Abstract
Background
Recent pieces of evidence about the efficacy of gait rehabilitation for incomplete spinal cord injury remain unclear. We aimed to estimate the treatment effect and find the best gait rehabilitation to regain velocity, distance, and Walking Index Spinal Cord Injury (WISCI) among incomplete spinal cord injury patients.
Method
PubMed and Scopus databases were searched from inception to October 2022. Randomized controlled trials (RCTs) were included in comparison with any of the following: conventional physical therapy, treadmill, functional electrical stimulation and robotic-assisted gait training, and reported at least one outcome. Two reviewers independently selected the studies and extracted the data. Meta-analysis was performed using random-effects or fixed-effect model according to the heterogeneity. Network meta-analysis (NMA) was indirectly compared with all interventions and reported as pooled unstandardized mean difference (USMD) and 95% confidence interval (CI). Surface under the cumulative ranking curve (SUCRA) was calculated to identify the best intervention.
Results
We included 17 RCTs (709 participants) with the mean age of 43.9 years. Acute-phase robotic-assisted gait training significantly improved the velocity (USMD 0.1 m/s, 95% CI 0.05, 0.14), distance (USMD 64.75 m, 95% CI 27.24, 102.27), and WISCI (USMD 3.28, 95% CI 0.12, 6.45) compared to conventional physical therapy. In NMA, functional electrical stimulation had the highest probability of being the best intervention for velocity (66.6%, SUCRA 82.1) and distance (39.7%, SUCRA 67.4), followed by treadmill, functional electrical stimulation plus treadmill, robotic-assisted gait training, and conventional physical therapy, respectively.
Conclusion
Functional electrical stimulation seems to be the best treatment to improve walking velocity and distance for incomplete spinal cord injury patients. However, a large-scale RCT is required to study the adverse events of these interventions.
Trial registration: PROSPERO number CRD42019145797.
Similar content being viewed by others
Introduction
Spinal cord injury temporarily or permanently impairs automatic nervous system, motor, and sensory nerve conduction [1]. Its incidence ranges from 440 to 526 per million population [2]. Levels, types, and extent of injury reflect the muscle strength, spasticity, and gait pattern [3, 4]. Gait rehabilitation may help the patients, especially those with incomplete spinal cord injury, to regain their functions and walking ability [5,6,7,8]. Nowadays, widely used gait improvement programs [5, 9, 10] are hands-on therapy, overground gait training, exercises, and stretching maneuvers. Other potential methods include treadmill; the body-weight-supported treadmill training; functional electrical stimulation; and robotic-assisted gait training. Their possible benefits over conventional physical therapy were neuronal coordination, muscle strength, motor function, balance, walking, and physiologic gait improvement [5, 9, 11].
The efficacy of various gait rehabilitations has been evaluated. Conventional physical therapy insignificantly enhanced the gait velocity compared to the body-weight-supported treadmill training with or without electrical stimulation [12]. On the other hand, robotic-assisted gait training improved Walking Index of Spinal Cord Injury (WISCI) more than conventional physical therapy and treadmill [11, 13]. Evidence is still limited for functional electrical stimulation compared to other methods [14,15,16], particularly robotic-assisted gait training [7, 17]. Moreover, there is no comprehensive review of gait training interventions focused on walking ability (i.e., velocity and distance) and safety among incomplete spinal cord injury [11]. A network meta-analysis would be able to demonstrate myriad effects of these interventions.
This systematic review and network meta-analysis of randomized controlled trials (RCTs) aimed to find the best intervention for incomplete spinal cord injury, such as conventional physical therapy, treadmill, functional electrical stimulation, and robotic-assisted gait training. The velocity improvement, distance and functional score of walking as well as safety issues were comprehensively assessed in this study.
Methods
Our systematic review and network meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines extension for network meta-analysis [18]. The study was registered at The International Prospective Register of Systematic Reviews; PROSPERO (ID: CRD42019145797).
One author identified all relevant studies in PubMed and Scopus databases as well as the research works published in academic journals and proceedings and previous systematic reviews with their reference lists up to October 2022. There was no language and status of publication restriction. Search terms comprised spinal cord injury, paralyzed, orthotic, physical therapy, electrical stimulation, treadmill, exoskeleton, robot, gait, velocity, walk and related terms as shown in Additional file 1: Table S1. Two reviewers selected RCTs based on titles and abstracts. If the decision could not be made, full articles were reviewed. Disagreements were resolved by discussion.
Eligible criteria were RCTs involving incomplete spinal cord injury patients with the American Spinal Cord Injury Association (ASIA) Impairment Scale classification grade B, C, and D [19] in comparison with any pairs of the following interventions: conventional physical therapy, functional electrical stimulation, treadmill, and robotic-assisted gait training. We excluded duplicated studies, those with insufficient data for pooling, and inaccessible full-text articles. The study factors were country of recruitment, sample sizes, study subject characteristics (age, time of injury, level of injury, ASIA impairment scale), and duration of interventions. The main outcome represented gait function including velocity (m/s), distance (m), WISCI [20], and WISCI II [21]. The secondary outcomes were any adverse events during gait training such as fall and pressure ulcer. Two reviewers independently retrieved the data using a standardized data extraction form. The quality assessment was separately evaluated by the two reviewers using the revised Cochrane risk-of-bias tool for randomized trials (RoB2) [22]. Each study was classified as having a low, high, or some concern of risk of bias. Any disagreements in data were resolved by team discussion.
Statistical analysis
The direct meta-analysis was performed for each pair of the interventions when there were at least three studies. For continuous outcomes (velocity, distance, and WISCI score), unstandardized mean difference (USMD) with 95% confidence interval (CI) was estimated. The fixed-effects or random-effects model was used according to no or present heterogeneity (Cochrane's Q test p value < 0.1 or Higgins I2 > 25%), respectively. Sources of heterogeneity were explored by fitting each co-variable (level of injury, duration of injury, ASIA impairment scale, and time of training) in a meta-regression model. An asymmetric funnel plot or p value of the Egger’s test less than 0.05 indicated publication bias.
Network meta-analysis was indirectly compared with relative treatment effects across studies. We used linear regression to estimate mean differences for continuous outcomes. Multivariate random-effects meta-analysis with consistency model was performed to pool the relative treatment effects across the studies. Transitivity was indirectly explored by assessing the distribution of the effect of factors (age, gender, ASIA impairment scale, time of injury, and time of training) on the interested outcome between intervention arms. Consistency, agreement between direct and indirect comparisons, was evaluated by a global chi-square test using a design–treatment interaction inconsistency model. In case of significant global chi-square test (p < 0.05), a loop-specific approach was used to identify the treatment arms and studies that mainly contributed to the inconsistency. If inconsistency factors (IF) were greater than or equal to 2, patients’ characteristics (i.e., age, level of injury, duration of injury, lesion of injury, and ASIA impairment scale) among treatment arms of the closed loop were explored. A sensitivity analysis was performed by excluding the studies with different characteristics and rechecked the inconsistency assumption with a design-by-treatment interaction model. The probability of being the best intervention was assessed by the probability closest to 100. Surface under the cumulative ranking curve (SUCRA) and rankogram plot were used for ranking treatments [23]. The treatment effect in the future for each treatment regimen was estimated by the predictive interval. Publication bias was checked by using an adjusted funnel plot.
All analyses were performed using the STATA software package, version 16.0 (StataCorp, College Station, Texas, USA). The level of significance was < 0.05 for a two-sided p value and < 0.1 for a one-sided p value of heterogeneity test.
Results
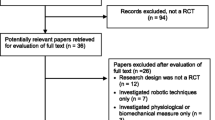
We identified 10,210 publications from PubMed (6766 studies) and Scopus (3444 studies) as shown in Additional file 1: Table S2. Of these, 720 duplicated studies were discarded. After screening titles, abstracts, and complete full-text review, 9469 studies were excluded leaving 17 RCTs (709 participants) for the review (Fig. 1). Fifteen studies were designed as parallel [17, 24,25,26,27,28,29,30,31,32,33,34,35,36,37], and the other two were crossover [38, 39]. Characteristics of included RCTs were the number of subjects ranging from 9 to 146, mean time of injury from 3 to 139 months, C1-L4 level, and ASIA impairment scale grade B-D (Table 1). They evaluated the effects of conventional physical therapy (94%) [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39], robotic-assisted gait training (59%) [17, 25, 29,30,31, 33,34,35, 37, 38], treadmill (47%) [17, 24, 26, 28, 31, 32, 36, 39], functional electrical stimulation + treadmill (12%) [17, 27], and functional electrical stimulation (6%) [17]. Common duration of interventions across the included studies ranged from 3 to 16 weeks. Details of intervention and outcomes for the analysis are presented in Table 2.
The risk-of-bias assessment is shown in Fig. 2. The overall results were of medium quality (29% high [26, 29, 32, 33, 37], 59% moderate [17, 24, 25, 27, 28, 34,35,36, 38, 39], and 12% low quality [30, 31]). High-quality studies demonstrated overall low risk of biases. Moderate-quality studies had some concerns about randomization process and/or deviation from the intended intervention, measurement of the outcome, and selection of the reported results, while low-quality studies had a high risk of deviation from the intended intervention and measurement of the outcome.
Direct meta-analysis
Direct meta-analysis involved 15 studies (601 patients) [24,25,26, 28,29,30,31,32,33,34,35,36,37,38,39] and three interventions (conventional physical therapy, treadmill, and robotic-assisted gait training). The studies reported the outcomes of velocity, distance, and WISCI without adverse events (Table 3). Publication biases are shown in Additional file 1: Fig. S1–S3.
Velocity
Conventional physical therapy insignificantly increased velocity compared to treadmill [24, 26, 28, 39] (pooled USMD − 0.03 m/s, 95% CI − 0.14, 0.19; no heterogeneity I2 = 0%, p value = 0.69) and robotic-assisted gait training [25, 29, 30, 33, 37, 38] (pooled USMD 0.04 m/s, 95% CI − 0.04, 0.12; heterogeneity I2 = 70%, p value < 0.01). Publication bias of both pairs was absent (p value = 0.435, and 0.655, respectively). Subgroup analysis (Additional file 1: Fig. S4, S5) showed that robotic-assisted gait training improved velocity more than conventional physical therapy in acute-phase patients (time of injury < 6 months) (pooled USMD 0.1 m/s, 95% CI 0.05, 0.14; no heterogeneity I2 = 0%, p value = 0.76) and underwent at least 2-month duration of intervention (pooled USMD 0.1 m/s, 95% CI 0.06, 0.14; no heterogeneity I2 = 0%, p value = 0.91). Regarding to the level of cervical, thoracic, and lumbar spinal cord injury, subgroup analysis showed no significant difference of velocity between robotic-assisted gait training and conventional physical therapy (Additional file 1: Fig. S6).
Distance
Conventional physical therapy was comparable with treadmill [26, 28, 31] (pooled USMD 76.00 m, 95% CI − 85.22, 236.96; heterogeneity I2 = 99%, p value < 0.01) and robotic-assisted gait training [25, 29, 31, 33, 37] (pooled USMD 65.34 m, 95% CI − 36.26, 166.92; heterogeneity I2 = 99%, p value < 0.001). There was no publication bias with p value = 0.930, and 0.075, respectively. Regarding subgroup analysis for acute-phase (Additional file 1: Fig. S7), robotic-assisted gait training provided longer walking distance than conventional physical therapy (pooled USMD 64.75 m, 95% CI 27.24, 102.27; heterogeneity I2 = 55%, p value = 0.14). Moreover, subgroup analysis for the level of cervical, thoracic, and lumbar spinal cord injury showed that robotic-assisted gait training improved distance more than conventional physical therapy (pooled USMD 40.45 m, 95% CI 14.69, 66.20; no heterogeneity I2 = 0%, p value = 0.89), Additional file 1: Fig. S8.
WISCI
Compared to conventional physical therapy, robotic-assisted gait training significantly increased WISCI [29, 33,34,35, 38] (pooled USMD 3.28, 95% CI 0.12, 6.45; heterogeneity I2 = 90%, p value < 0.01), whereas treadmill showed no significant differences [32, 36, 39] (pooled USMD − 0.08, 95% CI − 0.93, 0.78; no heterogeneity I2 = 0%, p value = 0.73). Publication bias of both pairs was absent (p value = 0.160, and 0.727, respectively). Regarding subgroup analysis for studies included cervical, thoracic, and lumbar spinal cord injury, robotic-assisted gait training improved WISCI score more than conventional physical therapy (pooled USMD 2.86, 95% CI 0.07, 5.66; heterogeneity I2 = 28.09%, p value = 0.24), Additional file 1: Fig. S9.
Network meta-analysis
Network meta-analysis included 13 studies (709 patients) [17, 24,25,26,27,28,29,30,31, 33, 37,38,39], 5 interventions (conventional physical therapy, functional electrical stimulation, treadmill, robotic-assisted gait training, functional electrical stimulation + treadmill), indirect and 9 direct comparisons (Fig. 3). Only velocity and distance outcomes had adequate studies to be pooled (Table 4).
Network of possible comparisons between intervention and comparator for a velocity and b distance. CPT = conventional physical therapy, FES = functional electrical stimulation, TM = treadmill, RAGT = robotic-assisted gait training, FES + TM = functional electrical stimulation combined with treadmill. The width of the lines is proportional to the numbers of studies and the size of the nodes is proportional to the sample size. Numbers along the lines refer to numbers of studies/numbers of patients corresponding to direct comparisons
Velocity
Twelve studies (10 RCTs [17, 24,25,26,27,28,29,30, 33, 37] and 2 crossover design [38, 39]) involving 559 patients and the velocity outcome were pooled. There was no evidence of inconsistency (global chi-square = 0.90, p value = 0.64). The network map was constructed for multiple comparisons of 5 interventions and also 9 direct comparisons (Fig. 3). Functional electrical stimulation showed insignificant treatment benefit when compared to conventional physical therapy, treadmill, robotic-assisted gait training, and functional electrical stimulation + treadmill with pooled USMD (95% CI) of 0.12 (− 0.07, 0.31), 0.07 (− 0.12, 0.25), 0.08 (− 0.10, 0.26), and 0.07 (− 0.12, 0.26) m/s, respectively (Table 4). From Fig. 4, functional electrical stimulation had the highest probability of being the best velocity improvement (66.6%), followed by treadmill (14.3%), functional electrical stimulation + treadmill (11.6%), robotic-assisted gait training (6.7%), and conventional physical therapy (0.8%).
Rankograms for a velocity and b distance network showing the probability for each intervention being the best to improve walking velocity in patients with incomplete spinal cord injury, CPT = conventional physical therapy, FES = functional electrical stimulation, TM = treadmill, RAGT = robotic-assisted gait training, FES + TM = functional electrical stimulation combined with treadmill
Distance
Data from 9 RCTs [17, 25,26,27,28,29, 31, 33, 37] including 496 patients and distance outcomes were analyzed. The design-by-treatment interaction model showed evidence of inconsistency (global chi-square = 60.50, df = 8, p value < 0.001). Sensitivity analysis was performed by excluding one study comparing robotic-assisted gait training and conventional therapy [31] since the authors did not report the number of participants in each ASIA impairment scale. The model inconsistency was reduced (global chi-square = 0.26, p value = 0.880). The network map was constructed for multiple comparisons of 5 interventions and 9 direct comparisons (Fig. 3). Treatment effect of functional electrical stimulation was superior to conventional physical therapy, treadmill, robotic-assisted gait training and functional electrical stimulation + treadmill with pooled USMD (95% CI) of 76.26 (− 59.68, 212.20), 2.90 (− 131.89, 137.70), 38.69 (− 95.66, 173.03), and 7.62 (− 132.66, 147.91) m, respectively (Table 4). Functional electrical stimulation was the highest probability of being the best intervention (39.7), followed by treadmill (29.8), functional electrical stimulation + treadmill (23.7), robotic-assisted gait training (6.7), and conventional physical therapy (0.1) (Fig. 4).
Nonsignificant predictive interval plots of velocity and distance outcomes indicated that future studies tend to produce similar results to ours (Additional file 1: Fig. S10, S11).
Discussion
Our systematic review and network meta-analysis evaluated the treatment effects among 5 gait training interventions to improve walking ability of incomplete spinal cord injury patients. Most of the included 17 RCTs were low to some of concerned risks of biases. From direct meta-analysis, robotic-assisted gait training tended to provide faster walking rate, longer distance, and significantly higher WISCI scores than conventional physical therapy. With regards to the network meta-analysis, there was no significant differences of velocity and distance between gait training interventions. Functional electrical stimulation tended to be the most effective gait training method for both velocity and distance outcomes (probability of being the best treatment 66.6% and 39.7%, respectively) followed by treadmill, functional electrical stimulation + treadmill, robotic-assisted gait training, and conventional physical therapy, respectively.
Robotic-assisted gait training provided better functional level than conventional physical therapy with nonsignificant different speed and distance [13]. Our reviews demonstrated significant 3.3 WISCI score improvement, and subgroup analyses among acute-phase patients with robotic-assisted gait training for at least 2 months significantly increased 0.1 m/s walking rate, and 64.75 m longer distance than the conventional physical therapy. Moreover, subgroup analyses of the level of cervical, thoracic, and lumbar spinal cord injury with robotic-assisted gait training significantly increased 40.45 m longer distance and 2.86 WISCI score than the conventional physical therapy. This might be due to low muscle tone during acute stage. Spasticity usually started 2–6 months after spinal cord injury [40,41,42]. Joint stiffness, muscle shortening [43], and neural plasticity [44, 45] would impact functional independence and gait training program [43, 46]. The higher the level of spinal cord injury, the more dysfunction of the body, and gait improvement. Either assistant or resistant robotic-assisted gait training could offer advantages in limb control, muscle strengths [47], physiological and reproducible gait patterns [48]. A network meta-analysis by Ma et al. [11] demonstrated that robotic-assisted gait training with overground training improved WISCI score with the highest SUCRA value (88.5) when compared to body weight support treadmill training and body-weight-supported overground training. Additional physical therapy, time of training, and velocity measurement might influence the effects of treatment. However, our subgroup analysis did not find significant difference.
According to our network meta-analysis, functional electrical stimulation was preferred to functional electrical stimulation + treadmill and treadmill in terms of velocity and distance without statistically significance. Functional electrical stimulation can enhance gait, muscle strength, and cardiorespiratory fitness for spinal cord injury patients [49]. Overground training might allow lifting, assisting, and walking variability [50, 51]. Field-Fote et al. [17] reported that functional electrical stimulation combined with overground training facilitated walking tasks greater than its combination with body-weight-supported treadmill training. However, including highly impaired participants in functional electrical stimulation + treadmill may cause inferior results [17]. Treadmill alone dominated the effect of walking velocity and distance compared to functional electrical stimulation + treadmill. This method triggers central pattern generators (CPGs) within the spinal cord [52] and improves stride and balance [53], whereas functional electrical stimulation improves foot drop and coordination of the lower limb movement [54,55,56]. Previous research works showed that combined functional electrical stimulation + treadmill could improve velocity and distance more than treadmill [17, 54]. Nevertheless, Kesar et al. [54] found nonsignificant difference of percent propulsion of ankle ground reaction force which correlated with velocity [57, 58]. This controversy still needs more evidence to support results. Robotic-assisted gait training and conventional physical therapy showed lower velocity and distance improvement than functional electrical stimulation. Even though these three gait rehabilitations build up muscle strengths and balance in spinal cord injury patients [11,12,13], functional electrical stimulation specifically activates weak muscles to improve foot clearance, stride length [59] as well as walking velocity and distance. Moreover, functional electrical stimulation can alleviate pain and spasticity which might reinforce gait training [60].
Strengths of our study are rigorous methodology as research questions focusing on incomplete spinal cord injury, following PRISMA guidelines, and including recently published RCTs without language restriction. Our search terms covered common gait training interventions and assessed specific outcomes as walking velocity, distance, and WISCI score. We investigated sources of heterogeneity by meta-regression and subgroup analysis and conducted sensitivity analysis by removing the heterogonous studies. Limitations of this review are small number of included studies in some comparison arms leading to imprecise/insignificant estimated treatment effects. Although 88% of included RCTs had low to some concern risk of bias, some of them did not report concealment (selection bias) and were unable to blind outcome assessors (measurement bias). Moreover, weighting methods for risk of biases did not apply for the analysis. The treatment effect of each intervention was based on average 2-month training which is inapplicable for long-term results. No included studies reported adverse events (fall, fracture, rash, and pressure ulcers) during the training period. We, therefore, are unable to presume gait training safety for incomplete spinal cord injury.
Robotic-assisted gait training significantly improves WISCI score more than conventional physical therapy. Velocity and distance of walking appear to be significant in acute phase of incomplete spinal cord injury patients. However, this gait training is very expensive (40,000–150,000 USD) [61] and requires cheaper price manufacturing with adapted home use. Among the 5 gait trainings, functional electrical stimulation tended to be the most effective intervention to improve velocity and distance of walking. Since functional electrical stimulation had low number of treatment arms and was not included in the direct meta-analysis, only indirect comparisons have been made in the network meta-analysis with nonsignificant difference from other interventions. The interpretation of the final results should be with caution. However, this modality is practical and cost-effective [62, 63] and provides good outcomes with affordable price. Further RCTs are recommended to compare robotic-assisted gait training and functional electrical stimulation to achieve a proper conclusion.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- WISCI:
-
Walking Index Spinal Cord Injury
- RCT:
-
Randomized controlled trial
- NMA:
-
Network meta-analysis
- USMD:
-
Unstandardized mean difference
- CI:
-
Confidence interval
- SUCRA:
-
Surface under the cumulative ranking curve
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- ASIA:
-
With the American spinal cord injury association
- RoB-2:
-
The revised Cochrane risk-of-bias tool for randomized trials
References
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34:535–46.
Kang Y, Ding H, Zhou H, Wei Z, Liu L, Pan D, et al. Epidemiology of worldwide spinal cord injury: a literature review. J Neurorestoratology. 2017;6:1–9.
Pérez-Nombela S, Barroso F, Torricelli D, de Los R-G, Del-Ama A, Gómez-Soriano J, et al. Modular control of gait after incomplete spinal cord injury: differences between sides. Spinal Cord. 2017;55:79–86.
Nas K, Yazmalar L, Şah V, Aydın A, Öneş K. Rehabilitation of spinal cord injuries. World J Orthop. 2015;6:8–16.
Amatachaya S, Keawsutthi MJJ, Therapy P. Gait rehabilitation for patients with incomplete spinal cord injury (iSCI): conventional and treadmill training. J Med Technol. 2007;19:100–7.
Noamani A, Lemay J-F, Musselman KE, Rouhani H. Rehabilitation. Postural control strategy after incomplete spinal cord injury: effect of sensory inputs on trunk–leg movement coordination. J Neuroeng Rehabil. 2020;17:1–12.
Field-Fote EC, Lindley SD, Sherman AL. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J Neurol Phys Ther. 2005;29:127–37.
Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. J Arch Phys Med Rehabil. 2005;86:672–80.
Cristante AF, Barros Filho TE, Marcon RM, Letaif OB, Rocha ID. Therapeutic approaches for spinal cord injury. Clinics. 2012;67:1219–24.
Mikolajczyk T, Ciobanu I, Badea DI, Iliescu A, Pizzamiglio S, Schauer T, et al. Advanced technology for gait rehabilitation: an overview. J Adv Mech Eng. 2018;10:1–19.
Ma D-N, Zhang X-Q, Ying J, Chen Z-J, Li L-X. Efficacy and safety of 9 nonoperative regimens for the treatment of spinal cord injury: a network meta-analysis. J Med. 2017;96:8679–87.
Harvey LA, Glinsky J, Bowden JL. The effectiveness of 22 commonly administered physiotherapy interventions for people with spinal cord injury: a systematic review. Spinal cord. 2016;54:914–23.
Nam KY, Kim HJ, Kwon BS, Park J-W, Lee HJ, Yoo AJJ, et al. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: a systematic review. J Neuroeng Rehabil. 2017;14:24–36.
Harvey LA. Physiotherapy rehabilitation for people with spinal cord injuries. J Physiother. 2016;62:4–11.
Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst Rev. 2012;33:768–77.
Zhang Y, Ji S, Peng X. Meta-analysis for efficacy of walking locomotor training on improving walking locomotion in chronic spinal cord injury. Chin J Rehabil Med. 2009;24:153–7.
Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. J Phys Ther. 2011;91:48–60.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:1–34.
Roberts TT, Leonard GR, Cepela DJ. Classifications in brief: american spinal injury association (ASIA) impairment scale. Clin Orthop Relat Res. 2017;475:1499–504.
Ditunno JF Jr, Ditunno PL, Graziani V, Scivoletto G, Bernardi M, Castellano V, et al. Walking index for spinal cord injury (WISCI): an international multicenter validity and reliability study. Spinal Cord. 2000;38:234–43.
Dittuno PL, Ditunno JF Jr. Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord. 2001;39:654–6.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS ONE. 2013;8:76654.
Alexeeva N, Sames C, Jacobs PL, Hobday L, DiStasio MM, Mitchell SA, et al. Comparison of training methods to improve walking in persons with chronic spinal cord injury: a randomized clinical trial. J Spinal Cord Med. 2011;34:362–79.
Chang S-H, Afzal T, Berliner J, Francisco GE. Exoskeleton-assisted gait training to improve gait in individuals with spinal cord injury: a pilot randomized study. Pilot Feasibil Stud. 2018;4:1–10.
Dobkin B, Barbeau H, Deforge D, Ditunno J, Elashoff R, Apple D, et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized spinal cord injury locomotor trial. Neurorehabil Neural Repair. 2007;21:25–35.
Kapadia N, Masani K, Catharine Craven B, Giangregorio LM, Hitzig SL, Richards K, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on walking competency. J Spinal Cord Med. 2014;37:511–24.
Lucareli P, Lima M, Lima F, De Almeida J, Brech G, Greve JDA. Gait analysis following treadmill training with body weight support versus conventional physical therapy: a prospective randomized controlled single blind study. Spinal Cord. 2011;49:1001–7.
Alcobendas-Maestro M, Esclarín-Ruz A, Casado-López RM, Muñoz-González A, Pérez-Mateos G, González-Valdizán E, et al. Lokomat robotic-assisted versus overground training within 3 to 6 months of incomplete spinal cord lesion: randomized controlled trial. J Neurorehabil Neural Repair. 2012;26:1058–63.
Tang Q, Huang Q, Hu C. Research on design theory and compliant control for underactuated lower-extremity rehabilitation robotic systems code:(51175368) 2012.01–2015.12. J Phys Ther Sci. 2014;26:1597–9.
Hornby G, Campbell D, Zemon D, Kahn J. Clinical and quantitative evaluation of robotic-assisted treadmill walking to retrain ambulation after spinal cord injury. Top Spinal Cord Inj Rehabil. 2005;11:1–17.
Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. J Neurol. 2006;66:484–93.
Esclarín-Ruz A, Alcobendas-Maestro M, Casado-Lopez R, Perez-Mateos G, Florido-Sanchez MA, Gonzalez-Valdizan E, et al. A comparison of robotic walking therapy and conventional walking therapy in individuals with upper versus lower motor neuron lesions: a randomized controlled trial. J Arch Phys Med Rehabil. 2014;95:1023–31.
Lin H, Zhang T, Chen Q. Effect of robot-assisted gait training on walking ability in patients with incomplete spinal cord injury. Acta Mech Solida Sin. 2016;42:1832–8.
Shin JC, Kim JY, Park HK, Kim NY. Effect of robotic-assisted gait training in patients with incomplete spinal cord injury. Ann Rehabil Med. 2014;38:719.
Senthilvelkumar T, Magimairaj H, Fletcher J, Tharion G, George J. Comparison of body weight-supported treadmill training versus body weight-supported overground training in people with incomplete tetraplegia: a pilot randomized trial. Clin Rehabil. 2015;29:42–9.
Edwards DJ, Forrest G, Cortes M, Weightman MM, Sadowsky C, Chang SH, et al. Walking improvement in chronic incomplete spinal cord injury with exoskeleton robotic training (WISE): a randomized controlled trial. Spinal Cord. 2022;60:522–32.
Labruyère R, van Hedel HJ. Strength training versus robot-assisted gait training after incomplete spinal cord injury: a randomized pilot study in patients depending on walking assistance. J Neuroeng Rehabil. 2014;11:1–12.
Yang JF, Musselman KE, Livingstone D, Brunton K, Hendricks G, Hill D, et al. Repetitive mass practice or focused precise practice for retraining walking after incomplete spinal cord injury? A pilot randomized clinical trial. Neurorehabil Neural Repair. 2014;28:314–24.
Hagen EM. Acute complications of spinal cord injuries. World J Orthop. 2015;6:17–23.
Dudley-Javoroski S, Shields RK. Muscle and bone plasticity after spinal cord injury: review of adaptations to disuse and to electrical muscle stimulation. J Rehabil Res Dev. 2008;45:283–96.
Hiersemenzel L-P, Curt A, Dietz V. From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology. 2000;54:1574–82.
Sezer N, Akkuş S, Uğurlu FG. Chronic complications of spinal cord injury. World J Orthop. 2015;6:24–33.
Su F, Xu W. Enhancing brain plasticity to promote stroke recovery. Front Neurol. 2020;11:554089.
Liu J, Yang X, Jiang L, Wang C, Yang M. Neural plasticity after spinal cord injury. Neural Regen Res. 2012;7:386–91.
Fang C-Y, Tsai J-L, Li G-S, Lien AS-Y, Chang Y-J. Effects of robot-assisted gait training in individuals with spinal cord injury: a meta-analysis. Biomed Res Int. 2020;2020:1–13.
Aach M, Cruciger O, Sczesny-Kaiser M, Höffken O, Meindl RC, Tegenthoff M, et al. Voluntary driven exoskeleton as a new tool for rehabilitation in chronic spinal cord injury: a pilot study. Spine J. 2014;14:2847–53.
Gandolla M, Guanziroli E, D’Angelo A, Cannaviello G, Molteni F, Pedrocchi A. Automatic setting procedure for exoskeleton-assisted overground gait: proof of concept on stroke population. Front Neurorobot. 2018;12:1–11.
Nightingale E, Raymond J, Middleton J, Crosbie J, Davis G. Benefits of FES gait in a spinal cord injured population. Spinal cord. 2007;45:646–57.
Jezernik S, Schärer R, Colombo G, Morari M. Adaptive robotic rehabilitation of locomotion: a clinical study in spinally injured individuals. Spinal cord. 2003;41:657–66.
Lewek MD, Cruz TH, Moore JL, Roth HR, Dhaher YY, Hornby TG. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Phys Ther. 2009;89:829–39.
Martin R, Sadowsky C, Obst K, Meyer B, McDonald J. Functional electrical stimulation in spinal cord injury: from theory to practice. Top Spinal Cord Inj Rehabil. 2012;18:28–33.
Mao Y-R, Lo WL, Lin Q, Li L, Xiao X, Raghavan P, et al. The effect of body weight support treadmill training on gait recovery, proximal lower limb motor pattern, and balance in patients with subacute stroke. Biomed Res Int. 2015;2015:1–10.
Kesar TM, Reisman DS, Perumal R, Jancosko AM, Higginson JS, Rudolph KS, et al. Combined effects of fast treadmill walking and functional electrical stimulation on post-stroke gait. Gait Posture. 2011;33:309–13.
Yan T, Hui-Chan CW, Li LS. Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: a randomized placebo-controlled trial. Stroke. 2005;36:80–5.
Daly JJ, Roenigk K, Holcomb P, John H, Rogers JM, Butler K, Gansen J, et al. A randomized controlled trial of functional neuromuscular stimulation in chronic stroke subjects. Stroke. 2006;37:172–8.
Browne MG, Franz JR. The independent effects of speed and propulsive force on joint power generation in walking. J Biomech. 2017;55:48–55.
Hsiao H, Zabielski TM Jr, Palmer JA, Higginson JS, Binder-Macleod SA. Evaluation of measurements of propulsion used to reflect changes in walking speed in individuals poststroke. J Biomech. 2016;49:4107–12.
Varoqui D, Niu X, Mirbagheri MM. Ankle voluntary movement enhancement following robotic-assisted locomotor training in spinal cord injury. J Neuroeng Rehabil. 2014;11:1–11.
Vasanthan LT, Nehrujee A, Solomon J, Tilak M. Electrical stimulation for people with spinal cord injury. Cochrane Database Syst Rev. 2019;2019:1–21.
Volpini M, Bartenbach V, Pinotti M, Riener R. Clinical evaluation of a low-cost robot for use in physiotherapy and gait training. J Rehabil Assist Technol Eng. 2017;4:1–11.
Taylor P, Humphreys L, Swain I. The long-term cost-effectiveness of the use of functional electrical stimulation for the correction of dropped foot due to upper motor neuron lesion. J Rehabil Med. 2013;45:154–60.
Elder W, Spetzler RF (2003) Functional electrical stimulation (FES). In: Michael J. Aminoff RBD (ed). Encyclopedia of the neurological sciences. pp 401–403.
Funding
None.
Author information
Authors and Affiliations
Contributions
TP, KK, PW, SV, TA, TW, and AT provided substantial contributions to the conception and design of the work; TP, KK, PW, SV, TA, TW, and AT contributed to acquisition, analysis, interpretation of data, and discussion; TP, PW, and TW drafted the work and substantively critiqued and revised the manuscript. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Table S1.
Search strategy in Medline via PubMed. Table S2. Search strategy in Scopus. Fig. S1. Funnel plots of (a) treadmill (TM) versus conventional physical therapy (CPT) (b) robotic assisted gait training (RAGT) versus conventional physical therapy and (c) contour funnel plot of plot robotic assisted gait training versus conventional physical therapy. Fig. S2. Funnel plots of (a) treadmill (TM) versus convention physical therapy (CPT) (b) robotic assisted gait training (RAGT) versus conventional physical therapy, and contour funnel plot of (c) treadmill versus convention physical therapy and (d) robotic assisted gait training versus conventional physical therapy for distance, Fig. S3. Funnel plots of Walking Index Spinal Cord injury (WISCI) outcome, (a) treadmill versus conventional physical therapy (b) robotic assisted gait training versus conventional physical therapy and contour funnel plot and (c) robotic assisted gait training versus conventional physical therapy. Fig. S4. Subgroup analysis of the time of injury between robotic assisted gait training versus conventional physical therapy on velocity of walking (m/s) in patients with incomplete spinal cord injury, Fig. S5. Subgroup analysis of time of training between robotic assist gait training versus conventional physical therapy on velocity of walking (m/s) in patients with incomplete spinal cord injury. Fig. S6. Subgroup analysis of the level of spinal cord injury between robotic assisted gait training versus conventional physical therapy on velocity of walking (m/s) in patients with incomplete spinal cord injury. Fig. S7. Subgroup analysis of time of injury between robotic assisted gait training versus conventional physical therapy on distance of walking (m) in patients with incomplete spinal cord injury.Fig. S8. Subgroup analysis of the level of spinal cord injury between robotic assisted gait training versus conventional physical therapy on distance of walking (m) in patients with incomplete spinal cord injury. Fig. S9. Subgroup analysis of the level of spinal cord injury between robotic assisted gait training versus conventional physical therapy on Walking Index Spinal Cord injury (WISCI) outcome in patients with incomplete spinal cord injury. Fig. S10. Predictive interval plots of mean difference for velocity (m/s) of 10 pairwise comparisons estimated; values in right column showed mean difference (95% confidence interval) (95% predictive interval). Fig. S11. Predictive interval plots of mean difference for distance (m) of 10 pairwise comparisons estimated; Values in right column showed mean difference (95% confidence interval) (95% predictive interval).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Patathong, T., Klaewkasikum, K., Woratanarat, P. et al. The efficacy of gait rehabilitations for the treatment of incomplete spinal cord injury: a systematic review and network meta-analysis. J Orthop Surg Res 18, 60 (2023). https://doi.org/10.1186/s13018-022-03459-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-022-03459-w