Abstract
Background
Total hip arthroplasty is a common orthopedic surgery for treating primary or secondary hip osteoarthritis. Bilateral total hip replacement could be performed in a single stage or two separate stages. Each surgical procedure's reliability, safety, and complications have been reported controversially. This study aimed to review the current evidence regarding the outcomes of simultaneous and staged bilateral total hip arthroplasty.
Methods
We conducted a meta-analysis using MEDLINE, EMBASE, Web of Science, and Scopus databases. Eligible studies compared complications and related outcomes between simultaneous and staged bilateral THA. Two reviewers independently screened initial search results, assessed methodological quality, and extracted data. We used the Mantel–Haenszel method to perform the meta-analysis.
Results
In our study, we included 29,551 patients undergoing simBTHA and 74,600 patients undergoing stgBTHA. In favor of the simBTHA, a significant reduction in deep vein thrombosis (DVT) and systemic, local, and pulmonary complications was documented. However, we evidenced an increased pulmonary embolism (PE) and periprosthetic fracture risk in simBTHA. In the simBTHA, total blood loss, length of hospital stay, and total cost were lower.
Conclusion
This meta-analysis shows that simultaneous bilateral THA accompanies fewer complications and lower total cost. Well-designed randomized controlled trials are needed to provide robust evidence.
Similar content being viewed by others
Background
Total hip arthroplasty (THA) is one of the most common orthopedics surgeries. It is the preferred cost-effective treatment for osteoarthritis and other end-stage hip abnormalities. Patients experience a significant improvement in joint function as well as the quality of life following THA [1]. Studies suggest a rising trend in the number of performed THAs during the last decade [2]. From 2000 to 2014, the number of annual performed THAs increased by 105% in the USA. It is also projected that by 2030, this number will increase by 71.2%, reaching 635,000 procedures per year [3]. Total hip replacement also imposes a high economic burden on healthcare systems, with US hospitals bearing a staggering cost of $ 15 billion annually [4].
Patients scheduled for bilateral THA usually undergo two different timing sets of surgeries: simultaneous or staged. Simultaneous BTHA is performed in single hospital admission and under the same anesthesia. On the other hand, staged BTHA is executed at separate intervals in two hospitalizations and under two distinct anesthesia [5]. In 1971, Charnley et al. introduced simultaneous THA for bilateral hip pathologies, a noteworthy revolution in orthopedic science [5, 6]. Since then, there has always been controversy over which method could have better outcomes.
In 2016, Shao et al. conducted a systematic review comparing simBTHA and stgBTHA. It was revealed that surgery time, deep vein thrombosis (DVT), and major systemic complications were significantly lower in simBTHA compared to stgBTHA [7]. In 2019, another systematic investigation performed by Huang et al. also demonstrated lower rates of DVT, pulmonary embolism (PE), and respiratory complications in simBTHA [8].
There is still debate concerning this critical issue, and many original studies have been conducted since the last published systematic review. Previous reviews have focused on systemic and surgical complications, blood loss, operation time, and mortality as their primary outcomes. Essential factors such as readmission, revision, hip joint function, and cost have been considered less. Thus, a thorough review of the available data is required to identify the best way to perform bilateral THAs. The forthcoming systematic review aims to make a more comprehensive and accurate comparison between simultaneous and staged BTHA with a higher sample size and additional related outcomes.
Method
The protocol of this study was registered on PROSPERO (CRD42022310240). We followed the Cochrane guidelines for meta-analysis during the process [9]. Our study phases were based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [10]. The PRISMA checklist is presented in Additional file 1.
Search strategy
We searched the electronic databases MEDLINE, Web of Science (WOS), Embase, and Scopus for relevant articles in any published language; the last updating search was performed on February 15, 2022. The keywords are exhibited in Additional file 2. In addition, we explored the reference part of the articles that fulfilled our eligibility criteria. We also used the “related articles” feature in PubMed to avoid probable missing.
Eligibility criteria
PICOS categories (population, intervention, comparator, outcomes, and study design) were applied to define our inclusion criteria. We included studies only if they were executed to compare mortality, complications, costs, or other possible outcomes between simBTHA and stgBTHA. Eligible study designs were randomized controlled trials (RCTs), non-randomized clinical trials, prospective and retrospective cohort studies, and case–control investigations. We did not impose any restrictions on the length of follow-up and year of publication. Exclusion criteria were reviews, research letters, conference abstracts, non-English articles, duplicate publications, irrelevant articles, non-human models, studies comparing simBTHA to unilateral THA, and resurfacing or revision surgery.
Systemic complications were defined as cardiovascular, pulmonary, gastrointestinal, urologic, and neurologic complications, hypotension, anemia, DVT, and PE. Notably, we did not include PE in the pulmonary complications in the meantime of analysis. Local complications in our study were defined as wound infection, decubitus ulcer, hematoma, dehiscence, neurapraxia, vascular injury, accidental laceration or puncture, chronic soft tissue pain, neuroma, wound drainage, superficial infection, and ectopic ossification.
Data extraction
We imported all the studies into Rayyan online tool [11] in order to screen conveniently. After resolving duplicates, two researchers (AR, AS) completed an initial independent review to determine if the studies met the inclusion criteria hinged upon the title and abstract. Then, the two prior reviewers (AR, AS) evaluated each in the full-text screening phase. In case of any discrepancy, a third reviewer (AG) became involved and resolved it.
We prepared an electronic spreadsheet according to the Cochrane's template for data extraction of intervention reviews. Two separate reviewers fulfilled the data extraction (AR, AG). We acquired the following data from the studies: first author's name, publication year, country, study design, the sample size, mean age, gender, mean body mass index (BMI), American Society of Anesthesiology (ASA) classification, the interval between stages, duration of follow-up, primary and secondary outcomes including mortality, DVT, PE, fracture, dislocation, deep infection, any other complications, revision, readmission, operation time, blood loss, blood transfusion, length of hospital stay (LOS), hospital cost, and functional measures. Raw data were reviewed by another researcher (AS) to settle any disagreement. We also tried to contact the corresponding authors of the included articles regarding raw data or missing information. Patients with an ASA score of 1 or 2 were categorized as ‘low risk,’ and patients with an ASA score of 3 or 4 were categorized as ‘high risk’ [12].
Methodology assessment
To assess the quality of each study, we employed the Newcastle–Ottawa Scale (NOS) for observational and non-randomized investigations. Briefly, the NOS evaluates a study according to three main characteristics: selection of groups, comparability, and outcome assessment [13]. We judged the quality of included studies according to the previous classification described in a meta-analysis by Simunovic et al. [14]. Studies with a score > 6 were categorized as high quality. Those with a score of 5 or 6 were classified as medium quality. Articles scored less than 5 were assigned as a low-quality study. Concerning randomized clinical trials (RCTs), we utilized the Cochrane Collaboration tool to assess the risk of bias. Two reviewers (AR, AS) independently assessed each study's quality. Disagreements were determined by consensus or involvement of the corresponding author (SHS).
Statistical analysis
We performed meta-analysis using the Comprehensive Meta-Analysis software (Biostat, Englewood, NJ, USA, Version 3.3) if three or more studies reported a particular outcome. For dichotomous variables, odds ratios (ORs) were calculated and pooled for all investigations. Meta-analysis of dichotomous variables was committed through the Mantel–Haenszel (MH) method, with 95% confidence intervals (CI). Meta-analysis of continuous data was performed by applying the mean and standard deviation of outcome measures with 95% confidence intervals (CI). For studies that reported only data ranges without standard deviations, we calculated SDs using the formula suggested by Walter & Yao [15]. A p value less than 0.05 was considered statistically significant. We analyzed heterogeneity among the studies using the I2 test [16]. I2 > 50% with a p value < 0.05 suggested high heterogeneity. A fixed-effects model was utilized if low statistical heterogeneity among the studies was discovered (I2 < 50%). A random-effects model was used if high heterogeneity became proven. We also detected potential publication bias by using Begg’s funnel plots and the Egger test [17].
Results
Search results
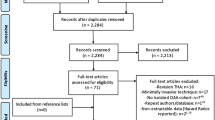
After deleting duplications, we identified 5324 potentially relevant titles from the mentioned databases. Based on the titles and abstracts, 5236 publications were excluded. Full texts of 88 remaining publications were screened. Finally, in this systematic review, 38 studies, including 104,151 patients (29,551 simBTHA and 74,600 stgBTHA), were entered into the quantitative analysis. A flowchart summarizing the selection process is provided in Fig. 1.
Study characteristics
Among the 38 included studies, 2 studies [18, 19], including 348 patients, were RCTs and 36 studies were non-RCTs [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. The baseline characteristics of the articles are displayed in Table 1. Studies were in the English language and were published from 1978 to 2022. The duration of follow-up was at least 3 months. The sample size of included studies ranged from 15 to 42,238. The mean age of participants was 57.6 years for simBTHA and 63.2 years for stgBTHA. The male-to-female ratio was 1:1.29. Raw data for ASA classification were reported in 14 studies [18, 19, 24, 25, 33,34,35, 37, 41, 42, 45,46,47, 49]. Regarding ASA score, 13% and 18% of patients in simBTHA and stgBTHA were considered high risk (ASA 3 or 4), respectively (Table 1).
Quality assessment
Randomization methods, outcome assessment blinding, incomplete outcome data, and selective data reporting were low risk for both RCTs. Although the allocation method was not reported in one RCT, all other included studies were observational, comprising one prospective cohort, seven registries, nineteen retrospective cohorts, and nine retrospective case controls. The risk-of-bias assessment results for both randomized and observational studies are summarized in Table 2.
Mortality and complications
Pooled analysis of 11 studies on DVT (OR = 0.639, p = 0.044, Fig. 2a), 12 studies on pulmonary complications (OR = 0.533, p < 0.001, Fig. 2c), 14 studies on systemic complications (OR = 0.803, p = 0.048, Fig. 3a), and 16 studies on local complications (OR = 0.736, p < 0.00, Fig. 3b) exhibited that these complications are lower in simBTHA. However, PE, reported in 12 studies (OR = 1.925, p < 0.001, Fig. 2b), and periprosthetic fracture, reported in 13 studies (OR = 1.306, p = 0.049, Fig. 4b), were higher in simBTHA. 90-day mortality, reported in eight studies (OR = 1.101, p = 0.815, Fig. 5), periprosthetic joint infection, reported in nine studies (OR = 1.112, p = 0.508, Fig. 4a), and dislocation, reported in 14 studies (OR = 0.760, p = 0.153, Fig. 4c), were similar between the two groups (Table 3).
Perioperative and postoperative relevant outcomes
The overall effect of included studies demonstrated that simBTHA was lower in terms of length of stay (MD = −4.777, p < 0.001, Fig. 6) (26 studies), operation cost (USD) (MD = −2464, p < 0.001, Fig. 7c) (11 studies), and blood loss (MD = −254.785, p < 0.001, Fig. 7a) (12 studies). Pooled data of nine studies showed that the simBTHA group experiences a mean 1.37 point improvement over the stgBTHA group in postoperative Harris Hip Score (HHS) (MD = 1.370, p = 0.006, Fig. 8a). There was no significant difference in the revision rate (OR = 1.033, p = 0.572, Fig. 9a) (ten studies), readmission rate (OR = 0.997, p = 0.980, Fig. 9b) (six studies), blood transfusion rate (MD = 0.114, p = 0.286, Fig. 7b) (12 studies), and postoperative limb length discrepancy (LLD) (MD = −0.391, p = 0.312, Fig. 8b) (seven studies) (Tables 4 and 5).
Systematic review of heterogeneous data
Based on 12 studies [18,19,20, 24, 25, 29, 34, 37, 38, 41, 42, 54], the mean operation time was 171.4 min for simBTHA and 191.4 min for stgBTHA. Cumulative operation time for both surgeries in stgBTHA was longer than simBTHA operation time in all studies except the study by Kim et al. [42]. Although postoperative Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores were reported to be similar between the two groups [35], two studies reported significantly higher scores of Oxford Hip Scores [56] or EuroQoL-5D index [42] in simBTHA compared to stgBTHA. In contrast, another study by Kamath et al. [37] stated no statistical difference between the two groups in mentioned functional outcomes. Functional recovery was faster in simBTHA, as walking without support started earlier [36] and walking capacity was better postoperatively [21, 28]. Rates of home-discharged patients for stgBTHA were higher in all studies [25, 26, 40, 41, 43, 49, 54].
For 90-day mortality, systemic complications, operation cost, LOS, blood loss, blood transfusion rate, HHS, LLD, and high heterogeneity existed between studies (I2 ranged from 59.909 to 99.729%). Begg’s funnel plots are shown in Additional file 3.
Discussion
SimBTHA has continued to attract attention since Charnley first introduced this type of orthopedic surgery. Many studies comparing simBTHA and stgBTHA have been conducted since then but, due to small sample size or other undetermined possible reasons, failed to obtain a definite conclusion. We conducted a comprehensive systematic review and meta-analysis of 38 comparative studies enrolling 104,151 patients. Findings of this updated meta-analysis generally concur and further extend that of previous reviews on the topic, providing several relevant results that have not been previously addressed.
Mortality and complications
The combined 90-day mortality rate was 0.22% for simBTHA and 1.57% for stgBTHA. Nonetheless, the 90-day mortality analysis failed to show any significant difference between the two groups. Since most included articles were retrospective studies, we should interpret the present results with caution. Previous studies have also posed no significant difference in mortality rate between the two groups [7, 32, 33, 48, 57].
Periprosthetic joint infection (PJI), as an uncommon complication of THA [58], can incur costs for the patient and healthcare system [59]. PJI can also lead to secondary surgery and even death [60]. No significant difference was observed regarding the PJI rate between the two groups. However, our results contrast with the previous review [7], which indicated a significantly higher infection rate in one-stage versus two-stage. Shao et al. [7] computed the risk in the cumulative number of superficial and deep infection cases, so their effect on subsequent procedures on hospitalization might be diverse. The overall PJI rate was 0.91% in the simBTHA group and 0.87% in the stgBTHA group. The overall PJI rate for both groups was higher than in previous studies [39, 61].
We investigated periprosthetic fracture between the two groups, and contrary to previous studies [5, 7, 41, 51], the incidence of fracture in simBTHA was higher than in stgBTHA. The unanticipated increased fracture risk in simBTHA can be attributed to the cemented or cementless fixation [62] and operation time in a single surgery. As in the previous meta-analyses [5, 7, 63], no clinically significant difference was seen in the occurrence of dislocation between the two groups in our study.
We found a significantly lower risk of DVT in simBTHA compared to stgBTHA. This finding is consistent with previous studies [7, 8]. Lower activity levels in stgBTHA due to pain in the contralateral hip can justify the elevated risk of DVT in stgBTHA [64]. Despite simBTHA patients having an associated lower risk of DVT, we observed an increased risk of PE in simBTHA compared to stgBTHA. Still, other investigations revealed no difference [5, 7, 57] or an elevated risk of PE in StgBTHA [8] PE, consuming a huge part of medical resources [65], can yield in-hospital and post-discharge mortality [66]. A large-scale data registry study by partridge et al. [48] suggested that simBTHA is associated with a greater risk of developing PE. This study included more than half of our study population and maybe has shifted the results toward itself. However, the quality of this study was high and might not have imposed bias on the results. We should consider that pharmacological thromboprophylaxis can reduce thromboembolic events [67], and many risk factors affect PE incidence [68].
The stgBTHA was associated with a higher risk for postoperative pulmonary complications. Malcolm et al. also reported a 1.42% respiratory complication rate for THA, similar to the simBTHA group in our study [69]. In our study, the pulmonary complications rate in simBTHA and stgBTHA was 1.69% and 2.38%, respectively.
On the other hand, a higher risk of systemic and local complications in the stgBTHA was evidenced. Similar results were reported by Aghayev et al. [28]. Poultsides et al. [43] and Guo et al. [47] also presented that the rate of systemic complications in simBTHA was lower than in stgBTHA.
Other outcomes
Combining the results of 10 studies revealed no significant differences in revision rate between the simBTHA and stgBTHA. Our findings are compatible with the previous study [46] published on this topic. Another study by Garland et al. [33] indicated a slightly higher risk of revision for stgBTHA. There were no significant differences among simBTHA and stgBTHA concerning readmission rates in keeping with previous studies [41, 47, 48].
Our research shows that simBTHA is superior to stgBTHA in terms of cumulative operation time, hospital cost, and LOS. The simBTHA surgery is performed in one session, while the stgBTHA surgery is performed in two sessions. Undergoing two operations, which obviously has a longer cumulative operation time, means a more extended anesthesia period which is correlated with increased risk of infection [70], venous thromboembolism (VTE) [71], neurologic deficit [72], revision, intraoperative blood loss, transfusion, and other critical adverse events [73, 74]. Operation time is a potentially modifiable risk factor that engages surgeons and healthcare systems interested in quality improvement. Sodhi et al. [75] saw that operation time is significantly associated with LOS, and LOS has also been a major driver of cost in THA [76]. Mean LOS for simBTHA was 4.8 days less than stgBTHA, which can justify more costs and complications in stgBTHA. However, operation time is varied by various factors such as operating technique, surgery approach, general or epidural anesthesia, patient's demographics, and surgeon's experience. Although almost all studies demonstrated a lower cost, and LOS in simBTHA, researchers utilized various methods to calculate these data. Therefore, high heterogeneity was observed in the pooled data.
The aggregate results of our study indicated that simBTHA outperformed stgBTHA in reducing perioperative total blood loss. Previous studies also showed a higher cumulative blood loss in stgBTHA compared to simBTHA [5, 18, 24]. Interestingly, in this meta-analysis, despite a lower total blood loss in simBTHA, analysis of transfusion units did not show any significant difference between the two groups. It should be taken into account that indications for blood transfusion in different studies were not the same. Another reason for similar rates of blood transfusion could be the interval between two operations in stgBTHA that provides enough time for hematopoiesis. In a retrospective study [39], comparing infection rates after THA, blood transfusion has found to be a powerful risk factor for PJI, and patients who underwent simBTHA had a higher blood transfusion rate than stgBTHA. In contrast, another study by Parvizi et al. [25] revealed that the cumulative blood transfusion was lower in simBTHA compared with stgBTHA. As higher blood loss is accompanied by more need for blood transfusion in which itself is associated with a higher risk for infection [77], immunosuppression [78], and even death [79], blood loss stands as a significant concern in major orthopedic surgeries [80].
Although the pooled results of analysis favored simBTHA in terms of the postoperative HHS, but a 1.37 point improvement is not clinically significant based on the prior evidence [81]. Kim et al. [42] found that the mean postoperative HHS was significantly higher in simBTHA than in stgBTHA, and they mentioned that better functional outcomes in simBTHA could be because of the accuracy of surgery, earlier starting rehabilitation for both operated hips, and reduced time lost from work in a simultaneous procedure. The diversity of functional outcome measure types did not allow us concluding precisely regarding hip joint function. Using a comprehensive and unified tool that includes important items for hip joint function evaluation can help us decide more precisely which type of surgery is appropriate for specific situation.
Concomitant to our results, several studies have exhibited no difference in LLD between simBTHA and stgBTHA [36, 37, 40]. However, LLD can yield patient dissatisfaction after THA [82]. It also has been indicated that LLD can worsen functional outcomes such as Oxford Hip Score [83].
The strength points of this meta-analysis comprise peer-reviewed comparative studies and a rigorous assessment of the methodological quality of the currently available data. This study enhanced the power to compare the clinical outcomes of simBTHA and stgBTHA through more excellent details. With respect to the previous meta-analysis [8], we used explicit exclusion and inclusion criteria. We also utilized a robust search strategy spanned multiple databases, yielding 38 published studies on the topic, twice the number of included studies in the previous meta-analysis.
Our study has several potential limitations. First, due to the limited number of RCTs, we included non-RCTs, too. As we know, retrospective studies vary in terms of quality, making our study susceptible to bias and confounding. Second, we also excluded non-English studies, which may cause language bias in our research. Third, lacking a specific definition for some outcomes like operation time and variety of measurements may bias our findings. Fourth, most of the studies did not report outcomes according to surgical approach, method of anesthesia, use of antibiotics and thrombosis prophylaxis, primary diagnosis, and demographic data. Although our goal was not to compare these data, they could have influenced the accuracy of our results. Fifth, some studies did not contain raw data for pooled analyses. Although we tried to contact the authors, we could not get these data. Sixth, each study’s criteria for blood transfusion were different or not mentioned. Seventh, the number of participants varied considerably among the included studies, ranging from 15 to 42,238. Eighth, National registry data studies have some missing information about patients and these studies may also underestimate complications rates which could have influenced the final result. Ninth, follow-up periods were heterogeneous among studies. Tenth, HHS measurements were done at different times, which might have biased our results. At last, we combined different complications to obtain two categories: systemic and local. However, some studies avoided reporting complications separately, so they put together all of them without paying attention to the different severity, which limits the conclusion's reliability.
Conclusion
Taken together, this meta-analysis demonstrated that simultaneous and staged THA have similar 90-day mortality, dislocation, and PJI rates. A statically significant risk reduction was identified in DVT, pulmonary, systemic, and local complications in the simBTHA group. Interestingly, stgBTHA is more promising in terms of PE and fracture rate. The present study also revealed that simBTHA is associated with lower total blood loss, length of stay, and total surgery cost. Reduced length of hospital stay and total surgery cost as essential advantages of simBTHA compared to stgBTHA may attract healthcare providers' and policy-makers' attention. After all, simBTHA remains noninferior to the stgBTHA in most postoperative outcomes. Anyhow, we recommend that well-designed randomized controlled trials should be conducted to elucidate the advantages of each surgery in order to help surgeons choose the proper surgical method hinged on their point of view and patient's benefits.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- THA:
-
Total hip arthroplasty
- simBTHA:
-
Simultaneous bilateral THA
- stgBTHA:
-
Staged bilateral THA
- DVT:
-
Deep vein thrombosis
- PE:
-
Pulmonary embolism
- Venous thromboembolism:
-
Venous thromboembolism
- BMI:
-
Body mass index
- ASA Classification:
-
American Society of Anesthesiology
- LOS:
-
Length of hospital stay
- HHS:
-
Harris hip score
- WOMAC:
-
The Western Ontario and McMaster Universities Arthritis Index
- LLD:
-
Limb length discrepancy
- VTE:
-
Venous thromboembolism
- PJI:
-
Periprosthetic joint infection
References
Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370:1508–19. https://doi.org/10.1016/s0140-6736(07)60457-7.
Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–5. https://doi.org/10.2106/jbjs.f.00222.
Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455–60. https://doi.org/10.2106/jbjs.17.01617.
Lavernia CJ, Hernandez VH, Rossi MD. Payment analysis of total hip replacement. Curr Opin Orthop. 2007;18:23–7. https://doi.org/10.1097/BCO.0b013e328011a270.
Tsiridis E, Pavlou G, Charity J, Tsiridis E, Gie G, West R. The safety and efficacy of bilateral simultaneous total hip replacement: an analysis of 2063 cases. J Bone Joint Surg Br. 2008;90:1005–12. https://doi.org/10.1302/0301-620x.90b8.20552.
Sarmiento A. Sir John Charnley and his legacy to total hip arthroplasty, 1970–1993. Curr Orthop Pract. 2014;25:115–8. https://doi.org/10.1097/BCO.0000000000000084.
Shao H, Chen CL, Maltenfort MG, Restrepo C, Rothman RH, Chen AF. Bilateral total hip arthroplasty: 1-stage or 2-stage? A meta-analysis. J Arthroplasty. 2017;32:689–95. https://doi.org/10.1016/j.arth.2016.09.022.
Huang L, Xu T, Li P, Xu Y, Xia L, Zhao Z. Comparison of mortality and complications between bilateral simultaneous and staged total hip arthroplasty: a systematic review and meta-analysis. Medicine. 2019;98: e16774. https://doi.org/10.1097/md.0000000000016774.
Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022 Available from www.training.cochrane.org/handbook.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed). 2009;339: b2535. https://doi.org/10.1136/bmj.b2535.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4.
Fitz-Henry J. The ASA classification and peri-operative risk. Ann R Coll Surg Engl. 2011;93:185–7.
Wells GA SB, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses - 2011. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Available from URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Simunovic N, Devereaux PJ, Sprague S, Guyatt GH, Schemitsch E, Debeer J, Bhandari M. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. Can Med Assoc J. 2010;182:1609–16. https://doi.org/10.1503/cmaj.092220.
Walter SD, Yao X. Effect sizes can be calculated for studies reporting ranges for outcome variables in systematic reviews. J Clin Epidemiol. 2007;60:849–52. https://doi.org/10.1016/j.jclinepi.2006.11.003.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327:557–60. https://doi.org/10.1136/bmj.327.7414.557.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315:629–34. https://doi.org/10.1136/bmj.315.7109.629.
Bhan S, Pankaj A, Malhotra R. One- or two-stage bilateral total hip arthroplasty: a prospective, randomised, controlled study in an Asian population. J Bone Joint Surg Br. 2006;88:298–303. https://doi.org/10.1302/0301-620x.88b3.17048.
Taheriazam A, Mohseni G, Esmailiejah AA, Safdari F, Abrishamkarzadeh H. Bilateral total hip arthroplasty: one-stage versus two-stage procedure. HIP Int. 2019;29:141–6. https://doi.org/10.1177/1120700018773427.
Shih CH, Ho WB. One-stage versus two-stage bilateral autophor ceramic total hip arthroplasty. Clin Orthop Relat Res. 1985;193:141–5.
Eggli S, Huckell CB, Ganz R. Bilateral total hip arthroplasty: one stage versus two stage procedure. Clin Orthop Relat Res. 1996;328:108–18.
Lorenze M, Huo MH, Zatorski LE, Keggi KJ. A comparison of the cost effectiveness of one-stage versus two-stage bilateral total hip replacement. Orthopedics. 1998;21:1249–52.
Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13:172–9. https://doi.org/10.1016/s0883-5403(98)90095-x.
Alfaro-Adrián J, Bayona F, Rech JA, Murray DW. One- or two-stage bilateral total hip replacement. J Arthroplasty. 1999;14:439–45. https://doi.org/10.1016/s0883-5403(99)90099-2.
Parvizi J, Tarity TD, Sheikh E, Sharkey PF, Hozack WJ, Rothman RH. Bilateral total hip arthroplasty: one-stage versus two-stage procedures. Clin Orthop Relat Res. 2006;453:137–41. https://doi.org/10.1097/01.blo.0000246529.14135.2b.
Berend KR, Lombardi AV Jr, Adams JB. Simultaneous vs staged cementless bilateral total hip arthroplasty: perioperative risk comparison. J Arthroplasty. 2007;22:111–5. https://doi.org/10.1016/j.arth.2007.03.043.
Hooper GJ, Hooper NM, Rothwell AG, Hobbs T. Bilateral total joint arthroplasty: the early results from the New Zealand National Joint Registry. J Arthroplasty. 2009;24:1174–7. https://doi.org/10.1016/j.arth.2008.09.022.
Aghayev E, Beck A, Staub LP, Dietrich D, Melloh M, Orljanski W, Röder C. Simultaneous bilateral hip replacement reveals superior outcome and fewer complications than two-stage procedures: a prospective study including 1819 patients and 5801 follow-ups from a total joint replacement registry. BMC Musculoskelet Disord. 2010;11:245. https://doi.org/10.1186/1471-2474-11-245.
Saito S, Tokuhashi Y, Ishii T, Mori S, Hosaka K, Taniguchi S. One- versus two-stage bilateral total hip arthroplasty. Orthopedics. 2010. https://doi.org/10.3928/01477447-20100625-07.
Johnston LR, Clift BA, Abboud RJ. Bilateral simultaneous hip replacement versus bilateral sequential hip replacement. A 7-year data review. Orthop Nurs. 2011;30:119–23. https://doi.org/10.1097/NOR.0b013e31820f5155 (quiz 124–115).
Lindberg-Larsen M, Joergensen CC, Husted H, Kehlet H. Simultaneous and staged bilateral total hip arthroplasty: a Danish nationwide study. Arch Orthop Trauma Surg. 2013;133:1601–5. https://doi.org/10.1007/s00402-013-1829-z.
Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29:142–8. https://doi.org/10.1016/j.arth.2013.04.001.
Garland A, Rolfson O, Garellick G, Kärrholm J, Hailer NP. Early postoperative mortality after simultaneous or staged bilateral primary total hip arthroplasty: an observational register study from the Swedish Hip Arthroplasty Register. BMC Musculoskelet Disord. 2015;16:77. https://doi.org/10.1186/s12891-015-0535-0.
Quadri TA, Rashid RH, Zubairi AJ, Umer M, Hashmi PM. Single stage bilateral total hip replacement: Is it an option or a risk? J Pak Med Assoc. 2015;65:S91-93.
Seol JH, Park KS, Yoon TR. Postoperative complications and cost-effectiveness of simultaneous and staged bilateral total hip arthroplasty using a modified minimally invasive two-incision technique. Hip Pelvis. 2015;27:77–82. https://doi.org/10.5371/hp.2015.27.2.77.
Agarwal S, Gupta G, Sharma RK. Comparison between single stage and two stage bilateral total hip replacement- our results and review of literature. Acta Orthop Belg. 2016;82:484–90.
Kamath AF, Monteiro EL, Spranger A, Impellizzeri F, Leunig M. Simultaneous versus staged bilateral direct anterior Total Hip Arthroplasty: Are early patient-centered outcomes equivalent? Acta Orthop Belg. 2016;82:497–508.
Martin GR, Marsh JD, Vasarhelyi EM, Howard JL, Lanting BA. A cost analysis of single-stage bilateral versus two-stage direct anterior total hip arthroplasty. HIP Int. 2016;26:15–9. https://doi.org/10.5301/hipint.5000292.
Triantafyllopoulos GK, Memtsoudis SG, Zhang W, Ma Y, Sculco TP, Poultsides LA. Same-day surgery does not increase deep infection risk in bilateral total hip arthroplasty patients. J Arthroplasty. 2016;31:237–41. https://doi.org/10.1016/j.arth.2016.01.069.
Brown ML, Plate JF, Holst DC, Bracey DN, Bullock MW, Lang JE. A retrospective analysis of the merits and challenges associated with simultaneous bilateral THA using the direct anterior approach. HIP Int. 2017;27:169–74. https://doi.org/10.5301/hipint.5000449.
Houdek MT, Wyles CC, Watts CD, Wagner ER, Sierra RJ, Trousdale RT, Taunton MJ. Single-anesthetic versus staged bilateral total hip arthroplasty: a matched cohort study. J Bone Joint Surg Am. 2017;99:48–54. https://doi.org/10.2106/jbjs.15.01223.
Kim SC, Lim YW, Jo WL, Park DC, Lee JW, Kang WW, Kim YS. Surgical accuracy, function, and quality of life of simultaneous versus staged bilateral Total hip Arthroplasty in patients with Osteonecrosis of the femoral head. BMC Musculoskelet Disord. 2017;18:266. https://doi.org/10.1186/s12891-017-1605-2.
Poultsides LA, Triantafyllopoulos GK, Memtsoudis SG, Do HT, Alexiades MM, Sculco TP. Perioperative morbidity of same-day and staged bilateral total hip arthroplasty. J Arthroplasty. 2017;32:2974-2979.e2971. https://doi.org/10.1016/j.arth.2017.05.028.
Schlegelmilch M, Rashiq S, Moreau B, Jarrín P, Tran B, Chuck A. Cost-effectiveness analysis of total hip arthroplasty performed by a Canadian short-stay surgical team in Ecuador. Adv Orthop. 2017;2017:5109895. https://doi.org/10.1155/2017/5109895.
Tan Z, Cao G, Wang G, Zhou Z, Pei F. Total hospital cost, length of stay, and complications between simultaneous and staged bilateral total hip arthroplasty: a nationwide retrospective cohort study in China. Medicine. 2019;98: e14687. https://doi.org/10.1097/md.0000000000014687.
Calabro L, Yong M, Whitehouse SL, Hatton A, de Steiger R, Crawford RW. Mortality and implant survival with simultaneous and staged bilateral total hip arthroplasty: experience from the Australian Orthopedic Association National Joint Replacement Registry. J Arthroplasty. 2020;35:2518–24. https://doi.org/10.1016/j.arth.2020.04.027.
Guo SJ, Shao HY, Huang Y, Yang DJ, Zheng HL, Zhou YX. Retrospective cohort study comparing complications, readmission, transfusion, and length of stay of patients undergoing simultaneous and staged bilateral total hip arthroplasty. Orthop Surg. 2020;12:233–40. https://doi.org/10.1111/os.12617.
Partridge TCJ, Charity JAF, Sandiford NA, Baker PN, Reed MR, Jameson SS. Simultaneous or staged bilateral total hip arthroplasty? An analysis of complications in 14,460 patients using national data. J Arthroplasty. 2020;35:166–71. https://doi.org/10.1016/j.arth.2019.08.022.
Villa JM, Pannu TS, Higuera CA, Suarez JC, Patel PD, Barsoum WK. Hospital adverse events and perioperative outcomes in bilateral direct anterior approach total hip arthroplasty. J Arthroplasty. 2020;35:762–6. https://doi.org/10.1016/j.arth.2019.10.005.
Hou JF, Hu C, Zhang Y, Tian LQ, Liu YZ, Zhang C, Li J. Cost analysis of staged versus simultaneous bilateral total knee and hip arthroplasty using a propensity score matching. BMJ Open. 2021;11: e041147. https://doi.org/10.1136/bmjopen-2020-041147.
Mou P, Zeng WN, Chen Y, Zhou Z. Synchronous or sequential cementless bilateral total hip arthroplasty for osseous ankylosed hips with ankylosing spondylitis. BMC Musculoskelet Disord. 2021;22:302. https://doi.org/10.1186/s12891-021-04142-7.
Inoue D, Grace TR, Restrepo C, Hozack WJ. Outcomes of simultaneous bilateral total hip arthroplasty for 256 selected patients in a single surgeon’s practice. Bone Joint J. 2021;103-b:116–21. https://doi.org/10.1302/0301-620x.103b7.bjj-2020-2292.r1.
Panchal S, Jogani AD, Mohanty SS, Rathod T, Kamble P, Keny SA. Is bilateral sequential total hip replacement as safe an option as staged replacement? A matched pair analysis of 108 cases at a tertiary care centre. Indian J Orthop. 2021;55:1250–5. https://doi.org/10.1007/s43465-021-00536-w.
Goh GS, Sutton RM, D’Amore T, Baker CM, Clark SC, Courtney PM. A time-driven activity-based costing analysis of simultaneous versus staged bilateral total hip and knee arthroplasty. J Arthroplasty. 2022. https://doi.org/10.1016/j.arth.2022.01.048.
Salvati EA, Hughes P, Lachiewicz P. Bilateral total hip-replacement arthroplasty in one stage. J Bone Joint Surg Am. 1978;60:640–4.
Wyatt MC, Hozack JW, Frampton C, Rothwell A, Hooper GJ. Is single-anaesthetic bilateral primary total hip replacement still safe? A 16-year cohort study from the New Zealand Joint Registry. ANZ J Surg. 2018;88:1289–93. https://doi.org/10.1111/ans.14864.
Haverkamp D, van den Bekerom MP, Harmse I, Schafroth MU. One stage bilateral total hip arthroplasty, is it safe? A meta-analysis. HIP Int. 2010;20:440–6. https://doi.org/10.1177/112070001002000405.
Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27:302–45. https://doi.org/10.1128/CMR.00111-13.
Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27:61-65.e61. https://doi.org/10.1016/j.arth.2012.02.022.
Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD, Tea I. Re-infection outcomes following one- and two-stage surgical revision of infected hip prosthesis: a systematic review and meta-analysis. PLoS ONE. 2015;10:e0139166–e0139166. https://doi.org/10.1371/journal.pone.0139166.
Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the medicare population. J Arthroplasty. 2009;24:105–9. https://doi.org/10.1016/j.arth.2009.04.027.
Hailer NP, Garellick G, Kärrholm J. Uncemented and cemented primary total hip arthroplasty in the Swedish Hip Arthroplasty Register. Acta Orthop. 2010;81:34–41. https://doi.org/10.3109/17453671003685400.
Wang T, Shao L, Xu W, Chen H, Huang W. Comparison of morphological changes of gluteus medius and abductor strength for total hip arthroplasty via posterior and modified direct lateral approaches. Int Orthop. 2019;43:2467–75. https://doi.org/10.1007/s00264-019-04331-z.
van Stralen KJ, Le Cessie S, Rosendaal FR, Doggen CJ. Regular sports activities decrease the risk of venous thrombosis. J Thromb Haemost. 2007;5:2186–92. https://doi.org/10.1111/j.1538-7836.2007.02732.x.
Cohen AT, Tapson VF, Bergmann JF, Goldhaber SZ, Kakkar AK, Deslandes B, Huang W, Zayaruzny M, Emery L, Anderson FA Jr. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet (London, England). 2008;371:387–94. https://doi.org/10.1016/s0140-6736(08)60202-0.
Pannucci CJ, Laird S, Dimick JB, Campbell DA, Henke PK. A validated risk model to predict 90-day VTE events in postsurgical patients. Chest. 2014;145:567–73. https://doi.org/10.1378/chest.13-1553.
Kapoor A, Ellis A, Shaffer N, Gurwitz J, Chandramohan A, Saulino J, Ishak A, Okubanjo T, Michota F, Hylek E, Trikalinos TA. Comparative effectiveness of venous thromboembolism prophylaxis options for the patient undergoing total hip and knee replacement: a network meta-analysis. J Thromb Haemost. 2017;15:284–94. https://doi.org/10.1111/jth.13566.
Barrett MC, Whitehouse MR, Blom AW, Kunutsor SK. Host-related factors for venous thromboembolism following total joint replacement: a meta-analysis of 89 observational studies involving over 14 million hip and knee replacements. J Orthop Sci. 2020;25:267–75. https://doi.org/10.1016/j.jos.2019.04.003.
Malcolm TL, Knezevic NN, Zouki CC, Tharian AR. Pulmonary complications after hip and knee arthroplasty in the United States, 2004–2014. Anesth Analg. 2020;130:917–24. https://doi.org/10.1213/ane.0000000000004265.
Wang Q, Goswami K, Shohat N, Aalirezaie A, Manrique J, Parvizi J. Longer operative time results in a higher rate of subsequent periprosthetic joint infection in patients undergoing primary joint arthroplasty. J Arthroplasty. 2019;34:947–53. https://doi.org/10.1016/j.arth.2019.01.027.
Jaffer AK, Barsoum WK, Krebs V, Hurbanek JG, Morra N, Brotman DJ. Duration of anesthesia and venous thromboembolism after hip and knee arthroplasty. Mayo Clin Proc. 2005;80:732–8. https://doi.org/10.1016/s0025-6196(11)61526-7.
Horlocker TT, Hebl JR, Gali B, Jankowski CJ, Burkle CM, Berry DJ, Zepeda FA, Stevens SR, Schroeder DR. Anesthetic, patient, and surgical risk factors for neurologic complications after prolonged total tourniquet time during total knee arthroplasty. Anesth Analg. 2006;102:950–5. https://doi.org/10.1213/01.ane.0000194875.05587.7e.
Bohl DD, Ondeck NT, Darrith B, Hannon CP, Fillingham YA, Della Valle CJ. Impact of operative time on adverse events following primary total joint arthroplasty. J Arthroplasty. 2018;33:2256-2262.e2254. https://doi.org/10.1016/j.arth.2018.02.037.
Surace P, Sultan AA, George J, Samuel LT, Khlopas A, Molloy RM, Stearns KL, Mont MA. The association between operative time and short-term complications in total hip arthroplasty: an analysis of 89,802 surgeries. J Arthroplasty. 2019;34:426–32. https://doi.org/10.1016/j.arth.2018.11.015.
Sodhi N, Anis HK, Gold PA, Garbarino LJ, Scuderi GR, Cushner FD, Higuera CA, Mont MA. Operative times can predict and are correlated with lengths-of-stay in primary total knee arthroplasty: a nationwide database study. J Arthroplasty. 2019;34:1328–32. https://doi.org/10.1016/j.arth.2019.03.024.
Meyers SJ, Reuben JD, Cox DD, Watson M. Inpatient cost of primary total joint arthroplasty. J Arthroplasty. 1996;11:281–5. https://doi.org/10.1016/S0883-5403(96)80079-9.
Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31:212–7. https://doi.org/10.1046/j.1537-2995.1991.31391165169.x.
Innerhofer P, Walleczek C, Luz G, Hobisch-Hagen P, Benzer A, Stöckl B, Hessenberger G, Nussbaumer W, Schobersberger W. Transfusion of buffy coat-depleted blood components and risk of postoperative infection in orthopedic patients. Transfusion. 1999;39:625–32. https://doi.org/10.1046/j.1537-2995.1999.39060625.x.
Madjdpour C, Spahn DR. Allogeneic red blood cell transfusions: efficacy, risks, alternatives and indications. Br J Anaesth. 2005;95:33–42. https://doi.org/10.1093/bja/aeh290.
Rosencher N, Kerkkamp HEM, Macheras G, Munuera LM, Menichella G, Barton DM, Cremers S, Abraham IL, Investigation FTO. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe*. Transfusion. 2003;43:459–69. https://doi.org/10.1046/j.1537-2995.2003.00348.x.
Singh JA, Schleck C, Harmsen S, Lewallen D. Clinically important improvement thresholds for Harris Hip Score and its ability to predict revision risk after primary total hip arthroplasty. BMC Musculoskelet Disord. 2016;17:1–8.
Mancuso CA, Jout J, Salvati EA, Sculco TP. Fulfillment of patients’ expectations for total hip arthroplasty. JBJS. 2009;91:2073–8.
Konyves A, Bannister G. The importance of leg length discrepancy after total hip arthroplasty. J Bone Joint Surg Br. 2005;87:155–7.
Acknowledgements
Not applicable.
Funding
This research received no fund from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Study concept and design were performed by SHS and SMJM. Literature review, collection, extraction, analysis, and interpretation of data were performed by AR, AS, AGR, and MS. The first draft of the manuscript was written by AR, AGR, and AS. Critical revision of the manuscript for important intellectual content was performed by SHS, SMJM, and MS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA Checklist.
Additional file 2.
Search String.
Additional file 3.
Begg’s funnel plots and Egger’s regression test.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ramezani, A., Ghaseminejad Raeini, A., Sharafi, A. et al. Simultaneous versus staged bilateral total hip arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res 17, 392 (2022). https://doi.org/10.1186/s13018-022-03281-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-022-03281-4