Abstract
Background
Accurate preoperative diagnosis of infected nonunion remains a challenge. Here, we evaluated the diagnostic potential of novel biomarkers for infected nonunion.
Methods
A cohort of 275 patients who underwent surgery for suspected septic nonunion after open reduction and internal fixation were enrolled. Preoperatively analyzed clinical parameters included white blood cell (WBC) count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), albumin, globulin, albumin-to-globulin ratio (AGR), plasma D-dimer, plasma fibrinogen, platelet count (PC), monocyte-lymphocyte ratio (MLR), neutrophil–lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR). Receiver operating characteristic (ROC) curves, sensitivity, and specificity were utilized to compare the diagnostic potential of those biomarkers.
Results
The WBC count and levels of CRP, ESR, NLR, MLR, PLR, PC, plasma D-dimer, plasma fibrinogen, and globulin in infected nonunion patients were significantly higher (p < 0.05) than those in aseptic patients. The albumin and AGR levels of the infected nonunion group were significantly lower (p < 0.05) than the aseptic group. The ROC curve analysis showed that the diagnostic accuracy of AGR and plasma fibrinogen was good. The combination of AGR with plasma fibrinogen had the highest area under the curve (AUC) (0.916). The sensitivity and specificity were 70.27% and 91.04% for AGR, and 67.57% and 84.08% for plasma fibrinogen, respectively. The combination of AGR with plasma fibrinogen showed a sensitivity of 86.49% and specificity of 92.54%. In patients with comorbidities, the diagnostic accuracy of the combination of AGR with plasma fibrinogen was also good.
Conclusions
AGR and plasma fibrinogen are promising biomarkers to improve the diagnosis of infected nonunion. The combination of AGR with plasma fibrinogen is a sensitive tool for screening infected nonunion.
Similar content being viewed by others
Background
Infected nonunion is one of the most challenging complications after open reduction and internal fixation (ORIF) for orthopedists. Ineffective control of infected nonunion can potentially result in higher hospitalization costs, longer treatment course, and higher morbidity and mortality rates than the primary procedure [1,2,3]. According to the 2018 Fracture-related Infection Consensus Definition [3], the gold standards for diagnosis of infected nonunion are histology and microbiology of deep tissue specimens, which are only available postoperatively and hence have no preoperative diagnostic value. Tibial fractures are the most frequent sites of nonunion and infected nonunion among limb fractures after ORIF [4,5,6]. Moreover, it is usually difficult to accurately differentiate between infected nonunion and aseptic nonunion preoperatively, especially when confirmatory clinical features such as a sinus tract and visible purulent discharge are absent [7, 8]. Thus, developing a non-invasive method that is more convenient, tolerable, reliable, economical, and faster for preoperatively diagnosing infected nonunion is of great importance, which can substantially influence treatment plans [9, 10].
Currently, white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) are cheap, easy to use, and widely available traditional blood inflammatory markers, which can provide preoperative information for diagnosing infection [11, 12]. These biomarkers are usually elevated in acute infected nonunion, but mostly remain normal in late or chronic infections, or are influenced by other infections or inflammation [13]. These blood markers showed poor sensitivity and specificity for the preoperative diagnosis of infected nonunion in prior studies [14, 15]. Therefore, new biomarkers for preoperatively diagnosing infected nonunion are particularly important, which can substantially affect treatment plans [16].
Coagulation-related parameters such as fibrinogen, D-dimer, and platelet count (PC) have been reported to be promising diagnostic markers of infection, and some studies have proved that fibrinogen, D-dimer, or PC are useful in the diagnosis of periprosthetic joint infection (PJI) [17,18,19]. Moreover, serum D-dimer and plasma fibrinogen have shown good performance in the diagnosis of infected nonunion in past studies [20, 21]. Alternative monocyte-lymphocyte ratio (MLR), neutrophil–lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) have been strongly associated with inflammation in many diseases such as hepatitis virus infection, rheumatic diseases, and infective endocarditis [22, 23]. Albumin is the main protein in human serum, and hypoalbuminemia, a historic index of malnutrition, has recently been associated with infection in orthopedics [24,25,26]. Globulin, which is a component of complements and ceruloplasmin, generally increases during the inflammatory process [27]. Hence, the albumin-to-globulin ratio (AGR), which considers both albumin and globulin levels, is negatively associated with chronic inflammation [28]. Furthermore, several studies have reported that globulin and AGR were useful biomarkers in the diagnosis of PJI [29, 30]. However, a comparison of the accuracy of all these blood biomarkers in diagnosing infected nonunion after internal fixation is still not available.
Therefore, this retrospective study was performed to assess the ability of WBC, CRP, ESR, albumin, globulin, AGR, plasma D-dimer, plasma fibrinogen, PC, MLR, NLR, and PLR to diagnose infected nonunion in patients undergoing reoperation after internal fixation and to compare the diagnostic performance of these ratios with those of WBC, CRP, and ESR.
Materials and methods
We conducted a retrospective study in which data on patients admitted to our hospital between January 2017 and February 2022 were analyzed. Ethical approval was obtained from the Clinical Research Ethics Committee of The Affiliated Drum Tower Hospital of Nanjing University Medical School. Our hospital’s institutional review board approved the study (2022–023) and waived the requirement for written informed consent because it was retrospective, involved only anonymized patient data, and posed no risk to patients [31]. The inclusion criteria were as follows: (1) patients aged ≥ 18 years and (2) those with nonunion after ORIF that required reoperation. The exclusion criteria were as follows: (1) age < 18 years (n = 3), (2) without complete blood workup (n = 7), and (3) unavailability of complete examinations (n = 4). Patients with nonunion after a joint fusion (n = 10), corrective osteotomy (n = 6), and pathological fracture (n = 3) were also excluded. Patients on antibiotic therapy 2 weeks prior to the reoperation (n = 11), and those with related comorbidities (n = 36) were also excluded. Finally, a total of 275 patients who underwent reoperation for a suspected infected nonunion were eligible for inclusion.
The clinical features of patients with nonunions were comprehensively interpreted by the attending physician after admission. Baseline demographic features, including age, sex, BMI, smoking, and fracture position were collected. A nonunion was determined as radiographic evidence of nonprogression of healing for at least 3 months, or lack of healing by 9 months since the initial injury [32]. According to the 2018 Infected Nonunion Consensus Definition [3], information on demographics, histological, and microbiological results from intraoperative sampling; visible pus; sinus tract; and serum inflammatory markers were recorded. The presence of an active infection was either confirmed by microbial detection in tissue and joint samples, or in the case of negative microbial detection, by the presence of a soft tissue defect with an exposed plate and existing joint sinus. The 275 patients were divided into two groups: 74 patients with infected nonunion and 201 aseptic patients.
Fasting venous blood samples were collected preoperatively, which were standard practices in our hospital. Within 1–2 h, the blood samples were sent to the Medical Laboratory Center for routine examination. The levels of WBC, CRP, ESR, albumin, globulin, AGR, plasma D-dimer, plasma fibrinogen, PC, MLR, NLR, and PLR were analyzed. Antibiotic use in patients was delayed by at most 2 weeks until after intraoperative specimens were collected, unless the patients needed anti-infective therapy urgently, in which case they were excluded. At least two (usually five) tissue specimens were cultured intraoperatively for at least 7 days after collection and even for 14 days in case of suspected infection associated with low-virulence or specific pathogens.
Statistical analysis
Categorical variables were expressed as frequencies and percentages and analyzed by Pearson’s chi-square test or Fisher’s exact test. Kolmogorov–Smirnov (KS) test was used to identify whether the data variables were normally or non-normally distributed. Continuous normally distributed variables were presented as mean ± SD (standard deviation), and analyzed by Student’s t-tests. Non-normally distributed data were presented as median (IQR), and Mann–Whitney U test was used to analyze numerical variables with non-normal distribution or unequal variance. All differences were considered significant at a value of p < 0.05. Receiver operating characteristic (ROC) curves were plotted to determine the diagnostic value of each biomarker for assessing infected nonunion, and the area under the curve (AUC) and 95% confidence interval (CI) were calculated to compare different biomarkers. Based on the AUC value, the discriminatory capacity was interpreted as excellent (0.9–1), good (0.8–0.89), fair (0.7–0.79), or poor (0.6–0.69), and values of 0.5–0.59 indicated that the marker had no discriminatory capacity. The Youden’s index (J = [sensitivity + specificity] − 1) was used to determine the optimal predictive cut-offs for calculating AUC. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each test was calculated. All statistical analyses were performed using STATA version 18.0.
Results
Basic demographics and clinical characteristics of the included patients among two groups are shown in Table 1. There was no significant difference in age, BMI, smoking history, and site of nonunion between the two groups (p > 0.05). In comparison with aseptic cases, there were more male patients (p = 0.032) and a higher number of previous open fractures in the infected nonunion group (p < 0.001).
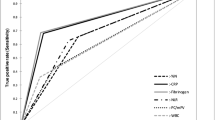
The WBC, CRP, ESR, NLR, MLR, PLR, PC, plasma D-dimer, plasma fibrinogen, and globulin levels were significantly increased in the infected nonunion group compared with the aseptic group (p < 0.05; Table 2). Nonetheless, the albumin and AGR levels of patients in the infected nonunion group were significantly lower than those in the aseptic group (p < 0.05). The composite biomarker combining AGR with plasma fibrinogen (0.916) had the highest AUC (Fig. 1 and Table 3). The combination of AGR with plasma fibrinogen showed the highest sensitivity (86.49%), PPV (81.01%), and NPV (94.90%), and the second highest specificity (92.54%).
Discussion
Infected nonunion is a major problem in orthopedic and trauma surgery and continues to be a significant clinical challenge [33]. We initially aimed to discover novel infected nonunion-specific biomarkers that can help preoperatively distinguish infected nonunion from other patients with aseptic nonunion after ORIF. A comprehensive review of the literature shows that our study is the first to compare albumin, globulin, AGR, plasma D-dimer, plasma fibrinogen, PC, MLR, NLR, and PLR with traditional inflammatory biomarkers, i.e., WBC, CRP, and ESR, for their ability to screen infected nonunion in patients undergoing reoperation after ORIF. Our research found that preoperative plasma fibrinogen and AGR were reliable blood biomarkers for screening infected nonunion, and the combination of AGR with plasma fibrinogen could further provide more accurate and more specific evaluation for diagnosing infected nonunion in these patients.
Albumin and globulin, easily accessible and reliable biomarkers in the basic metabolic panel, have proven to be critical markers associated with inflammation and infection [34, 35]. Albumin, a negative acute-phase reactant, is widely considered to be an inflammatory and nutritional biomarker in humans [36]. Hypoalbuminemia is correlated with malnutrition, hepatopathy, kidney disease, and all types of inflammation [26, 37]. Globulin, another major serum protein component, consists of various immunoglobulins and acute-phase proteins [38, 39]. Collectively, both decreased albumin and increased globulin played essential roles in response to inflammation and infection functions to dramatically decrease the AGR, suggesting that AGR indicates the body’s inflammatory state more accurately [40, 41]. Previous studies have validated the potential role of globulin and AGR in association with PJI and may serve as potential adjuvant biomarkers in the diagnosis of PJI [29, 30]. In our study, we observed that albumin and AGR were significantly lower, while globulin was considerably higher in patients with infected nonunion than aseptic patients. However, ROC curve analysis revealed that only AGR showed acceptable predictive value for the diagnosis of infected nonunion, with high AUC (0.845), fair sensitivity and PPV (70.27% and 74.29%, respectively), but high specificity and NPV (91.04% and 89.27%, respectively).
Many studies have indicated that systemic and local infections result in fibrinolytic activity, and coagulation-related parameters such as fibrinogen, D-dimer, and PC have been shown to be promising diagnostic biomarkers for the diagnosis of PJI in some studies [17, 42,43,44,45,46,47]. We found that the AUC of plasma fibrinogen (0.805) was larger than that of plasma D-dimer (0.752) and PC (0.702). However, plasma fibrinogen had a moderate sensitivity and PPV (67.57% and 60.98%, respectively), but a high specificity and NPV (84.08% and 87.56%, respectively).
MLR, NLR, and PLR have been demonstrated as stable and cost-effective biomarkers that reflect the inflammatory response as they mediate inflammation by various biochemical mechanisms [48, 49]. Previous research suggested that NLR may perform better than CRP for diagnosing early PJI [50]. In the contrast, most studies showed that MLR, NLR, and PLR showed limited diagnostic value in the preoperative diagnosis of infected nonunion, which was similar to our result [11, 22]. Our result showed that the diagnostic performance of MLR, NLR, and PLR was limited, as the AUC, sensitivity, and specificity of MLR were 0.595, 31.08, and 78.26%, respectively; those of NLR were 0.687, 56.76, and 52.50%, respectively; and those of PLR were 0.725, 68.92, and 42.86%, respectively.
WBC, CRP, and ESR are the most commonly used biomarkers of infected nonunion. Unfortunately, they are usually affected by other factors such as physiological stress, treatment, and other diseases [7, 51]. Peripheral WBC, CRP, and ESR are typically normal in low-grade infections and afford little value in diagnosing infected nonunion. Moreover, different fractures may have different risks of infection, so it may make sense to analyze these laboratory parameters in the different subgroups of fractures.
This study has some limitations. First, all patients with suspected infected nonunion were collectively analyzed, and thus, these results may not be applicable to all possible subgroups. Second, this study was retrospective, with inherent biases, because electronic medical records may contain incorrect or non-existent information for individual patients. Third, the sample size in our study was relatively small, so we did not include data on any probable effect of antibiotic use or different comorbidities. Last, there are heterogeneities among the patients with different fracture sites. Therefore, multicenter, prospective, comparative studies with larger samples are required to more thoroughly determine the accuracy of these biomarkers for predicting infected nonunion of different fracture sites.
In conclusion, our results show that AGR and plasma fibrinogen may be reliable biomarkers to screen for infected nonunion. The combination of preoperative AGR with plasma fibrinogen may provide more accurate and specific evaluation of infected nonunion. Larger and prospective studies should be carried to verify our findings.
Availability of data and materials
The final dataset will be available from the corresponding author upon reasonable request.
References
Struijs PA, Poolman RW, Bhandari M. Infected nonunion of the long bones. J Orthop Trauma. 2007;21(7):507–11.
Motsitsi NS. Management of infected nonunion of long bones: the last decade (1996–2006). Injury. 2008;39(2):155–60.
Metsemakers WJ, Morgenstern M, McNally MA, Moriarty TF, McFadyen I, Scarborough M, Athanasou NA, Ochsner PE, Kuehl R, Raschke M, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury. 2018;49(3):505–10.
Perisano C, Greco T, Polichetti C, Inverso M, Maccauro G. Antibiotic-coated nail in open tibial fracture: a retrospective case series. J Funct Morphol Kinesiol. 2021;6(4):97.
Greco T, Cianni L, Polichetti C, Inverso M, Maccauro G, Perisano C. Uncoated vs. antibiotic-coated tibia nail in open diaphyseal tibial fracture (42 according to AO classification): a single center experience. Biomed Res Int. 2021;2021:7421582.
Greco T, Vitiello R, Cazzato G, Cianni L, Malerba G, Maccauro G, Perisano C. Intramedullary antibiotic coated nail in tibial fracture: a systematic review. J Biol Regul Homeost Agents. 2020;34(3 Suppl 2):63–9 (advances in musculoskeletal diseases and infections - sotimi 2019).
Wang S, Yin P, Quan C, Khan K, Wang G, Wang L, Cui L, Zhang L, Zhang L, Tang P. Evaluating the use of serum inflammatory markers for preoperative diagnosis of infection in patients with nonunions. Biomed Res Int. 2017;2017:9146317.
Olszewski D, Streubel PN, Stucken C, Ricci WM, Hoffmann MF, Jones CB, Sietsema DL, Tornetta P 3rd. Fate of patients with a “surprise” positive culture after nonunion surgery. J Orthop Trauma. 2016;30(1):e19-23.
McNally MA. Decision-making in infected nonunion: is the surgery more important than the condition? Bone Jt J. 2016;98-b(4):435–6.
Patzakis MJ, Zalavras CG. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg. 2005;13(6):417–27.
Sigmund IK, Dudareva M, Watts D, Morgenstern M, Athanasou NA, McNally MA. Limited diagnostic value of serum inflammatory biomarkers in the diagnosis of fracture-related infections. Bone Jt J. 2020;102-b(7):904–11.
Govaert GAM, Kuehl R, Atkins BL, Trampuz A, Morgenstern M, Obremskey WT, Verhofstad MHJ, McNally MA, Metsemakers WJ. Diagnosing fracture-related infection: current concepts and recommendations. J Orthop Trauma. 2020;34(1):8–17.
van den Kieboom J, Bosch P, Plate J, IJpma F, Kuehl R, McNally MA, Metsemakers WJ, Govaert G. Diagnostic accuracy of serum inflammatory markers in late fracture-related infection: a systematic review and meta-analysis. Bone Jt J. 2018;100-b(12):1542–50.
Stevenson MC, Slater JC, Sagi HC, Palacio Bedoya F, Powers-Fletcher MV. Diagnosing fracture-related infections: where are we now? J Clin Microbiol. 2022;60(2): e0280720.
Jiang N, Wang BW, Chai YM, Wu XB, Tang PF, Zhang YZ, Yu B. Chinese expert consensus on diagnosis and treatment of infection after fracture fixation. Injury. 2019;50(11):1952–8.
Basilico M, Vitiello R, Oliva MS, Covino M, Greco T, Cianni L, Dughiero G, Ziranu A, Perisano C, Maccauro G. Predictable risk factors for infections in proximal femur fractures. J Biol Regul Homeost Agents. 2020;34(3 Suppl. 2):77–81 (advances in musculoskeletal diseases and infections - sotimi 2019).
Hu Q, Fu Y, Tang L. Serum D-dimer as a diagnostic index of PJI and retrospective analysis of etiology in patients with PJI. Clin Chim Acta; Int J Clin Chem. 2020;506:67–71.
Xu H, Xie J, Yang J, Chen G, Huang Q, Pei F. Plasma Fibrinogen and platelet count are referable tools for diagnosing periprosthetic joint infection: a single-center retrospective cohort study. J Arthroplasty. 2020;35(5):1361–7.
Greig D, Trikha R, Sekimura T, Cevallos N, Kelley BV, Mamouei Z, Yeaman MR, Bernthal NM. Platelet deficiency represents a modifiable risk factor for periprosthetic joint infection in a preclinical mouse model. J Bone Jt Surg Am. 2021;103(11):1016–25.
Wang XJ, Wang Z, Zhang ZT, Qiu XS, Chen M, Chen YX. Plasma fibrinogen as a diagnostic marker of infection in patients with nonunions. Infect Drug Resist. 2020;13:4003–8.
Wang Z, Zheng C, Wen S, Wang J, Zhang Z, Qiu X, Chen Y. Usefulness of serum D-dimer for preoperative diagnosis of infected nonunion after open reduction and internal fixation. Infect Drug Resist. 2019;12:1827–31.
Xu H, Xie JW, Liu L, Wang D, Huang ZY, Zhou ZK. Combination of CRP with NLR is a sensitive tool for screening fixation-related infection in patients undergoing conversion total hip arthroplasty after failed internal fixation for femoral neck fracture. Bone Jt J. 2021;103-b(9):1534–40.
Yu BZ, Fu J, Chai W, Hao LB, Chen JY. Neutrophil to lymphocyte ratio as a predictor for diagnosis of early periprosthetic joint infection. BMC Musculoskelet Disord. 2020;21(1):706.
Yuwen P, Chen W, Lv H, Feng C, Li Y, Zhang T, Hu P, Guo J, Tian Y, Liu L, et al. Albumin and surgical site infection risk in orthopaedics: a meta-analysis. BMC Surg. 2017;17(1):7.
Yang G, Zhu Y, Zhang Y. Prognostic risk factors of surgical site infection after primary joint arthroplasty: a retrospective cohort study. Medicine. 2020;99(8): e19283.
Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181–93.
Li K, Fu W, Bo Y, Zhu Y. Effect of albumin-globulin score and albumin to globulin ratio on survival in patients with heart failure: a retrospective cohort study in China. BMJ Open. 2018;8(7): e022960.
Ukibe NR, Ndiuwem CK, Ogbu II, Ukibe SN, Ehiaghe FA, Ikimi CG. Prognostic value of some serum protein fractions as Early Index of Clinical Recovery in Pulmonary Tuberculosis subjects. Indian J Tuberc. 2020;67(2):167–71.
Ye Y, Chen W, Gu M, Xian G, Pan B, Zheng L, Zhang Z, Sheng P. Serum globulin and albumin to globulin ratio as potential diagnostic biomarkers for periprosthetic joint infection: a retrospective review. J Orthop Surg Res. 2020;15(1):459.
Wang H, Zhou H, Jiang R, Qian Z, Wang F, Cao L. Globulin, the albumin-to-globulin ratio, and fibrinogen perform well in the diagnosis of periprosthetic joint infection. BMC Musculoskelet Disord. 2021;22(1):583.
Fong M, Braun KL, Chang RM. Native Hawaiian preferences for informed consent and disclosure of results from genetic research. J Cancer Educ: Off J Am Assoc Cancer Educ. 2006;21(1 Suppl):S47-52.
Bell A, Templeman D, Weinlein JC. Nonunion of the femur and tibia: an update. Orthop Clin N Am. 2016;47(2):365–75.
Jain AK, Sinha S. Infected nonunion of the long bones. Clin Orthop Relat Res. 2005;431:57–65.
Wang Y, Li C, Wang W, Wang J, Li J, Qian S, Cai C, Liu Y. Serum albumin to globulin ratio is associated with the presence and severity of inflammatory bowel disease. J Inflamm Res. 2022;15:1907–20.
Santaolalla A, Sollie S, Rislan A, Josephs DH, Hammar N, Walldius G, Garmo H, Karagiannis SN, Van Hemelrijck M. Association between serum markers of the humoral immune system and inflammation in the Swedish AMORIS study. BMC Immunol. 2021;22(1):61.
Sheinenzon A, Shehadeh M, Michelis R, Shaoul E, Ronen O. Serum albumin levels and inflammation. Int J Biol Macromol. 2021;184:857–62.
Park J, Kim HJ, Kim J, Choi YB, Shin YS, Lee MJ. Predictive value of serum albumin-to-globulin ratio for incident chronic kidney disease: a 12-year community-based prospective study. PLoS ONE. 2020;15(9): e0238421.
Zhou T, Yu ST, Chen WZ, Xie R, Yu JC. Pretreatment albumin globulin ratio has a superior prognostic value in laryngeal squamous cell carcinoma patients: a comparison study. J Cancer. 2019;10(3):594–601.
Wu PP, Hsieh YP, Kor CT, Chiu PF. Association between albumin-globulin ratio and mortality in patients with chronic kidney disease. J Clin Med. 2019;8(11):1991.
Hoffman LK, Ghias MH, Cohen SR, Lowes MA. Polyclonal hyperglobulinaemia and elevated acute-phase reactants in hidradenitis suppurativa. Br J Dermatol. 2018;178(2):e134–5.
Schmilovitz-Weiss H, Cohen M, Pappo O, Sulkes J, Braun M, Tur-Kaspa R, Ben-Ari Z. Serum globulin levels in predicting the extent of hepatic fibrosis in patients with recurrent post-transplant hepatitis C infection. Clin Transplant. 2007;21(3):391–7.
Kirschenbaum LA, McKevitt D, Rullan M, Reisbeck B, Fujii T, Astiz ME. Importance of platelets and fibrinogen in neutrophil-endothelial cell interactions in septic shock. Crit Care Med. 2004;32(9):1904–9.
Gaertner F, Ahmad Z, Rosenberger G, Fan S, Nicolai L, Busch B, Yavuz G, Luckner M, Ishikawa-Ankerhold H, Hennel R, et al. Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell. 2017;171(6):1368-1382.e1323.
Xu H, Shang G, Wang Y, Xiang S. Plasma fibrinogen is a reliable marker for diagnosing periprosthetic joint infection and determining the timing of second-stage revision. Int J Infect Dis: IJID : Off Publ Int Soc Infect Dis. 2021;108:220–5.
Yang F, Zhao C, Huang R, Ma H, Wang X, Wang G, Zhao X. Plasma fibrinogen in the diagnosis of periprosthetic joint infection. Sci Rep. 2021;11(1):677.
Wu H, Meng Z, Pan L, Liu H, Yang X, Yongping C. Plasma Fibrinogen performs better than plasma D-dimer and fibrin degradation product in the diagnosis of periprosthetic joint infection and determination of reimplantation timing. J Arthroplasty. 2020;35(8):2230–6.
Grzelecki D, Walczak P, Grajek A, Szostek M, Dudek P, Bartosz P, Olewnik Ł, Czubak-Wrzosek M, Marczak D, Tyrakowski M. Elevated plasma D-dimer concentration has higher efficacy for the diagnosis of periprosthetic joint infection of the knee than of the hip-A single-center, retrospective study. J Orthop Res: Off Publ Orthop Res Soc. 2021;39(2):291–8.
Inose H, Kobayashi Y, Yuasa M, Hirai T, Yoshii T, Okawa A. Procalcitonin and neutrophil lymphocyte ratio after spinal instrumentation surgery. Spine. 2019;44(23):E1356-e1361.
Huang Y, Liu A, Liang L, Jiang J, Luo H, Deng W, Lin G, Wu M, Li T, Jiang Y. Diagnostic value of blood parameters for community-acquired pneumonia. Int Immunopharmacol. 2018;64:10–5.
Gallo J, Juranova J, Svoboda M, Zapletalova J. Excellent AUC for joint fluid cytology in the detection/exclusion of hip and knee prosthetic joint infection. Biomed Pap Med Fac Univ Palacky Olomouc Czechoslovakia. 2017;161(3):310–9.
Metsemakers WJ, Kuehl R, Moriarty TF, Richards RG, Verhofstad MHJ, Borens O, Kates S, Morgenstern M. Infection after fracture fixation: current surgical and microbiological concepts. Injury. 2018;49(3):511–22.
Acknowledgements
We thank the contribution of all the doctors and nurses in our department.
Funding
This work was supported by the Key Project of the Medical Science and Technology Development Foundation, Nanjing Department of Health, Jiangsu, China [Grant No. ZKX16034].
Author information
Authors and Affiliations
Contributions
Conception and design were the responsibility of Z-W and G-YX. Data collection was the responsibility of Z-W and H-JM. Statistical analysis was the responsibility of Z-W. Analysis and interpretation were the responsibility of Z-W. Composition of the manuscript was the responsibility of Z-W. Revision of the manuscript for important intellectual content and approval of the final draft were done by all authors. G-YX took responsibility for the paper as a whole. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, China (Approval No.: 2022–023). All patients signed an informed consent form approved by the Institutional Review Board.
Consent for publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Z., Mao, H. & Xu, G. Combination of albumin-to-globulin ratio and plasma fibrinogen is a sensitive tool for preoperative screening of infected nonunion in patients undergoing reoperation after open reduction and internal fixation: a retrospective study. J Orthop Surg Res 17, 471 (2022). https://doi.org/10.1186/s13018-022-03363-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-022-03363-3