Abstract
Background
Trigger finger is a common disease with a lifetime prevalence of 2%. One of the frequently preferred non-surgical treatments is blinded injection around the A1 pulley. This study aims to compare the clinical results of ultrasound-guided and blinded corticosteroid injection in the trigger finger.
Methods
In this prospective clinical study, 66 patients who had persistent symptoms of a single trigger finger were included. Patients with similar baseline characteristics such as age, gender, triggering period, and comorbidities were randomized. 34 patients had ultrasound-guided (UG), and 32 had blinded injections (BG). QDASH, VAS, time to return to work, and complications were compared between the groups.
Results
The mean age was 52,66 (29–73) years. There were 18 male and 48 female patients. In the UG, the triggering resolved faster, returning to work was earlier, and the medication period was shorter (p < 0.05). A total of 17 patients who had diabetes mellitus received re-injections, 11 of which were in BG and 6 in UG (p < 0.05). Although statistically significantly lower scores were obtained in UG at the 1st and 4th weeks in the QDASH and VAS scores (p < 0.05), at the 12th and 24 weeks, there was no significant difference (p > 0.05).
Conclusion
Using ultrasound guidance for corticosteroid injections is more effective for treating trigger fingers than the blinded method, leading to better results and a faster return to work in the early stages of treatment.
Similar content being viewed by others
Background
Trigger finger (TF) is the inflammation and swelling of the retinacular sheath that gradually restricts the mobility of flexor tendons [1]. This sheath forms a roller system intended to maximize the strength and effectiveness of the flexor tendon [2]. The annular pulley (A1) in the volar of the metacarpal head is the most affected in the trigger finger, where the affected finger suffers from pain, clicking, stiffening and immobility [3].TF diagnosis can be easily made with meticulous physical examination and careful examination of the patient history. Findings in physical examination range from sensitivity in the A1 pulley to stuck fingers in flexion [4]. Before resorting to surgery, the initial treatment applied to treat trigger fingers is the conservative treatment regimen encompassing activity modification, non-steroidal anti-inflammatory drugs, joint immobilization, and corticosteroid injections [3]. However, most patients with advanced trigger fingers are treated with release surgery [5]. Since the introduction of corticosteroids in 1953, corticosteroid injection has proven to be an effective treatment, although histopathological examinations of A1 pulleys found no inflammatory component [6]. Therefore, blinded corticosteroid injection is applied around the A1 pulley using the palpation technique and reduces symptoms between 60 and 90% [7, 8]. Upon the widespread access to ultrasonography in recent years, studies have reported the results they obtained focusing on the accuracy of ultrasound (USG)-guided corticosteroid injection [9,10,11]. USG offers to view the tendon sheath's interior. On the other hand, USG has an extra advantage in showing the potential accompanying pathologies (e.g. ganglion cysts, tendon sheath tumors, tendon sheath effusions, etc.) [12]. There are very few prospective studies comparing the ring ultrasound-guided and blinded injection in the relevant literature. The present study is intended to clinically compare the USG-guided and blinded injection methods to investigate the feasibility of the former approach.
Materials and methods
The present study was designed as a prospective controlled study and initiated upon the approval of the local ethics committee. The present study included successive patients with Quinnel stage 2,3 or 4 trigger fingers who did not respond to the conservative treatment (splint, non-steroidal anti-inflammatory, drugs and exercise) and gave their consent on the patient consent form. The two groups of patients treated with blinded injection (BG) or USG-guided injection (UG) were compared in terms of demographic data, trigger recovery time, QDASH (Quick Disability of Arm, Shoulder and Hand) scores, VAS (Visual Analogue Scale) scores, time until return to work, complications, and other diseases. QDASH scores of the patients were recorded before the injection and at 1–4 to 12–24 weeks after the injection. Their VAS scores were recorded before and after injection.
Patients over the age of 18 who had no history of treatment or surgery for trigger fingers were included in the study. The exclusion criteria herein were having congenital trigger fingers, having multiple trigger fingers, having a neurological disease, having finger contracture, and having rheumatoid arthritis.
In the blinded injection, patients were positioned in a sitting position with the elbow in 90o flexion, the forearm in supination, and the application area was disinfected twice while applying the palpation technique (on the distal palmar crease and volar side of the metacarpophalangeal joint for the first finger) on the A1 pulley. A mixture of 1 mL of betamethasone sodium phosphate 40 mg/mL and 1 mL of prilocaine hydrochloride 20 mg/mL was used. Fingers were passively stretched and extended before and during the injection to prevent intratendinous application. The syringe was also retracted to ensure the injection was not intravenously applied.
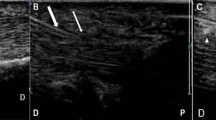
The Samsung trademark ultrasound device and the LA2-14A linear probe with a frequency of 2.0–14.0 MHz were used for ultrasound-guided injections. In the USG-guided injection, patients were positioned in a sitting position with their elbow in 90o flexion, and their forearm in supination, and the application area was disinfected by putting a silicon support under the hand. A distal-to-proximal approach was used for all UG patients. After ensuring that the USG was positioned in parallel with the flexor tendon, the injection was applied under the A1 pulley in parallel with the tendon and into the tendon sheath using a 5 mL syringe with a 27-gauge needle (Fig. 1).
In both groups of the two techniques, the patients were examined for their VAS and QDASH scores at the time of their first-week follow-up, and in those who demonstrated no improvement, the injection was repeated.
Statistical analysis
Excel (Microsoft Corporation, WA, USA) was used to collect and manage the study data, and SPSS version 24.0 (IBM Corporation, Armonk, NY, USA) was used to perform data analyses. The quantitative data were presented as mean ± standard deviation, whereas the qualitative data were presented as frequencies with percentages. The Independent t-test was used to detect the differences in normally distributed numerical values. In contrast, the Mann–Whitney U test was used to detect the differences in non-normally distributed numerical values. Statistical significance was set at a p-value of < 0.05.
Results
The study included 66 fingers of 66 patients, 18 males and 48 females between October 2020 and May 2021. There were 34 fingers in UG and 32 in BG. The demographics of patients are presented in Table 1.
The mean age was 52,66 (29–73) years. The triggering symptoms were relieved in 59.4% of fingers in the first 3 days, 82.6% in the first week. In addition, 11 patients in BG and 6 in UG required re-injection, and all patients who required re-injection had diabetes mellitus. Patients requiring reinjection in both groups received a second injection in the first week. Thereafter, trigger symptoms were relieved by 91.3% in the second week. Only 4 patients had persistent triggering and pain symptoms after the second injection. 2 patients in BG and 2 patients in UG with persistent symptoms were treated with open surgery. All of them had diabetes mellitus and Quinell stage 3 scores. Patients in the BG required significantly more repeat injections than those in the UG (p = 0.000). Two patients in the BG had numbness at the affected finger, which was resolved spontaneously in the second week. 94,2% of patients discontinued NSAIDs after the first week. All patients but two in the blinded group were able to return to work after the 1st week. Between the group comparisons, in the UG, it was found that triggering resolved faster (p = 0.000), returning to work was earlier (p = 0.004), and medication was shorter (p = 0.013). In the QDASH and VAS evaluation, it was observed that statistically significantly lower scores were obtained in the UG at the 1st (p = 0.017, p = 0.019) and 4th(p = 0.005, p = 0.009) weeks (Figs. 2 and 3) (Table 2). However, there was no significant difference in VAS and QDASH scores at the 12th (p = 0.073, p = 0.826) and 24th weeks (p = 0.494, p = 0.320).
Discussion
The present study revealed that ultrasound-guided injections in the treatment of trigger fingers achieved faster clinical recovery and an earlier time to return to work compared to the blinded method. The patients who had ultrasound-guided injections demonstrated better recovery at the end of the first 4-week period, particularly regarding VAS scores. After the first month, both groups were found to have similar functional results and VAS values. The most substantial impact of the current prospective controlled study is that considering those who applied ultrasound-guided injection had shorter recovery time, shorter time to return to work and lower rates of repeated injection. The USG-guided injection method was found to be superior in the early period, while the clinical outcomes were the same in both techniques in the long run.
There are many treatment methods available to treat the trigger finger. However, one of the most frequently preferred treatment options is corticosteroid injection [13]. Although the exact action mechanism of corticosteroids is not well-defined in the treatment of trigger fingers, they are thought to act with their anti-inflammatory characteristics. First described by Howard et al., a corticosteroid injection into the tendon sheath offers advantages such as ease of application, applicability in an office setting, low cost, and low rate of complications [14,15,16]. In addition, ultrasound guidance has become a popular technique to avoid iatrogenic injury when the tendon sheath is located in an anatomically difficult position [9, 10, 17]. However, related literature revealed some studies reporting that out-of-sheath injections also have similar efficacy [18, 19].
Callegari et al. compared open surgery, ultrasound-guided corticosteroid, and hyaluronic acid injection and reported that the ultrasound-guided corticosteroid and hyaluronic acid injection achieved satisfactory outcomes in 14 out of 15 patients (93.3%) [17]. Besides, Bodor and Flossman reported that the ultrasound-guided A1 pulley injection technique is a very effective and minimally invasive treatment option for the trigger fingers, with a 94% success rate within 6 months [10]. The surgical release was applied for all the patients in both groups when the treatment failed. Surgery is more suitable in patients with Quinell stage 4 according to the relevant literature. At this point, we may speculate that associated diabetes, rather than the stage, may play a more critical role in the surgical treatment of stage 3 patients.
In a study where Flensted et al. retrospectively evaluated the recurrence rate after corticosteroid injection, 330 (61%) of the 539 patients had recurrent trigger fingers [20]. The mean recurrence time is 312 days, and the third trigger finger was affected the most commonly. They also argued that this result may be linked to thyroid disease, shoulder diseases, and carpal tunnel syndrome. Similar studies in the literature show that the trigger finger is associated with diabetes, thyroid diseases, carpal tunnel syndrome, and shoulder diseases [15, 21, 22]. As the patients with accompanying upper extremity diseases are excluded from the study, findings concerning associated carpal tunnel syndrome and shoulder diseases were not presented. A notable finding of the present study is that all patients (17) who required repeated injections had associated diabetes mellitus, and 13 (76%) also had associated hypothyroidism.
This study has limitations. The follow-up period is short and the sample size is relatively small. Besides, the USG application has a learning curve and is not available in all orthopedics clinics. The strength of the present study is that it has been well designed as a prospective controlled study including all the follow-ups of the subjects.
The ultrasound-guided corticosteroid injection method was superior to the blinded method in the treatment of the trigger finger due to having a shorter recovery time, an earlier return to work, and fewer injection repetitions. Despite the USG-guided injection method did not differ from the blinded method regarding medium-term clinical outcomes, further prospective studies including larger case series for long-term results are still needed.
Availability of data and materials
Not applicable.
References
Newport ML, Lane LB, Stuchin SA. Treatment of trigger finger by steroid injection. J Hand Surg Am. 1990;15:748–50.
Lin GT, Amadio PC, An KN, Cooney WP. Functional anatomy of the human digital flexor pulley system. J Hand Surg Am. 1989;14:949–56.
Makkouk AH, Oetgen ME, Swigart CR, Dodds SD. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92–6.
Quinnell RC. Conservative management of trigger finger. Practitioner. 1980;224:187–90.
Paulius KL, Maguina P. Ultrasound-assisted percutaneous trigger finger release: is it safe? Hand (NY). 2009;4:35–7.
Drossos K, Remmelink M, Nagy N, de Maertelaer V, Pasteels JL, Schuind F. Correlations between clinical presentations of adult trigger digits and histologic aspects of the A1 pulley. J Hand Surg Am. 2009;34:1429–35.
Rozental TD, Zurakowski D, Blazar PE. Trigger finger: prognostic indicators of recurrence following corticosteroid injection. J Bone Joint Surg Am. 2008;90:1665–72.
Kerrigan CL, Stanwix MG. Using evidence to minimize the cost of trigger finger care. J Hand Surg Am. 2009;34:997–1005.
Godey SK, Bhatti WA, Watson JS, Bayat A. A technique for accurate and safe injection of steroid in trigger digits using ultrasound guidance. Acta Orthop Belg. 2006;72:633–4.
Bodor M, Flossman T. Ultrasound-guided first annular pulley injection for trigger finger. J Ultrasound Med. 2009;28:737–43.
Mardani Kivi M, Lahiji FA, Jandaghi AB, Saheb Ekhtiari K, Hashemi MK. Efficacy of sonographically guided intra-flexoral sheath corticosteroid injection in the treatment of trigger thumb. Acta Orthop Traumatol Turc. 2012;46:346–52.
Lee SA, Kim BH, Kim S-J, Kim JN, Park S-Y, Choi K. Current status of ultrasonography of the finger. Ultrasonography. 2016;35:110–23.
Huisstede BMA, Hoogvliet P, Coert JH, Fridén J. Multidisciplinary consensus guideline for managing trigger finger: results from the European handguide study. Phys Ther. 2014;94:1421–33.
Howard LDJ, Pratt DR, Bunnell S. The use of compound F (hydrocortone) in operative and non-operative conditions of the hand. J Bone Joint Surg Am. 1953;35-A:994–1002.
Nimigan AS, Ross DC, Gan BS. Steroid injections in the management of trigger fingers. Am J Phys Med Rehabil. 2006;85:36–43.
Benson LS, Ptaszek AJ. Injection versus surgery in the treatment of trigger finger. J Hand Surg Am. 1997;22:138–44.
Callegari L, Spanò E, Bini A, Valli F, Genovese E, Fugazzola C. Ultrasound-guided injection of a corticosteroid and hyaluronic acid: a potential new approach to the treatment of trigger finger. Drugs R D. 2011;11:137–45.
Taras JS, Raphael JS, Pan WT, Movagharnia F, Sotereanos DG. Corticosteroid injections for trigger digits: is intrasheath injection necessary? J Hand Surg Am. 1998;23:717–22.
Kazuki K, Egi T, Okada M, Takaoka K. Clinical outcome of extrasynovial steroid injection for trigger finger. Hand Surg. 2006;11:1–4.
Flensted F, Jensen CH, Daugaard H, Vedel J-C, Jørgensen RW. Factors associated with increased risk of recurrence following treatment of trigger finger with corticosteroid injection. J Hand Microsurg. 2021;13:109–13.
Titchener AG, White JJE, Hinchliffe SR, Tambe AA, Hubbard RB, Clark DI. Comorbidities in rotator cuff disease: a case-control study. J Shoulder Elbow Surg. 2014;23:1282–8.
Zhang D, Collins J, Earp BE, Blazar P. Relationship of carpal tunnel release and new onset trigger finger. J Hand Surg Am. 2019;44:28–34.
Funding
There is no funding source.
Author information
Authors and Affiliations
Contributions
MT: Design of the work, drafting the work, approval of the version to be published, agreement to be accountable for all aspects of the work. KT: Interpretation of data for the work, revising the work critically for important intellectual content, approval of the version to be published, agreement to be accountable for all aspects of the work. ÖDA: Interpretation of data for the work, drafting the work, approval of the version to be published, agreement to be accountable for all aspects of the work. HÇT: Interpretation of data for the work, revising the work critically for important intellectual content, approval of the version to be published, agreement to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics and consent to participate
This study was approved by the institutional review board of the No. 2/2020.K-060 of Istinye University.
Competing interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tunçez, M., Turan, K., Aydın, Ö.D. et al. Ultrasound guided versus blinded injection in trigger finger treatment: a prospective controlled study. J Orthop Surg Res 18, 459 (2023). https://doi.org/10.1186/s13018-023-03950-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-03950-y