Abstract
Background
The efficacy and safety profile of mesenchymal stem cells (MSCs) augmentation in chondral procedures are controversial. This systematic review updated the current evidence on MSCs augmentation for chondral procedures in patients with symptomatic chondral defects of the knee.
Methods
This study followed the PRISMA guidelines. The literature search was updated in August 2022. Two independent authors accessed PubMed, Google scholar, Embase, and Scopus. No additional filters or time constrains were used for the search. A cross reference of the bibliographies was also performed. All the clinical studies investigating surgical procedures for chondral defects of the knee augmented with MSCs were accessed. Defects of both tibiofemoral and patellofemoral joints were included. The following patient reported outcomes measures (PROMs) were retrieved at baseline and last follow-up: Visual Analogic Scale (VAS), Tegner Activity Scale, Lysholm Knee Scoring System, International Knee Documentation Committee (IKDC). Return to daily activities and data on hypertrophy, failure, revision surgery were also collected. Failures were defined as the recurrence of symptoms attributable to the index procedure. Revisions were defined as any reoperation at the site of the index procedure.
Results
A total of 15 clinical studies (411 procedures) were included. Patients returned to their prior sport activity at 2.8 ± 0.4 months. All the PROMs improved at last follow-up: Tegner (P = 0.0002), Lysholm (P < 0.0001), the IKDC (P < 0.0001), VAS (P < 0.0001). At a mean of 30.1 ± 13.9 months, 3.1% (2 of 65 patients) reported graft hypertrophy, 3.2% (2 of 63) were considered failures. No surgical revision procedures were reported. Given the lack of available quantitative data for inclusion, a formal comparison of surgical procedures was not conducted.
Conclusion
MSCs augmentation in selected chondral procedures could be effective, with a low rate of complications. Further investigations are required to overcome the current limitations to allow the clinical translation of MSCs in regenerative medicine.
Similar content being viewed by others
Introduction
Chondral defects of the knee are common. Most are traumatic, and involve the medial femoral condyle [1, 2]. Given the poor healing capability of the articular cartilage, these defects have limited chance to heal [3, 4]. Chondral defects may cause persistent pain, reducing the quality of life, and sport participation, and may predispose to early onset osteoarthritis [1, 3]. Microfractures have been proposed for lesions up to 2.5 cm2 [5,6,7]. For larger defects, autologous chondrocyte implantation (ACI) has been proposed [3, 8]. ACI is a two-step surgical procedure which necessitates chondral harvesting from a non-weight-bearing area of the knee and external chondrocytes expansion in a dedicated laboratory [9, 10]. Autologous matrix-induced chondrogenesis (AMIC) is also widely performed to regenerate chondral defects [11,12,13]. AMIC combines microfractures with an acellular membrane scaffold in a single-session surgery [13, 14]. AMIC exploits the regenerative potential of the mesenchymal stem cells arising from the subchondral bone [15, 16]. These procedures have been augmented with MSCs to enhance their regenerative potential. Mesenchymal stem cells (MSCs) derived from bone marrow aspirate concentrate (BMAC) or adipose tissue have also been used to enhance chondral procedures with promising results [17,18,19,20,21]. The use of MSCs seeded on a bio-degradable scaffold, with or without growth factors augmentation, has shown promise in animal and clinical studies [22,23,24,25,26,27]. MSCs are able to maintain multipotency during culture expansion [27], and to differentiate into chondrocytes [28]. Moreover, these cells are characterized by self-renewal capacity, plasticity and the ability to migrate toward injury sites, where they demonstrate trophic effects and immunomodulatory potential, inhibiting T and B cell proliferation and NK cell activation [29,30,31]. The current literature evidences a high ratio of preclinical to clinical studies on this topic, suggesting that we are in a transition phase to clinical application in human. Thus, this systematic review updated the current evidence on MSCs application in chondral defects of the knee. The outcome of interest was to assess efficacy and safety of MSCs augmentation for chondral procedures in patients with symptomatic chondral defects of the knee.
Methods
Search strategy
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA guidelines [32]. The PICOT algorithm was preliminary pointed out:
-
P (Population): symptomatic knee chondral defects;
-
I (Intervention): MSCs augmentation of surgical procedures for chondral defects of the knee;
-
C (Comparison): efficacy and safety;
-
O (Outcomes): PROMs, return to sport;
-
T (Timing): minimum 12 months follow-up.
Data source and extraction
Two authors (F.M. and R.G.) independently performed the literature search. The last update of the search was conducted in August 2022 accessing PubMed, Google scholar, Embase, and Scopus. The following keywords were used in combination using the Boolean operator AND/OR: knee OR/AND chondral defects AND (focal OR surgery OR pain OR sports OR surgery OR therapy OR management OR arthroscopy OR augmentation OR enhance OR application) AND (ACI OR autologous chondrocyte implantation OR matrix-induced OR periosteum OR membrane OR chondral OR collagen OR stem cells OR bone marrow OR adipose OR peripheral OR blood OR concentrate OR mesenchymal) AND (visual analogic scale OR PROM OR patient reported outcome measures OR outcome OR revision OR failure). No additional filters or time constrains were used for the search. The same authors independently screened the resulting articles. If title and abstract matched the topic, the full-text article was accessed. A cross reference of the bibliographies was also performed. Disagreements were debated and solved by a third author (M.N.).
Eligibility criteria
All the clinical studies investigating surgical procedures for chondral defects of the knee augmented with MSCs were accessed. According to the authors language capabilities, articles in English, German, Italian, French and Spanish were eligible. Level I to IV of evidence, according to Oxford Center of Evidence-Based Medicine [33], were considered. Studies that combined multiple chondral strategies or MSCs were not eligible, nor were those addressing multiple chondral defects. We included only studies that enhanced chondral procedures with MSCs. Studies which employed innovative hydrogel were not eligible, nor were those including synthetic scaffolds/polymers [34,35,36,37,38,39]. Studies were included irrespectively of the size, location (tibiofemoral and patellofemoral), and depth (chondral and osteochondral) of the chondral defect, the cell delivery methods used, and the concomitant procedures. Moreover, studies were included irrespective of the sources of MSCs (adipose, bone marrow, synovial, peripheral blood), culture, expansion, and implantation modalities. Only articles reporting quantitative data under the outcomes of interest were considered.
Data extraction
Two authors (F.M. and R.G.) independently performed data extraction. Study generalities (author, year, journal, type of study, length of the follow-up) and patients baseline characteristics (number of procedures, mean BMI, age, and defect size, mean duration of the symptoms, percentage of female and right side) were collected. The following data were retrieved at baseline and at last follow-up: visual analog scale (VAS), Lysholm Knee Scoring Scale [40], Tegner Activity Scale [41] and International Knee Documentation Committee (IKDC) [42]. Data concerning patients return to daily activity were also collected. Further, data on complications were also collected: hypertrophy, failure, arthroplasty, revision surgery. Failures were defined as the recurrence of symptoms attributable to the index procedure. Revisions were defined as any reoperation at the site of the index procedure.
Methodology quality assessment
The methodological quality assessment was performed by one author (R.G.) using the risk of bias graph tool of the Review Manager Software (The Nordic Cochrane Collaboration, Copenhagen). This tool evaluated the major risk of bias of each included study. The following risks of bias were evaluated: selection (random sequence generation and the allocation concealment), detection (assessor blinding), attrition, reporting, and other (additional risk of bias) source of bias. Disagreements were solved by a third senior author (N. M.). The risk of bias was evaluated in percentage as low (green), high (red), or unclear (yellow).
Statistical analysis
The statistical analyses were performed using the IBM SPSS software (version 25). To evaluate possible differences of continuous variables (post vs pre-operative data), the mean difference (MD) effect measure was adopted. The t-test was performed, with P values of < 0.05 considered statistically significant. The confidence interval (CI) was set at 95% in all the comparisons. For binary data (rate of complication), the number of events reported in each single study was evaluated.
Results
Search result
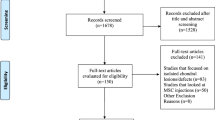
The literature search resulted in 1071 articles. Of them, 267 were duplicates. A further 778 studies were not eligible: study design (N = 231), not focused on knee (N = 101), not focused on chondral defects management (N = 199), not enhanced with MSCs (N = 221), combined treatment (N = 3), type of surgical intervention (N = 20), language limitation (N = 2), uncertain results (N = 1). A further 11 studies were excluded because lacking of quantitative data under the outcomes of interest. This left 15 clinical investigations for the present study: two RCTs, seven prospective and six retrospective studies. The literature search results are shown in Fig. 1.
Methodological quality assessment
The risk of selection, detection, attrition, reporting, and other bias were evaluated. The risk of selection bias was moderate. Indeed, 40% (6/15) of the included studies were retrospective, and 87% (13/15) did not perform randomization. Assessor blinding was seldom performed, increasing the risk of performance bias. The risk of reporting and detection biases was low-moderate, as was the risk of the risk of other biases. Concluding, the methodological assessment showed an acceptable quality (Fig. 2).
Patient demographics
Data from 411 procedures were retrieved. The mean duration of symptoms before the procedure was 7.3 ± 6.9 months. The mean follow-up was 30.1 ± 13.9 months. The mean age of the patients was 36.3 ± 9.8 years, the mean BMI was 25.2 ± 1.4 kg/m2. 41% (169 of 411 patients) were women, while 52% (214 of 411) of patients the defect was located on the right side. The mean defect size was 4.6 ± 2.3 cm2 (Table 1).
Outcomes of interest
Patients returned to their prior sport activity at a mean of 2.8 ± 0.4 months. At a mean of 30.1 ± 13.9 months, the Tegner scale increased of 2.8 points (P = 0.0002), the Lysholm score of 32.9 points (P < 0.0001), the IKDC of 36.8 (P < 0.0001). The VAS (0–10) decreased of − 4.6 (< 0.0001). The results of PROMs are shown in greater detail in Table 2.
Complications
Few studies reported data on complications. 3.1% (2 of 65 patients) developed graft hypertrophy. 3.2% (2 of 63 patients) experienced persistent symptomatic pain at the site of the index procedure and were considered failure. No revision procedure was reported.
Discussion
According to the main findings of the present study, chondral procedures augmented with MSCs demonstrated efficacy and feasibility. Irrespective of the surgical procedure, a statistically significant improvement in PROMs was evidenced, with return to previous sporting activities within three months.
MSCs are able to maintain multipotency during culture expansion [27], and to differentiate into chondrocytes [28]. Moreover, these cells are characterized by self-renewal capacity, plasticity and the ability to migrate toward injury sites, where they demonstrate trophic effects and immunomodulatory potential, inhibiting T and B cell proliferation and NK cell activation [29,30,31]. Animal studies have demonstrated that, compared to the other techniques, chondral procedures augmented with MSCs give rise to a greater thickness, a smoother surface and greater integration of the neo-cartilage with the surrounding native cartilage, probably from improved type II collagen content and orientation [27]. The properties and differentiation abilities of MSCs may differ according to their source [29]. Several methods are used to obtain and deliver MSCs. Bone marrow (BM), synovial, and adipose tissues are well established sources of MSCs. BM-MSCs are commonly harvested from the posterior iliac crest. After harvesting, BM-MSCs are centrifuged to remove erythrocytes and plasma cells, and subsequently seeded in either a hyaluronic acid membrane [2, 43, 44], or collagen type I/III matrix [18, 19]. In other protocols, MSC from BMA are first cultured and later inserted into the defect [25, 45, 46]. Although the MSC yield per unit is not high [31], they can be easily collected, and have a good osteogenic and chondrogenic potential [29]. On the other hand, the differentiation ability of BM-MSCs seems to decrease with age, the harvesting procedure can be painful, and the amount of de novo hyaline cartilage can be limited [28]. In the present study, only one article documented the successful use of synovium derived MSCs [47]. The harvesting technique is less painful than BM harvesting since the synovial tissue is harvested arthroscopically from the supracondylar region of the femoral condyle of the operated knee, to be later minced, digested, centrifuged, and cultured. These amplified MSCs are then suspended within a culture medium and pipetted onto a collagen membrane, thereby giving rise to a membrane cell construct, known as matrix-induced autologous mesenchymal cell implantation (mAMI). Synovial derived MSCs demonstrate a greater chondrogenic and less osteogenic potential than those deriving from bone marrow [29, 47]. Furthermore, they seem to maintain greater differentiation potential regardless of age, yield greater amount of MSC per unit, and decrease total costs and culture time [29, 30]. Other authors used adipose derived MSCs from the subcutaneous adipose buttock tissue [48]. Lipoaspirate is collected using syringe suction, the stromal vascular structure containing MSCs is separated from mature adipocytes by centrifugation, and later suspended in platelet rich plasma. Adipose-MSCs can be isolated in large quantity, are easily available, possess anti-inflammatory properties and have a similar proliferative profile to BM-MSCs, and do not seem to be influenced by age [29, 49]. On the other hand, they seem to have a lower chondrogenic potential compared to BM-MSCs and synovial MSCs [29].
Allogenic MSCs have also been used [20, 50]. A mix of allogenic MSCs, autologous chondrons, derived from the patient’s recycled cartilage tissue, and fibrin glue has been delivered directly at the lesion site in a single surgical session, avoiding donor site morbidity. Despite the allogenicity of these cells, the authors reported no adverse immune response and a good tissue integration, comparable, if not superior, to ACI procedures [20, 50]. Other authors, instead, used autologous BMAC for augmentation. Harvested autologous BM from the posterior iliac crest undergoes centrifugation to concentrate pluripotent mesenchymal stem cells, growth factors, platelets, and white blood cells [51]. BMAC can be later used on its own and injected over the chondral defect, or can be combined with either microfractures [43], chondral grafting, or biphasic scaffolds [18, 19, 44, 51]. Although these procedures have been associated with low complication rates [51], it is still not clear whether they really enhance the regeneration of hyaline-like cartilage tissue [52, 53]. Moreover, the composition of BMAC varies widely among individuals [51]; therefore, clinical results may be different according to patient demographics, especially when younger subjects are compared to older individuals [52].
Cell delivery can be performed through different methodologies with a variable degree of invasiveness, from arthroscopy [2, 43, 48] to mini arthrotomy [18,19,20, 50], or formal arthrotomy [25, 45, 46, 54]. Irrespective to the type of cell therapy, the use of a periosteal flap mandates a parapatellar arthrotomy, leading to increased donor site morbidity and greater invasiveness. Better efficacy, lesser donor site morbidity, and fewer complications are achieved when the periosteal flap is substituted with a bio-degradable scaffold, either collagen type I/III or hyaluronan, to fill the defect. These membrane cell constructs can be delivered either through mini arthrotomy [18, 19, 44, 47] or arthroscopically [43]. A mini arthrotomy is also employed when recycled cartilage is needed to obtain autologous chondrons [20, 50].
We are aware that the present study presents several limitations. The retrospective nature of most of the included studies negatively impacted the reliability of the results, leading to high risk of selection bias. The sample size was small in most studies, with 27% (4/15) reporting data on less than 10 patients. In only 27% (4/15) of studies was the follow-up longer than 24 months. Hence, long term complications have not been investigated. In addition, the size, location (tibiofemoral and patellofemoral), and depth (chondral and osteochondral) of the chondral defects, and the cell delivery methods used are heterogeneous, and preclude statistical analysis [27, 29, 49, 51, 52, 55, 56]. The type of chondral procedure was heterogeneous in the included studies, which also represents an important source of bias. Most authors used a collagen or hyaluronic membrane to stabilize the blood coat within the knee cavity. Whether the nature of the membrane used influence the outcome is unclear, and future investigations are required. Histological analysis was seldom performed, representing another limitation. Moreover, in most of the included studies concomitant procedures, such as meniscectomy, synovectomy, anterior cruciate ligament repair, and high tibial osteotomy, were performed [2, 18, 19, 43,44,45,46,47,48]. Several MSCs sources (adipose, bone marrow, synovial, peripheral blood), culture, expansion, and implantation modalities have been described, but seldom compared to one another. Thus, it is difficult to understand whether a given technique is superior to another. Further studies should investigate the optimal cell source and dosage [27]. Between studies, heterogeneity in the eligibility criteria and timing of assessment of outcome was evident. Patient selection was not standardized. The role of age on the regenerative potential of MSCs and autologous chondrocytes is still unclear, and comparative investigations should be undertaken. Imaging and histological analyses were undertaken at different time intervals between the studies, representing a further limitation. Further investigations are required to establish the superiority of MSCs augmentation over the isolated procedures in a clinical setting. Given these limitations, results from the present study must be interpreted with caution.
Conclusion
MSCs augmentation in selected chondral procedures could be effective, with a low rate of complication. Further investigations are required to overcome current limitations to the clinical translation of MSCs in the regenerative medicine.
Availability of data and materials
No new data were generated or analyzed in support of this review.
Abbreviations
- Microfractures:
-
Microfractures
- ACI:
-
Autologous chondrocyte implantation
- AMIC:
-
Autologous matrix-induced chondrogenesis
- BMAC:
-
Bone marrow aspirate concentrate
- MSCs:
-
Mesenchymal stem cells
- BM:
-
Bone marrow
- mAMI:
-
Matrix-induced autologous mesenchymal cell implantation
- PROMs:
-
Patient reported outcome measures
References
Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177–82.
Buda R, Vannini F, Cavallo M, et al. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am. 2010;92(Suppl 2):2–11.
Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil. 2002;10:432–63.
Steinert AF, Ghivizzani SC, Rethwilm A, et al. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213.
Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391:S362-369.
Knutsen G, Engebretsen L, Ludvigsen TC, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86:455–64.
Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthr Cartil. 2006;14:1119–25.
Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95.
Mastbergen SC, Saris DB, Lafeber FP. Functional articular cartilage repair: here, near, or is the best approach not yet clear? Nat Rev Rheumatol. 2013;9:277–90.
Tuli R, Li WJ, Tuan RS. Current state of cartilage tissue engineering. Arthritis Res Ther. 2003;5:235–8.
Migliorini F, Eschweiler J, Maffulli N, et al. Autologous matrix-induced chondrogenesis (AMIC) and microfractures for focal chondral defects of the knee: a medium-term comparative study. Life (Basel). 2021;11:183.
Migliorini F, Eschweiler J, Maffulli N, et al. Management of patellar chondral defects with autologous matrix induced chondrogenesis (AMIC) compared to microfractures: a four years follow-up clinical trial. Life (Basel). 2021;11:141.
Gotze C, Nieder C, Felder H, et al. AMIC for focal osteochondral defect of the talar shoulder. Life (Basel). 2020;10:328.
de Girolamo L, Schonhuber H, Vigano M, et al. Autologous matrix-induced chondrogenesis (AMIC) and AMIC enhanced by autologous concentrated bone marrow aspirate (BMAC) allow for stable clinical and functional improvements at up to 9 years follow-up: results from a randomized controlled study. J Clin Med. 2019;8:392.
Schiavone Panni A, Del Regno C, Mazzitelli G, et al. Good clinical results with autologous matrix-induced chondrogenesis (AMIC) technique in large knee chondral defects. Knee Surg Sports Traumatol Arthrosc. 2018;26:1130–6.
Schagemann J, Behrens P, Paech A, et al. Mid-term outcome of arthroscopic AMIC for the treatment of articular cartilage defects in the knee joint is equivalent to mini-open procedures. Arch Orthop Trauma Surg. 2018;138:819–25.
Wakitani S, Imoto K, Yamamoto T, et al. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr Cartil. 2002;10:199–206.
Gobbi A, Karnatzikos G, Scotti C, et al. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2:286–99.
Gobbi A, Karnatzikos G, Sankineani SR. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am J Sports Med. 2014;42:648–57.
de Windt TS, Vonk LA, Slaper-Cortenbach IC, et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35:256–64.
Bonetti MA, Rovere G, Fulchignoni C, et al. Autologous fat transplantation for the treatment of trapeziometacarpal joint osteoarthritis. Orthop Rev (Pavia). 2020;12:8666.
Kim YS, Park EH, Kim YC, et al. Clinical outcomes of mesenchymal stem cell injection with arthroscopic treatment in older patients with osteochondral lesions of the talus. Am J Sports Med. 2013;41:1090–9.
Koh YG, Choi YJ, Kwon OR, et al. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med. 2014;42:1628–37.
Koh YG, Jo SB, Kwon OR, et al. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29:748–55.
Haleem AM, Singergy AA, Sabry D, et al. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1:253–61.
Miljkovic ND, Cooper GM, Marra KG. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthr Cartil. 2008;16:1121–30.
Anderson JA, Little D, Toth AP, et al. Stem MSCs for knee cartilage repair: the current status of preclinical and clinical studies. Am J Sports Med. 2014;42:2253–61.
Contentin R, Demoor M, Concari M, et al. Comparison of the chondrogenic potential of mesenchymal stem cells derived from bone marrow and umbilical cord blood intended for cartilage tissue engineering. Stem Cell Rev Rep. 2020;16:126–43.
Filardo G, Madry H, Jelic M, et al. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21:1717–29.
Freitag J, Shah K, Wickham J, et al. Evaluation of autologous adipose-derived mesenchymal stem cell therapy in focal chondral defects of the knee: a pilot case series. Regen Med. 2020;15:1703–17.
Khan WS, Johnson DS, Hardingham TE. The potential of stem cells in the treatment of knee cartilage defects. Knee. 2010;17:369–74.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
Howick JCI, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, Moschetti I, Phillips B, Thornton H, Goddard O, Hodgkinson M. 2011. The 2011 Oxford levels of evidence. Oxford Centre for Evidence-Based Medicine. https://www.cebmnet/indexaspx?o=5653.
Pipino G, Risitano S, Alviano F, et al. Microfractures and hydrogel scaffolds in the treatment of osteochondral knee defects: a clinical and histological evaluation. J Clin Orthop Trauma. 2019;10:67–75.
Eglin D, Grad S, Gogolewski S, et al. Farnesol-modified biodegradable polyurethanes for cartilage tissue engineering. J Biomed Mater Res A. 2010;92:393–408.
Grad S, Kupcsik L, Gorna K, et al. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: potential and limitations. Biomaterials. 2003;24:5163–71.
Kwan H, Chisari E, Khan WS. Cell-free scaffolds as a monotherapy for focal chondral knee defects. Materials (Basel). 2020;13:306.
Bekkers JE, Tsuchida AI, Malda J, et al. Quality of scaffold fixation in a human cadaver knee model. Osteoarthr Cartil. 2010;18:266–72.
Shah SS, Lee S, Mithoefer K. Next-generation marrow stimulation technology for cartilage repair: basic science to clinical application. JBJS Rev. 2021;9:e2000090.
Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–4.
Briggs KK, Lysholm J, Tegner Y, et al. The reliability, validity, and responsiveness of the Lysholm score and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37:890–7.
Higgins LD, Taylor MK, Park D, et al. Reliability and validity of the International Knee Documentation Committee (IKDC) Subjective Knee Form. Joint Bone Spine. 2007;74:594–9.
Enea D, Cecconi S, Calcagno S, et al. Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee. 2013;20:562–9.
Gobbi A, Scotti C, Karnatzikos G, et al. One-step surgery with multipotent stem cells and Hyaluronan-based scaffold for the treatment of full-thickness chondral defects of the knee in patients older than 45 years. Knee Surg Sports Traumatol Arthrosc. 2017;25:2494–501.
Nejadnik H, Hui JH, Feng Choong EP, et al. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110–6.
Teo BJ, Buhary K, Tai BC, et al. Cell-based therapy improves function in adolescents and young adults with patellar osteochondritis dissecans. Clin Orthop Relat Res. 2013;471:1152–8.
Akgun I, Unlu MC, Erdal OA, et al. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg. 2015;135:251–63.
Koh YG, Kwon OR, Kim YS, et al. Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy. 2016;32:97–109.
Makris EA, Gomoll AH, Malizos KN, et al. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34.
de Windt TS, Vonk LA, Slaper-Cortenbach ICM, et al. Allogeneic MSCs and recycled autologous chondrons mixed in a one-stage cartilage cell transplantion: a first-in-man trial in 35 patients. Stem Cells. 2017;35:1984–93.
Cavinatto L, Hinckel BB, Tomlinson RE, et al. The role of bone marrow aspirate concentrate for the treatment of focal chondral lesions of the knee: a systematic review and critical analysis of animal and clinical studies. Arthroscopy. 2019;35:1860–77.
Chahla J, Dean CS, Moatshe G, et al. Concentrated bone marrow aspirate for the treatment of chondral injuries and osteoarthritis of the knee: a systematic review of outcomes. Orthop J Sports Med. 2016;4:2325967115625481.
Chahla J, Mannava S, Cinque ME, et al. Bone marrow aspirate concentrate harvesting and processing technique. Arthrosc Tech. 2017;6:e441–5.
Buda R, Baldassarri M, Perazzo L, et al. A useful combination for the treatment of patellofemoral chondral lesions: realignment procedure plus mesenchymal stem cell-retrospective analysis and clinical results at 48 months of follow-up. Eur J Orthop Surg Traumatol. 2019;29:461–70.
Migliorini F, Berton A, Salvatore G, et al. Autologous chondrocyte implantation and mesenchymal stem cells for the treatments of chondral defects of the knee—a systematic review. Curr Stem Cell Res Ther. 2020;15:547–56.
Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259–71.
Enea D, Cecconi S, Calcagno S, et al. One-step cartilage repair in the knee: collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee. 2015;22:30–5.
Skowronski J, Rutka M. Osteochondral lesions of the knee reconstructed with mesenchymal stem cells-results. Ortop Traumatol Rehabil. 2013;15:195–204.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
FM contributed to literature search, data extraction, methodological quality assessment, statistical analyses, writing; NM contributed to supervision, revision, final approval; RG contributed to literature search, data extraction, methodological quality assessment; JE contributed to supervision; ML contributed to supervision VV contributed to supervision. All author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
Professor Maffulli is the Editor in Chief of the Journal of Orthopedic Surgery and Research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Filippo, M., Laura, M., Riccardo, G. et al. Mesenchymal stem cells augmentation for surgical procedures in patients with symptomatic chondral defects of the knee: a systematic review. J Orthop Surg Res 17, 415 (2022). https://doi.org/10.1186/s13018-022-03311-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-022-03311-1