Abstract
Background
Immune-mediated conditions associated to Corona Virus Disease-19 (COVID-19) have been reported, including vasculitis, antiphospholipid antibody syndrome, myositis, and lupus. Emerging studies have reported the potential occurrence of reactive arthritis in patients previously infected with COVID-19. This systematic review summarised the current evidence on the occurrence of reactive arthritis in patients previously infected by COVID-19.
Methods
This study was conducted according to the 2020 PRISMA guidelines. All the clinical investigations describing the occurrence of reactive arthritis following COVID-19 were accessed. In September 2022, the following databases were accessed: PubMed, Web of Science, Google Scholar, Embase. The generalities of the study were extracted: author, year and journal of publication, country of the main author, study design, sample size, mean age, number of women, main results of the study. The following data on COVID-19 severity and management were retrieved: type of treatment, hospitalization regimes (inpatient or outpatient), admission to the intensive care unit, need of mechanical ventilation, pharmacological management. The following data on reactive arthritis were collected: time elapsed between COVID-19 infection to the onset of reactive arthritis symptoms (days), pharmacological management, type of arthritis (mono- or bilateral, mono- or polyarticular), extra-articular manifestations, presence of tenosynovitis or enthesitis, synovial examination at microscopic polarised light, imaging (radiography, magnetic resonance, sonography), clinical examination, laboratory findings.
Results
Data from 27 case reports (54 patients) were retrieved, with a mean age of 49.8 ± 14.5 years. 54% (29 of 54 patients) were women. The mean time span between COVID-19 infection and the occurrence of reactive arthritis symptoms was 22.3 ± 10.7 days. Between studies diagnosis and management of reactive arthritis were heterogeneous. Symptoms resolved within few days in all studies considered. At last follow-up, all patients were minimally symptomatic or asymptomatic, and no additional therapy or attentions were required by any patient.
Conclusion
Poor evidence suggests that COVID-19 could target the musculoskeletal system causing reactive arthritis at its post infectious stage. COVID-19 can act as a causative agent or as a trigger for development of reactive arthritis even without presence of antibodies of rheumatological disorders. Treating physicians should have a high index of suspicion while treating post infectious COVID-19 patient with arthralgia.
Level of evidence
Level IV, systematic review.
Similar content being viewed by others
Introduction
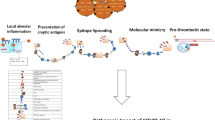
Immune-mediated conditions associated to Corona Virus Disease-19 (COVID-19) infection have been reported, including vasculitis, antiphospholipid antibody syndrome, myositis, and lupus [1, 29]. The underlying immune mechanisms behind the occurrence of immune-mediated manifestations following COVID-19 deserve further investigation. COVID-19 could induce transient immunosuppression [20], which may induce to immune-mediated innate response, with a marked elevation of IL-2, IL-2R, IL-6, IL-7, IL-8 IL-10, IP10, MIP1A, and TNF-α [7, 9, 20, 28, 40, 50]. These interleukins are key in the pathogenesis of psoriasis and psoriatic arthritis [6, 34, 48]. Hence, elevated levels of Interleukin-17 in serum have been observed also in patients with Middle East respiratory syndrome, whereas patients with COVID-19 demonstrated elevated circulating Th17 cells [44].
Emerging studies have reported the potential occurrence of reactive arthritis in patients previously infected with COVID-19. A syndrome consistent with reactive arthritis has been described following HIV, Dengue, Chickungunya and Parvo virus B19 [33, 38, 39, 52]. An extra-articular infection, typically sexually transmitted or gastro intestinal, may trigger reactive arthritis [56]. Common pathogens are Shigella, Chlamydia Trichomatis, Streptococcus, Yersinia, Salmonella and Compylobacter species [5, 31]. However, reactive arthritis may happen also following viral infections [3, 39].
This systematic review summarises the current evidence on the occurrence of reactive arthritis in patients previously infected by COVID-19.
Methods
Eligibility criteria
All the clinical investigations describing the occurrence of reactive arthritis following COVID-19 were accessed. According to the author´ language capabilities, articles in English, German, Italian, French and Spanish were eligible. Studies with level I–IV of evidence, according to Oxford Centre of Evidence-Based Medicine [27], were considered. Reviews, animals, in vitro, biomechanics, computational, and cadaveric studies were not eligible. Only clinical investigation which reported reactive arthritis clinically defined by signs of joint inflammation (dolor, rubor, calor, tumour, functio lesa, either alone or in combination) or by imaging modalities (ultrasounds and/or magnetic resonance) were eligible.
Search strategy
This study was conducted according to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [45]. The following algorithm was preliminary pointed out:
-
Problem reactive arthritis following COVID;
-
Intervention diagnosis and management;
-
Outcome clinical outcome.
In September 2022, the following databases were accessed: PubMed, Web of Science, Google Scholar, Embase. No time constrain was set for the search. The following matrix of keywords were used in each database to accomplish the search using the Boolean operator AND/OR: (COVID-19 OR COVID OR SARS-CoV2 OR pandemic) AND (reactive) AND (osteoarthritis OR arthritis OR knee pain OR ankle pain OR spine pain OR shoulder pain OR hand pain). No additional filters were used in the databases search.
Selection and data collection
Two authors (F. M. and H. S.) independently performed the database search. All the resulting titles were screened by hand and, if suitable, the abstract was accessed. The full-text of the abstracts which matched the topic were accessed. If the full-text was not accessible or available, the article was not considered for inclusion. A cross reference of the bibliography of the full-text articles was also performed for inclusion. Disagreements were debated and mutually solved by the authors. In case of further disagreements, a third author (F.H.) took the final decision.
Data items
Two authors (F.M. and N.M.) independently performed data extraction. The generalities of the study were extracted: author, year and journal of publication, country of the main author, study design, sample size, mean age, number of women, main results of the study. The following data on COVID-19 severity and management were retrieved: type of treatment, hospitalization regimes (inpatient or outpatient), admission to the intensive care unit (ICU), need of mechanical ventilation, pharmacological management. The following data on reactive arthritis were collected: time elapsed between COVID-19 infection to reactive arthritis symptoms (days), pharmacological management, type of arthritis (mono- or bilateral, mono- or polyarticular), extra-articular manifestations, presence of tenosynovitis or enthesitis, synovial examination at microscopic polarised light, imaging (radiography, magnetic resonance, sonography), clinical examination, laboratory findings.
Synthesis methods
The statistical analysis was performed by the main author (F.M.) using the software IBM SPSS (version 25). The arithmetic mean and standard deviation were used for continuous variables.
Results
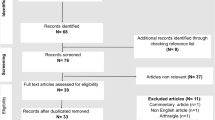
The literature search resulted in 8704 articles. Of them, 1989 were excluded as they were duplicates. A further 6688 articles were excluded with reason: no matching the topic of interest (N = 6357), study type not adequate (N = 301), with uncertain results or diagnosis (N = 15), not reporting any data on the outcomes of interests (N = 12), language limitation (N = 3). Finally, 27 case reports were included in the present systematic review. The results of the literature search are shown in Fig. 1.
Study characteristics and synthesis of results
Data from 54 patients were retrieved, with a mean age of 49.8 ± 14.5 years. 54% (29 of 54 patients) were women. Most studies were conducted in India, Pakistan, Italy and United Kingdom. Two cases per country were reported in Japan, Libanon, Saudi Arabia, Turkey, USA, whereas Denmark Iran Germany, Kazakhstan, Portugal, and Singapore reported only one case each. The generalities and demographic of the included studies are reported in Table 1.
Between patient severity of COVID-19 was heterogeneous. Most patients were affected by mild COVID-19 and did not require hospitalisation. Few studies observed the occurrence of reactive arthritis in inpatients who experienced more severe COVID-19 [22, 26, 35, 43, 53, 54, 57, 58, 63] and required intensive cares and/or mechanical ventilation [22, 43]. The mean time span between COVID-19 infection and the occurrence of reactive arthritis symptoms was 22.3 ± 10.7 days. An overview of the pharmacological management, body location, and main findings of the included studies is shown in Table 2.
Discussion
Low quality evidence suggests that COVID-19 infection can act as a causative agent or as a trigger for the development of reactive arthritis even in patients who did not show the antibodies of rheumatological disorders. The diagnosis was made by exclusion: all patients shared a previous COVID-19 infection approximatively 22 days prior of the symptoms. This length is similar to what described in other reactive arthritis, which is approximately between few days to 4 weeks after an infection [25, 60]. All cases resolved within few days in all studies considered. At last follow-up, all patients were minimally symptomatic or asymptomatic, and no additional therapy or attentions were required by any patient.
The pharmacological management was heterogeneous. The therapy lasted five to 42 days. NSAIDs should be used as first line and are recommended in treatment of peripheral arthritis [16, 21, 36]. Indeed, among the included studies, NSAIDS, including etoricoxib, celecoxib, indomethacin, naproxen, and ibuprofen were the most commonly administered compounds [11, 17, 19, 22, 23, 26, 30, 35, 37, 42, 43, 49, 53, 57, 58]. The most common orally administered steroids were 10–80 mg prednisolone [4, 10, 17, 19, 23, 26, 42, 54, 57], and 4–8 mg of methylprednisolone [2, 15]. Two authors performed intra-articular injections of steroids (e.g. triamcinolone) [41, 43], and one administered 120 mg methylprednisolone intra-muscular daily [11]. The intra-articular use of steroids in mono- or oligoarthritis demonstrated efficacy in previous studies [47, 59]. Opioids (e.g. oxycodone) were also administered orally [13, 58]. Disease-modifying antirheumatic drugs (DMARDs) such as sulfasalazine can also be used [55]. Recently, biologics have also been introduced in the management of ReA [64]. Among the included studies, even if less commonly administered, intra-muscular and oral dilaudid [13], neurontin [13], TNF \(\alpha\) inhibitor [51], certolizumab [51], 1 g sulfasalazine [17], 20 mg leflunomide [42], and 200–400 mg hydroxychloroquine daily [42, 46] were also used. One author also administered 15 mg methotrexate weekly [2, 46].
At plain radiography, an effusion was reported in two patients in plain radiographies [26, 37]. Most patients did not evidence any bony involvement [4, 17, 22, 30, 35, 41, 43, 53], whereas in two cases mild sclerotic changes were noted [37, 51]. At magnetic resonance imaging, mild effusion, with aspecific soft tissue swelling and subcutaneous and bone marrow oedema were evidenced [11, 13, 17, 41, 51, 57]. At joint sonography, synovitis and articular effusion were reported [15, 22, 35, 41, 57] [49]. Four studies documented the involvement of surrounding tendons [13, 15, 17, 46]. Most of patients who had reactive arthritis at the ankle evidenced enthesitis of the Achilles tendon [23, 26, 43, 46, 49]. A mild inflammatory hypercellularity [22, 43] of synovial aspirate, with no evidence of crystal deposition [22, 26, 35, 37, 63], was found in most patients at polarized light microscopic examination.
The location of reactive arthritis was heterogeneous. Similar to other spondiloarthropathies, reactive arthritis has a tendency to affect the lower extremities and can present with enthesitis and dactylitis [21, 32, 56]. Indeed, the knee was the most common location of the pain, followed by the hand and the ankle. Less common locations were the spine and sacroiliac joint, foot, wrist and the hip. Articular involvement in reactive arthritis can be either monoarthritis or oligoarthritis [21, 32]. Hence, most patients had an oligoarthritis [2, 11, 17, 19, 22, 23, 26, 30, 42, 43, 46, 49, 51, 53, 54, 57, 58, 61, 63], and some a monoarticular involvement [10, 13,14,15, 35, 37, 41]. With regards to the body location (right and left) of reactive arthritis, the distribution of the involved joints was bilateral [2, 17, 19, 23, 30, 42, 43, 46, 51, 54, 58, 61], or involved only one side of the body [10, 13,14,15, 22, 26, 35, 37, 41, 49, 57]. There was no symmetrical distribution in most bilateral cases. At clinical examination, warmth, redness, swelling, and decreased range of movement was reported by most authors [10, 13, 15, 22, 23, 30, 37, 41, 53, 58, 61, 63]. Most patients had no associated extra-articular manifestations. A few patients reported associated psoriasis [14, 46], bilateral conjunctivitis [4, 46], myalgia [46], balanitis [4, 37], and symmetrical vasculitis at the calf [54, 61] and trunk [61]. Additional symptoms, including fever, cough, nausea, diarrhoea, and dysgeusia were inconstant.
Serological results were also heterogeneous. C-reactive protein was positive in some patients [2, 4, 11, 15, 17, 23, 26, 35, 46, 49, 53, 54, 63], but not in other patients [13, 19, 22, 30, 43]. Almost all authors investigated the presence of antibodies of rheumatological disorders, and their presence was inconstant. Of 54 patients, 14 (26%) had antibodies. One patient presented anti-carbamylated protein antibody (ACPA) [2], one presented antinuclear antibodies (ANA) [13], two rheumatoid factor (RF) [2, 58], and 11 positive HLA-B27 [11, 17, 46, 54]. These data are consistent with the current evidence, which estimated that 30% to 50% of patients with reactive arthritis have associated positive HLA-B27 [8, 24], and that the disease is five times more prevalent in patients who are HLA-B27 positive compared to the general population [12, 18, 62]. Most patients have no history of autoimmunity, inflammatory bowel disease or travel history, and had been prescribed no new medication.
The results of the present systematic review must be considered in the light of some important limitations. All included studies are case reports, and the results are subjected to several confounders. Case reports lack of generalisability to larger populations of patients, with high risk of over- or mis-interpretation when generalized to clinical practice. In conclusion, the results of the present study lack of generalisability to larger populations of patients, with high risk of over- or mis-interpretation when generalized to clinical practice. Between studies heterogeneity in diagnosis, treatment, examinations, and patient’s ethnicity were evident. Established diagnostic or classification criteria for reactive arthritis are missing; the American College of Rheumatology (ACR) proposed general principles in a 1999 workshop [56]. A registry to collect information on de novo autoimmune presentations would be highly informative. Deeper understanding of the immune mechanism related to COVID-19 may useful opportunity to further investigate the immunopathogenic mechanisms capable of promoting or contrasting the development of specific rheumatic diseases. Close monitoring on the prevalence and expressiveness of rheumatic disorders is required.
Conclusion
Poor evidence suggests that COVID-19 could target the musculoskeletal system causing reactive arthritis at its post infectious stage. COVID-19 can play as a causative agent or as a trigger for reactive arthritis development even in patients without presence of antibodies of rheumatoid disorders. The treating physician or rheumatologist should have a high index of suspicion while treating any post infectious COVID-19 patient with arthralgia.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available throughout the manuscript.
References
Ahmed S, Zimba O, Gasparyan AY. COVID-19 and the clinical course of rheumatic manifestations. Clin Rheumatol. 2021;40:2611–9.
Baimukhamedov C, Barskova T, Matucci-Cerinic M. Arthritis after SARS-CoV-2 infection. Lancet Rheumatol. 2021;3:e324–5.
Barth WF, Segal K. Reactive arthritis (Reiter’s syndrome). Am Fam Phys. 1999;60(499–503):507.
Basheikh M. Reactive arthritis after COVID-19: a case report. Cureus. 2022;14:e24096.
Bawazir Y, Towheed T, Anastassiades T. Post-streptococcal reactive arthritis. Curr Rheumatol Rev. 2020;16:2–8.
Beringer A, Miossec P. Systemic effects of IL-17 in inflammatory arthritis. Nat Rev Rheumatol. 2019;15:491–501.
Bordoni V, Sacchi A, Cimini E, Notari S, Grassi G, Tartaglia E, et al. An inflammatory profile correlates with decreased frequency of cytotoxic cells in coronavirus disease 2019. Clin Infect Dis. 2020;71:2272–5.
Carter JD, Hudson AP. Reactive arthritis: clinical aspects and medical management. Rheum Dis Clin North Am. 2009;35:21–44.
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–9.
Cincinelli G, Di Taranto R, Orsini F, Rindone A, Murgo A, Caporali R. A case report of monoarthritis in a COVID-19 patient and literature review: simple actions for complex times. Medicine (Baltimore). 2021;100:e26089.
Coath FL, Mackay J, Gaffney JK. Axial presentation of reactive arthritis secondary to COVID-19 infection. Rheumatology (Oxford). 2021;60:e232–3.
Colmegna I, Cuchacovich R, Espinoza LR. HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin Microbiol Rev. 2004;17:348–69.
Danssaert Z, Raum G, Hemtasilpa S. Reactive arthritis in a 37-year-old female with SARS-CoV2 infection. Cureus. 2020;12:e9698.
De Stefano L, Rossi S, Montecucco C, Bugatti S. Transient monoarthritis and psoriatic skin lesions following COVID-19. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-218520.
Di Carlo M, Tardella M, Salaffi F. Can SARS-CoV-2 induce reactive arthritis? Clin Exp Rheumatol. 2021;39(Suppl 128):25–6.
Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377:2127–37.
El Hasbani G, Jawad A, Uthman I. Axial and peripheral spondyloarthritis triggered by sars-cov-2 infection: a report of two cases. Reumatismo. 2021;73:59–63.
Feltkamp TE. Factors involved in the pathogenesis of HLA-B27 associated arthritis. Scand J Rheumatol Suppl. 1995;101:213–7.
Fragata I, Mourao AF. Coronavirus disease 19 (COVID-19) complicated with post-viral arthritis. Acta Reumatol Port. 2020;45:278–80.
Garcia LF. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441.
Garcia-Kutzbach A, Chacon-Suchite J, Garcia-Ferrer H, Iraheta I. Reactive arthritis: update 2018. Clin Rheumatol. 2018;37:869–74.
Gasparotto M, Framba V, Piovella C, Doria A, Iaccarino L. Post-COVID-19 arthritis: a case report and literature review. Clin Rheumatol. 2021;40:3357–62.
Gibson M, Sampat K, Coakley G. A self-limiting symmetrical polyarthritis following COVID-19 infection. Rheum Adv Pract. 2020;4(rkaa052):014.
Hannu T. Reactive arthritis. Best Pract Res Clin Rheumatol. 2011;25:347–57.
Hill Gaston JS, Lillicrap MS. Arthritis associated with enteric infection. Best Pract Res Clin Rheumatol. 2003;17:219–39.
Honge BL, Hermansen MF, Storgaard M. Reactive arthritis after COVID-19. BMJ Case Rep. 2021;14:e241375.
Howick J CI, Glasziou P, Greenhalgh T, Carl Heneghan, Liberati A, Moschetti I, Phillips B, Thornton H, Goddard O, Hodgkinson M (2011) The 2011 oxford CEBM levels of evidence. Oxford centre for evidence-based medicine. https://www.cebm.net/index.aspx?o=5653
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
Hyrich KL, Machado PM. Rheumatic disease and COVID-19: epidemiology and outcomes. Nat Rev Rheumatol. 2021;17:71–2.
Jali I. Reactive Arthritis After COVID-19 Infection. Cureus. 2020;12:e11761.
Jubber A, Moorthy A. Reactive arthritis: a clinical review. J R Coll Physicians Edinb. 2021;51:288–97.
Kim PS, Klausmeier TL, Orr DP. Reactive arthritis: a review. J Adolesc Health. 2009;44:309–15.
Kishimoto M, Mor A, Abeles AM, Solomon G, Pillinger MH, Lee MJ. Syphilis mimicking Reiter’s syndrome in an HIV-positive patient. Am J Med Sci. 2006;332:90–2.
Kishimoto T. IL-6: from arthritis to CAR-T-cell therapy and COVID-19. Int Immunol. 2021;33:515–9.
Kocyigit BF, Akyol A. Reactive arthritis after COVID-19: a case-based review. Rheumatol Int. 2021;41:2031–9.
Koehler L, Kuipers JG, Zeidler H. Managing seronegative spondarthritides. Rheumatology (Oxford). 2000;39:360–8.
Liew IY, Mak TM, Cui L, Vasoo S, Lim XR. A case of reactive arthritis secondary to coronavirus disease 2019 infection. J Clin Rheumatol. 2020;26:233.
Mateo L, Roure S. Chronic arthritis in chikungunya virus infection. Reumatol Clin (Engl Ed). 2019;15:113–6.
Mathew AJ, Ravindran V. Infections and arthritis. Best Pract Res Clin Rheumatol. 2014;28:935–59.
McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19:102537.
Mukarram IG, Mukarram MS, Ishaq K, Riaz SU. Post COVID-19 reactive arthritis: an emerging existence in the spectrum of musculoskeletal complications of SARS-CoV-2 infection. J Clin Stud Med Case Rep. 2020;7:101.
Mukarram MS, Ishaq Ghauri M, Sethar S, Afsar N, Riaz A, Ishaq K. COVID-19: an emerging culprit of inflammatory arthritis. Case Rep Rheumatol. 2021;2021:6610340.
Ono K, Kishimoto M, Shimasaki T, Uchida H, Kurai D, Deshpande GA, et al. Reactive arthritis after COVID-19 infection. RMD Open. 2020;6:e001350.
Pacha O, Sallman MA, Evans SE. COVID-19: a case for inhibiting IL-17? Nat Rev Immunol. 2020;20:345–6.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Pal A, Roongta R, Mondal S, Sinha D, Sinhamahapatra P, Ghosh A, et al. Does post-COVID reactive arthritis exist? Experience of a tertiary care centre with a review of the literature. Reumatol Clin. 2022. https://doi.org/10.1016/j.reuma.2022.03.004.
Palazzi C, Olivieri I, D’Amico E, Pennese E, Petricca A. Management of reactive arthritis. Expert Opin Pharmacother. 2004;5:61–70.
Pandolfi F, Franza L, Carusi V, Altamura S, Andriollo G, Nucera E. Interleukin-6 in rheumatoid arthritis. Int J Mol Sci. 2020;21:5238.
Parisi S, Borrelli R, Bianchi S, Fusaro E. Viral arthritis and COVID-19. Lancet Rheumatol. 2020;2:e655–7.
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8.
Saikali W, Gharib S. The first non-radiographic axial spondyloarthrits with COVID-19. Immun Inflamm Dis. 2021;9:628–31.
Salazar V, Jagger BW, Mongkolsapaya J, Burgomaster KE, Dejnirattisai W, Winkler ES, et al. Dengue and Zika virus cross-reactive human monoclonal antibodies protect against Spondweni virus infection and pathogenesis in mice. Cell Rep. 2019;26(1585–1597):e1584.
Saricaoglu EM, Hasanoglu I, Guner R. The first reactive arthritis case associated with COVID-19. J Med Virol. 2021;93:192–3.
Schenker HM, Hagen M, Simon D, Schett G, Manger B. Reactive arthritis and cutaneous vasculitis after SARS-CoV-2 infection. Rheumatology (Oxford). 2021;60:479–80.
Schmitt SK. Reactive arthritis. Infect Dis Clin North Am. 2017;31:265–77.
Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev. 2014;13:546–9.
Shokraee K, Moradi S, Eftekhari T, Shajari R, Masoumi M. Reactive arthritis in the right hip following COVID-19 infection: a case report. Trop Dis Travel Med Vaccines. 2021;7:18.
Sureja NP, Nandamuri D. Reactive arthritis after SARS-CoV-2 infection. Rheumatol Adv Pract. 2021;5:rka001.
Toivanen A. Managing reactive arthritis. Rheumatology (Oxford). 2000;39:117–9.
Toivanen A, Toivanen P. Reactive arthritis. Best Pract Res Clin Rheumatol. 2004;18:689–703.
Waller R, Price E, Carty S, Ahmed ADC. Post COVID-19 reactive arthritis. Rheumatol Adv Pract. 2020;4:rkka52.
Wendling D, Prati C, Chouk M, Verhoeven F. Reactive arthritis: treatment challenges and future perspectives. Curr Rheumatol Rep. 2020;22:29.
Yokogawa N, Minematsu N, Katano H, Suzuki T. Case of acute arthritis following SARS-CoV-2 infection. Ann Rheum Dis. 2021;80:e101.
Zeng H, Luo B, Zhang Y, Xie Z, Ye Z (2020) Treatment of reactive arthritis with biological agents: a review. Biosci Rep 40:
Acknowledgements
None
Registration and protocol
The present review was not registered.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
FM contributed to conception and design, literature search, data extraction, drafting, final approval; NM contributed to literature search, data extraction, revision, final approval; FH contributed to supervision, final approval; RV contributed to revision, final approval; AB contributed to supervision, final approval; Hanno Schenker contributed to drafting, final approval. All authors have agreed to the final version to be published and agree to be accountable for all aspects of the work
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study complies with ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have any competing interests for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Migliorini, F., Bell, A., Vaishya, R. et al. Reactive arthritis following COVID-19 current evidence, diagnosis, and management strategies. J Orthop Surg Res 18, 205 (2023). https://doi.org/10.1186/s13018-023-03651-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-03651-6