In this study, 95 (92.2%) patients achieved bone healing at 12 months of follow-up. The bone healing rate was comparable in both groups (IMT: 92.7% vs. one-stage: 91.9%, P = 0.089), although the bone healing Lane and Sandhu score was superior in the IMT group (8.68 ± 0.96 vs. 7.81 ± 1.76, P = 0.002) (Table 2). Within the total follow-up, 19 (18.4%) patients had complications after treatment, of whom 8 (7.8%) patients had nonunion, 3 (2.9%) patients had infection and 2 (6.9%) patients had material failure. An additional revision to achieve final success was needed among these patients, three of whom received one-stage autologous grafting and ten of whom received two-stage IMT treatment again. In addition, six patients had mild pain at the donor site (anterior iliac) area. For all these complications, there was no significant difference between the two groups (Table 2).
Quality of life and healthcare burden
Before and after surgery over a minimum of a 1-year follow-up period, patients’ healthy quality of life was longitudinally surveyed. Among all atrophic nonunion patients, the preoperative self-reported SF-12 scores, including physical composite (mean: 22.16 ± 6.84) and mental composite scores (mean: 25.24 ± 5.97), were very poor and were significantly below the total scores. Both the physical composite scores (mean: 21.36 ± 5.96 vs. 49.64 ± 3.75) and mental composite scores (mean: 24.85 ± 5.54 vs. 46.14 ± 4.46) were significantly improved at 12 months after treatment in the IMT groups as well as in the one-stage autologous grafting group, with no significant difference between the two groups (Table 3). In addition, since the staging method of IMT causes an increase in the number of operations, the total hospital stays (median: 8 days vs. 14 days, P < 0.01) and direct healthcare costs (¥30,432 vs. ¥56,327, P = 0.026) were significantly greater in the IMT group (Table 3).
Discussion
Atrophic nonunion [1, 9, 17] is mostly caused by inadequate local blood supply and low osteogenic potential, and low toxic infection may also be another important cause. At present, the revision surgery strategy is mainly based on the "diamond theory," which was first proposed by Professor Giannoudis [6]. In the management of nonunion, the author indicated that factors such as peripheral blood vessels, osteoinductive factors, osteoblasts, bone conduction matrix and mechanical stability are most important and need special attention. In this study, all atrophic nonunion patients were treated according to the "diamond theory" [5, 6], and both methods applied in our cohorts aimed to improve biological conditions to achieve final bone healing.
Currently, the treatment options for atrophic nonunion include autologous bone grafts, the use of biological agents and stem cell transplantation. Obviously, the treatment of biological agents [21] and stem cell [22] transplantation are expensive and often have high technical requirements. Recently, Łukasz Szelerski et al. [23, 24] also reported a new method of Ilizarov to treat nonunion, and excellent long-term union was achieved in their cohorts. The greatest benefit of this approach is that there is no need for additional bone grafting, and the damage of the implant to the local blood supply can be reduced. However, the long duration of Ilizarov stabilization (a mean of 7.9 months) in these patients is a concern, and atrophic nonunion also seems to be a risk factor with this method in their reports. Among these, autologous bone grafting [1, 5, 17] is a standard method that provides all the essential properties of bone healing, such as osteoblasts, bone conduction matrix, and bone induction factors. Previously, this standard treatment was often performed within one step (one-stage autologous bone grafting).
However, IMT provides another two-stage treatment strategy [11,12,13,14,15]. In this two-stage surgical method, bone cement is first implanted in the nonunion site after debridement, while bone grafting is performed in the second stage after 6–8 weeks. Many reports [13, 18, 25] have indicated that IMT can significantly improve the success of bone defect treatment compared to one-stage bone grafting in the management of large bone defects. The key to osteogenesis is that an induced membrane [10, 14] can form around the cement; it is highly vascularized and can secrete various growth factors, including transforming growth factor beta 1 (TGF-β1), fibroblast growth factor 2 (FGF-2), bone morphogenetic protein 2 (BMP-2) and vascular endothelial growth factor (VEGF), and it can mobilize precursor cells to promote bone healing. Moreover, PMMA bone cement can also include antibiotics to treat the underlying infection [11, 15]. Comparing the IMT to other surgical procedures, the recent review [26] showed that better functional results were achieved in bone defects due to infection with IMT treatment. Therefore, IMT is theoretically ("diamond theory") more in line with the treatment of atrophic nonunion to improve the biological environment of the fracture site.
In this study, the two-stage method of IMT was used to treat atrophic aseptic nonunion and compared with one-stage autologous bone grafting. Before that, the baseline demographic (e.g. age [1]) and clinical characteristics (e.g. hardware used [27]) were compared, since these can be potential confounding factors affecting the bone union. The cure rate (92.7% vs. 91.9%, P = 0.089) and total complications (17.1% vs. 19.4%, P = 0.770) were comparable in both groups; however, the final Lane and Sandhu score was superior within the IMT treatment group (8.68 ± 0.96 vs. 7.81 ± 1.76, P = 0.002). Previously, Moghaddam [28, 29] et al. reported that the success rate of atrophic nonunion treatment with one-stage autologous bone grafting ranged from 80 to 90%. Our results are supportive of the idea that two-stage IMT serves as an alternative in treating atrophic nonunion since it can achieve good healing by improving the local biological environment.
In addition to the above clinical results, patients’ health quality of life [30, 31] and treatment burden were also assessed in this study. It is clear that the two-stage method of IMT, compared to one-stage autologous bone grafting, significantly increases the number of operations, hospital stays and total medical costs. With respect to patients’ quality of life, we found that both the SF-12 physical composite and mental composite were below the 50th percentile of the total score. These findings were similar in other studies on femoral [32] and tibial [3] nonunions, indicating a detrimental effect on patients’ quality of life. Both the stage method of IMT and one-stage autologous bone grafting can significantly increase patients’ quality of life after surgery; however, no statistically significant difference was found within groups in this study. In comprehensive patient clinical outcomes, quality of life and treatment burden, we suggest that the staging method of IMT seems to not be a preferred option in the management of atrophic nonunion. More evidence-based research and cost-utility analysis are needed to further guide clinical decision-making.
Based on the "diamond theory," the stage method of IMT should be regarded as a supplement to one-stage autologous bone grafting in the management of atrophic nonunion. According to our experience and previous results in the literature, IMT may be more suitable for the following clinical settings: (1) patients with a high risk of infection (e.g. suffering severe open fractures and long-term smoking), as IMT can treat potential low toxicity infections [15]. The results from our total cohort revealed that a proportion of atrophic nonunion patients (n = 17, 16.5%) had infections with low toxicity. Another study [9] indicated that as many as 57% of patients were associated with low-grade infection; (2) patients with large bone defects or without enough autologous bone grafts who require the addition of bone substitutes (e.g. allogeneic bone) since IMT [10, 33, 34] can further improve the local biological environment; and (3) patients who failed previous one-stage autologous bone grafting for unknown reasons. Of note, the treatment options should also reflect some sensitivity to patient tolerance, including patient compliance, increased surgery or costs [35] and patient quality of life [36], as emphasized above in this study.
There are some limitations to this study. First, the total number of patients was relatively small. Although nonunion is a serious complication of fracture and the overall population is large, patients with hypertrophic nonunion or infectious nonunion were excluded from our study. Second, our present study is a retrospective study, and incomplete clinical data collection may occur within the retrospective analysis. Finally, patients choosing the different surgical protocols may have bias, although we have been very neutral to surgical information for both methods. Overall, this study provides a comparison of the two-stage induced membrane technique and one-stage autografting in the management of atrophic nonunion, and our findings will provide basic information for patient and clinician decision-making as well as patient consultation.
Conclusion
The stage method of IMT should be regarded as a supplement to one-stage autologous bone grafting in the management of atrophic nonunion. Current data also indicate that the staging method of IMT may not be a preferred option in comprehensive considering patient clinical outcomes and healthcare burden, although it serves as an alternative in treating atrophic nonunion. The IMT may be more suitable in some high-risk clinical settings by improving the local biological environment. The results of future large-sample, randomized controlled studies and cost-utility analyses are needed to better guide clinical decision-making.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Wiedemann B, Ignatius A, Leung F, Taitsman LA, Smith RM, Pesantez R, et al. Non-union bone fractures. Nat Rev Dis Primers. 2021;7:57.
Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–43.
Brinker MR, Hanus BD, Sen M. The devastating effects of tibial nonunion on health-related quality of life. J Bone Joint Surg Am. 2013;95:2170–6.
Schottel PC, O’Connor DP, Brinker MR. Time trade-off as a measure of health-related quality of life_long bone nonunions have a devastating impact. J Bone Joint Surg Am. 2015;97:1406–10.
Tanner MC, Hagelskamp S, Vlachopoulos W, Miska M, Findeisen S, Grimm A, et al. Non-union treatment based on the “Diamond Concept” is a clinically effective and safe treatment option in older adults. Clin Interv Aging. 2020;15:1221–30.
Andrzejowski P, Giannoudis PV. The “diamond concept” for long bone non-union management. J Orthop Traumatol. 2019;20:21.
Lu J, Guo SC, Wang QY, Sheng JG. J-bone graft with double locking plate: a symphony of mechanics and biology for atrophic distal femoral non-union with bone defect. Cancer Manag Res. 2020;15:144.
Reed AA, Joyner CJ, Isefuku S, Brownlow HC, Simpson AH. Vascularity in a new model of atrophic nonunion. J Bone Joint Surg Br. 2003;85:604–10.
Dapunt U, Spranger O, Gantz S, Burckhardt I, Zimermann S, Schmidmaier G, et al. Are atrophic long-bone nonunions associated with low-grade infections? Therap Clin Risk Manag. 2015;11:1843.
Alford AI, Nicolaou D, Hake M, McBride-Gagyi S. Masquelet’s induced membrane technique: review of current concepts and future directions. J Orthop Res. 2021;39:707–18.
Inci F, Yildirim AO, Kocak C, Yavuz IA, Ceyhan E, Oken OF, et al. Treatment strategies of defect nonunion with vascular damaged by induced membrane technique: Is two-stage treatment sufficient? Injury. 2020;51:1103–8.
Siboni R, Joseph E, Blasco L, Barbe C, Bajolet O, Diallo S, et al. Management of septic non-union of the tibia by the induced membrane technique What factors could improve results? Orthop Traumatol Surg Res OTSR. 2018;104:911–5.
Masquelet A, Kanakaris NK, Obert L, Stafford P, Giannoudis PV. Bone repair using the Masquelet technique. J Bone Joint Surg Am. 2019;101:1024–36.
Aho OM, Lehenkari P, Ristiniemi J, Lehtonen S, Risteli J, Leskela HV. The mechanism of action of induced membranes in bone repair. J Bone Joint Surg Am. 2013;95:597–604.
Raven TF, Moghaddam A, Ermisch C, Westhauser F, Heller R, Bruckner T, et al. Use of Masquelet technique in treatment of septic and atrophic fracture nonunion. Injury. 2019;50(Suppl 3):40–54.
Simpson A, Robiati L, Jalal MMK, Tsang STJ. Non-union: indications for external fixation. Injury. 2019;50(Suppl 1):S73–8.
Bégué T, Mouchantaf M, Aurégan JC. Aseptic humeral shaft nonunion. Orthop Traumatol Surg Res OTSR. 2023;109:103462.
Wu H, Shen J, Yu X, Fu J, Yu S, Sun D, et al. Two stage management of Cierny-Mader type IV chronic osteomyelitis of the long bones. Injury. 2017;48:511–8.
Ferbert T, Münch C, Findeisen S, Pauly W, Miska M, Grossner T, et al. Effect of tricalcium phosphate on healing of non-unions: an observational study of over 400 non-unions. Therap Clin Risk Manag. 2023;19:395–404.
Helbig L, Sun H, Godbout C, Ryan G, Hoit G, Higgins J, et al. The induced membrane technique: optimization of bone grafting in a rat model of segmental bone defect. Ther Clin Risk Manag. 2022;53:1848–53.
Singh R, Bleibleh S, Kanakaris NK, Giannoudis PV. Upper limb non-unions treated with BMP-7: efficacy and clinical results. Injury. 2016;47(Suppl 6):S33–9.
Gómez-Barrena E, Rosset P, Gebhard F, Hernigou P, Baldini N, Rouard H, et al. Feasibility and safety of treating non-unions in tibia, femur and humerus with autologous, expanded, bone marrow-derived mesenchymal stromal cells associated with biphasic calcium phosphate biomaterials in a multicentric, non-comparative trial. Biomaterials. 2019;196:100–8.
Szelerski Ł, Pajchert Kozłowska A, Żarek S, Górski R, Mochocki K, Dejnek M, et al. A new criterion for assessing Ilizarov treatment outcomes in nonunion of the tibia. Arch Orthop Trauma Surg. 2020;141:879–89.
Szelerski Ł, Żarek S, Górski R, Mochocki K, Górski R, Morasiewicz P, et al. Surgical treatment outcomes of the Ilizarov and internal osteosynthesis methods in posttraumatic pseudarthrosis of the tibia—a retrospective comparative analysis. J Orthop Surg Res. 2020;15:179.
Giannoudis PV, Faour O, Goff T, Kanakaris N, Dimitriou R. Masquelet technique for the treatment of bone defects: tips-tricks and future directions. Injury. 2011;42:591–8.
Careri S, Vitiello R, Oliva MS, Ziranu A, Maccauro G, Perisano C. Masquelet technique and osteomyelitis: innovations and literature review. Eur Rev Med Pharmacol Sci. 2019;23:210–6.
Perisano C, Cianni L, Polichetti C, Cannella A, Mosca M, Caravelli S, Maccauro G, Greco T. Plate augmentation in aseptic femoral shaft nonunion after intramedullary nailing: a literature review. Bioengineering (Basel). 2022;9(10):560.
Moghaddam A, Zietzschmann S, Bruckner T, Schmidmaier G. Treatment of atrophic tibia non-unions according to ‘diamond concept’: results of one- and two-step treatment. Injury. 2015;46:S39–50.
Moghaddam A, Thaler B, Bruckner T, Tanner M, Schmidmaier G. Treatment of atrophic femoral non-unions according to the diamond concept: results of one- and two-step surgical procedure. J Orthop. 2017;14:123–33.
Khanna DTJ. Health-related quality of life—an introduction. Am J Manag Care. 2007;13(Suppl 9):S218–22.
Lerner RKEJ, Polomano RC, Cheatle MD, Heppenstall RB. Quality of life assessment of patients with posttraumatic fracture nonunion, chronic refractory osteomyelitis, and lower-extremity amputation. Clin Orthop Relat Res. 1993;295:28–36.
Brinker MR, Trivedi A, O’Connor DP. Debilitating effects of femoral nonunion on health-related quality of life. J Orthop Trauma. 2017;31:e37–42.
Zhao Z, Wang G, Zhang Y, Luo W, Liu S, Zeng Z, et al. Induced membrane technique combined with antibiotic-loaded calcium sulfate-calcium phosphate composite as bone graft expander for the treatment of large infected bone defects: preliminary results of 12 cases. Ann Transl Med. 2020;8:1081.
Wang W, Zuo R, Long H, Wang Y, Zhang Y, Sun C, et al. Advances in the Masquelet technique: myeloid-derived suppressor cells promote angiogenesis in PMMA-induced membranes. Acta Biomater. 2020;108:223–36.
Akacha M, Binkowitz B, Claggett B, Hung HMJ, Mueller-Velten G, Stockbridge N. Assessing treatment effects that capture disease burden in serious chronic diseases. Therap Innov Regul Sci. 2019;53:387–97.
Bergner M. Quality of life, health status, and clinical research. Med Care. 1989;27:S148–56.
Funding
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: this work was supported by the Chongqing Natural Science Foundation Program (cstc2021jcyj-msxmX0541, CSTB2023NSCQ-BHX0202) and the National Natural Science Foundation Program (No. 82202707). The author thanks all the participants in this study.
Author information
Authors and Affiliations
Contributions
HZ contributed to the investigation, data curation and writing–original draft. JF, JS and XW were involved in the investigation and resources. SW curated the data. HW, YH and CH: assisted in the conceptualization, formal analysis, writing—review and editing. All authors reviewed the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the First Affiliated Hospital of Army Medical University, Chongqing, China (No. KY2022073).
Competing interests
All authors declared that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, H., Fu, J., Jie, S. et al. Induced membrane technique versus one-stage autografting in management of atrophic nonunion of long bone in the lower limb: clinical and health burden outcomes. J Orthop Surg Res 18, 853 (2023). https://doi.org/10.1186/s13018-023-04296-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-04296-1