Abstract
Background
This study aimed to analyze the in vitro effect of micro-strain stress on the proliferation and functional marker expression in chondrocytes isolated from human osteoarthritis cartilage samples.
Methods
Chondrocytes isolated from human osteoarthritis cartilage samples were subjected to loading with different types of micro-strain stress. The proliferation activity was assessed by flow cytometry, and the functional expression of chondrocyte markers was detected by qRT-PCR and western blot.
Results
Flow cytometry results showed stimulation of proliferation of human osteoarthritic chondrocytes when an adequate micro-strain stress was applied. qRT-PCR and western blot results showed that micro-strain stress promotes human osteoarthritic chondrocyte functional marker expression. These features coincide with the upregulation of multiple proteins and genes affecting cell proliferation and functional chondrocyte marker expression, including cyclin D1, collagen II, and Rock.
Conclusion
Adequate micro-strain stress could activate the Rho/Rock signaling pathway in osteoarthritic chondrocytes, thus transmitting mechanical signals to the cytoskeleton. This process leads to cytoskeleton reorganization, and transmission of the mechanical signals to the downstream effectors to promote proliferation and functional marker expression of osteoarthritic chondrocytes.
Similar content being viewed by others
Introduction
Osteoarthritis is the most common type of joint disease, and its pathology is characterized by chondrocyte apoptosis and cartilage matrix destruction [1,2,3]. Cartilage tissue, which covers the joint surface, is a specialized connective tissue without blood vessels. It is stimulated continuously by endogenous and exogenous mechanical stimuli. Mechanical stimulation in a specific range plays a critical role in the maintenance of the structural integrity of articular cartilage [4]. Some in vitro studies have shown that loading cultured chondrocytes with different types of mechanical stimulation could produce varying degrees of cell biological changes [5,6,7].
The cellular mechanism of how osteoarthritic chondrocytes sense mechanical stress stimulation signals and transfer them into the cells to regulate bone remodeling is not thoroughly understood. The cytoskeleton is a critical component involved in the maintenance of cell morphology and various cellular functions [8, 9]. After mechanical stimulation, the cytoskeletal structures undergo dynamic rearrangement, polymerization, and depolymerisation [10]. The cytoskeleton plays a vital role in the maintenance of the intracellular structures and could rapidly transmit stress from the cytoskeleton to effectors in cells, thus causing various biological effects [11, 12]. In studies on vascular endothelial cells, osteoblasts, and bone marrow mesenchymal stem cells, it has been shown that cytoskeletal-mediated mechanical signaling plays a crucial role in the reactivity of various cell lines to mechanical stimulation [13]. At present, the biological effects of stress stimulation on osteoarthritic chondrocytes and the underlying role of the cytoskeleton are still unclear, and further experimental studies are needed urgently.
In this study, the varying extent of micro-strain stress was applied to cultured human osteoarthritic chondrocytes and their effects on cell proliferation and functional expression of markers were evaluated. The regulatory effect of differing micro-strain stress on the cytoskeleton of osteoarthritic chondrocytes was studied by confocal fluorescence microscopy to explore the underlying role of the cytoskeleton in mediating the mechanical stress signals to regulate the physiological functions of osteoarthritic chondrocytes. The role of the Rho/Rock signaling pathway in the above process was studied through the use of the Rho/Rock signaling pathway inhibitor Y-27632.
Materials and methods
Study objects
After obtaining the informed consent from patients with osteoarthritis of the knee, a small sample of cartilage tissue was removed during surgery. Tissues were collected aseptically, and sections prepared for Hematoxylin and Eosin (H&E) staining. Chondrocytes were isolated from the remaining tissues and cultured within two hours.
The Ethical Committee of Tianjin Hospital approved this study. Following the Declaration of Helsinki guidelines, consents were obtained from either the patient or family member before enrollment in the study.
Isolation, culture, and identification of chondrocytes
The tissue was soaked in D-Hank's solution containing antibiotics for 5 min and rinsed twice with D-Hank's solution to remove the blood and fat from the cartilage tissue. The tissue was cut into 1 × 1 × 1 mm3 sized tissue blocks and digested with 0.02% EDTA and 0.25% trypsin at 37℃ for 30 min. The trypsin–EDTA solution was removed, after which 0.2% collagen II enzyme was added, and the tissue digested overnight at 4℃. The tissue was digested almost completely after incubation at 37℃ for 2 h, and the addition of 1 ml FBS stopped the digestion. The resulting sample was filtered using a 200-mesh filter, and the filtrate transferred to a tube and centrifuged at 1500 r/min for 7 min, and the supernatant discarded. The cells were suspended in an H-DMEM medium containing 10% FBS, and cells counted. A 25 cm2 cell culture flask was inoculated with 5 × 104/ml of cells. After 24 h, half the volume was replaced with fresh media changes every two days. When the cells reached 80–90% confluence, the cells were trypsinized and passaged.
The cells derived were identified by toluidine blue staining, Safranin O staining, and collagen II immunofluorescence staining. The experimental methods were carried out following the kit instructions. Cells at passage three were used for subsequent cell mechanical loading tests.
Cell mechanical loading
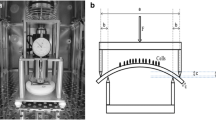
We performed the mechanical loading of cells following the methods described earlier by our group [14,15,16]. The conditions used were: mechanical loading frequency, 0.25 HZ; time, 2 h/day; duration, three days. The study was divided into five groups: group A, 0% micro-strain; group B, 5% micro-strain; group C, 10% micro-strain; group D, 15% micro-strain; group E, 10% micro-strain plus Rock inhibitor Y-27632.
Immunofluorescence detection
Cells of each group were washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde. Following fixation, the cells were washed with PBS and permeabilized with Triton X-100. After washing with PBS, the cells were blocked with 1% goat serum and incubated at room temperature with FITC labeled phalloidin. After PBS wash, the cells were stained with DAPI, and the immunofluorescent cytoskeletal proteins were observed under confocal microscopy.
Cell cycle analysis
The cell cycle analysis was performed as described previously [15]. After mechanical loading, cells from each group were cultured in the incubator for another 24 h, allowing adequate recovery time. The cells were digested enzymatically, centrifuged at 2,000 r/min for 5 min, and the supernatant discarded. The cells were rinsed twice with sterile D-Hank’s solution and fixed in 75% cold ethanol. The cells were centrifuged at 2,000 r/min for 5 min, and 500 μl RNAase was added to the cell pellet and incubated at 37℃ for 30 min. The cells were centrifuged at 2,000 r/min for 5 min, and 500 μl propidium iodide (PI) was added and incubated at room temperature in the dark for 30 min. The cell cycle analysis was carried out by flow cytometry. The data were calculated by Flow Plus software. The proliferative index, the proportion (%) of cells in the S phase were calculated based on the G0/G1 phase and G2/M phase, using the formula: S(%) = 1 − [G0/G1(%) + G2/M(%)], PI(%) = S(%) + G2/M(%).
Quantitative real-time (qRT) PCR
We extracted total RNA from each group of cells using Trizol following the manufacturer’s instructions. The total RNA was reverse transcribed to cDNA using Prime Script RT reagent kit. The primers used for the genomic analysis of the chondrocytes were as follows: β-actin, F 5′-CCTCGCCTTTGCCGATCC-3′ and R 5′-GGATCTTCATGAGGTAGTCAGTC-3′; cyclin D1, F 5′-AAAGGAAGCAAGAACCCAT-3′ and R 5′-GTCCGAGATTATCATTACCC-3′; collagen II, F 5′-TTCAGCTATGGAGATGACAATC-3′ and R 5′-AGAGTCCTAGAGTGACTGAG-3′. The reaction conditions were as outlined in the SYBR Premix Ex Taq. The amplification and melting curves were analyzed after the reaction was completed. Gene expression values were analyzed for target gene expression by the 2−△△Ct method.
Western blot analysis
We extracted total proteins from each group of cells using RIPA lysis method. The proteins were quantified by BCA protein quantitation kit. An equal amount of protein from each group was separated on SDS-PAGE and transferred to nitrocellulose (NC) membranes. After blocking with 5% milk, the NC membranes were incubated with primary antibodies against β-actin, cyclin D1, collagen II, and Rock, followed by appropriate HRP-conjugated secondary antibodies. The membranes were treated with ECL, and bands were visualized.
Statistical analysis
SPSS 18.0 software was used for analysis. Data were expressed as mean ± standard deviation (x ± s), one-way ANOVA was used for multi-group comparison, and the LSD-t method was used for multi-group comparison. P < 0.05 was considered statistically significant.
Results
Characterization and identification of cartilage and chondrocytes
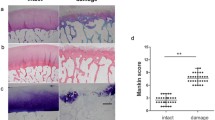
Gross observation: the articular cartilage lost its original luster, and its color darkened markedly. Articular cartilage surface defects of the lateral femoral condyle were noted, and subchondral bone was exposed, as shown in Fig. 1A. H&E staining showed the formation of longitudinal cracks in the cartilage, showing a fibrillation-like change and disordered cell arrangement. In the deep layer of the cartilage, cell aggregation, as shown in Fig. 1B was observed. The results, when combined with the clinicopathological data of the patient, showed moderate and severe degeneration of the articular cartilage.
Characterization and identification of cartilage and chondrocytes. A Gross view of knee osteoarthritis cartilage. B The H&E staining of knee osteoarthritis cartilage. C Toluidine blue staining of osteoarthritic chondrocytes. D Safranin O staining of osteoarthritic chondrocytes. E Collagen II immunofluorescence staining of osteoarthritic chondrocytes
Toluidine blue staining results showed blue cytoplasm, confirming that cells secrete glycosaminoglycan (Fig. 1C). Safranin O staining results showed red cytoplasm, thus identifying than cells secrete proteoglycan (Fig. 1D). Collagen II immunofluorescence staining results showed green fluorescent cytoplasm suggesting secretion of collagen II (Fig. 1E). The above results confirmed that the cultured cells were chondrocytes.
Micro-strain stress causes reorganization of osteoarthritic chondrocyte cytoskeleton
The expression and distribution of cytoskeletal proteins in chondrocytes under micro-strain stress were evaluated by immunofluorescence, and the images captured by confocal microscopy: cells in group A showed uniform fluorescence staining, scattered and distributed in the cytoplasm without any directionality (Fig. 2A). The cells in group B showed a fine fiber staining pattern, distributed in a specific direction (Fig. 2B). The cells in group C showed intense fluorescence staining, and the actin fibers were relatively thick, evenly distributed and arranged in bundles in parallel, and distributed in the direction of stress (Fig. 2C). The cells in group D were composed mainly of fine fibers, which were distributed in a specific direction (Fig. 2D). The above results suggested that the application of adequate micro-strain stress could lead to the reorganization of the cytoskeleton.
Micro-strain stress promotes the proliferation of osteoarthritic chondrocytes
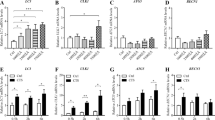
Cell cycle analysis by flow cytometry was used to examine the micro-strain stress effect on the proliferation of osteoarthritic chondrocytes. PI staining shows the ability of cells to proliferate. With increased micro-strain stress, PI staining increased gradually, reaching peak levels in group C, and decreased gradually (Fig. 3A). Based on the qRT-PCR results, changes in Cyclin D1 gene expression were similar to changes in PI staining. With increasing micro-strain stress, the Cyclin D1 gene expression increased gradually, and reached a peak in group C, then progressively decreased (Fig. 3B). Western blot results showed that with an increase in micro-strain stress, Cyclin D1 protein expression increased gradually, and peaked in group C, then decreased gradually (Fig. 3C). These results suggested that adequate micro-strain stress could promote the proliferation of osteoarthritic chondrocytes.
Effects of micro-strain stress on the proliferation of osteoarthritic chondrocytes. A Proliferative index of osteoarthritic chondrocytes after different micro-strain stress for 3-days (*P < 0.05). B Cyclin D1 mRNA expression of osteoarthritic chondrocytes after different micro-strain stress for 3-days (*P < 0.05). C Cyclin D1 protein expression of osteoarthritic chondrocytes after different micro-strain stress for 3-days
Micro-strain stress promotes the functional expression of osteoarthritic chondrocyte markers
The effects of micro-strain stress on functional expression of osteoarthritic chondrocyte markers were examined by detecting the collagen II gene and protein expression in each group. qRT-PCR results showed that with increasing micro-strain stress, collagen II gene expression increased gradually, and reached a peak in group C, then steadily decreased (Fig. 4A). Western blot results showed a gradual increase in collagen II protein expression with increased micro-strain stress, which reached a peak in group C, then decreased gradually (Fig. 4B). These results showed the functional expression of osteoarthritic chondrocyte markers after the application of adequate micro-strain stress.
Effects of micro-strain stress on functional marker expression in osteoarthritic chondrocytes. A Collagen II mRNA expression in osteoarthritic chondrocytes after different micro-strain stress for 3-days (*P < 0.05). B Collagen II and Rock protein expression in osteoarthritic chondrocytes after different micro-strain stress for 3-days. C Schematic diagram of possible signaling pathways
Expected mechanism
Phalloidin immunofluorescence results showed that compared to group C, the crude fibers in group E cells disappeared and were replaced by tiny fibers, which were distributed diffusely in the cytoplasm with no directionality (Fig. 2E). The cell cycle results showed a lower proliferative index value in group E compared to group C (P < 0.05, Fig. 3A). The cyclin D1 and collagen II gene and protein expression in group E were lower compared to group C as shown by qRT-PCR and western blot results (P < 0.05, Fig. 3B, C) and (P < 0.05, Fig. 4A, B), respectively. Western blot results showed a high expression of Rock in groups C and D, moderate expression in group B, and low expression in groups A and C (Fig. 4B). These results revealed the involvement of the Rho/Rock signaling pathway in the above biological changes (Fig. 4C).
Discussion
The present study used a novel micro-strain stress loading system designed by our research group [14,15,16]. The osteoarthritic chondrocyte proliferation and functional marker expression were detected after subjecting cells to varying micro-strain stress during culture. The results showed that the application of adequate micro-strain stress could promote osteoarthritic chondrocyte proliferation and functional marker expression. The cyclin D1 and collagen II mRNA and protein expression increased significantly under micro-strain stress and peaked in the 10% micro-strain group. With increasing micro-strain stress, the cytoskeleton exhibited rearrangement. However, the above-described changes were suppressed when the Rock inhibitor Y-27632 was used before the application of micro-strain stress.
Articular cartilage is in a complex physiological and mechanical environment in the body [17, 18]. The mechanical stimuli are important factors in maintaining the normal structure and function of articular cartilage [19, 20]. Xu et al. [21] applied intermittent cyclic mechanical tension (0.5 Hz, 10% deformation, 4 h/d, 6d/week) to rat endplate chondrocytes, and showed that tension stimulation promotes the proliferation of endplate chondrocytes. Thomopoulos et al. [22] applied cyclic tensile loading tension (1 Hz, 10% deformation, 7d) to bone marrow stromal cells in a 3D in vitro model, showing that the cyclic tensile strain could promote spindle cell formation, increase collagen I and glycosaminoglycan synthesis. Therefore, we believe that an appropriate micro-strain stress could promote the proliferation and matrix anabolism of chondrocytes, and also maintain the normal structure and function of chondrocytes. However, micro-strain stress beyond the chondrocytes bearing range might inhibit cell proliferation, damage structural function and integrity of cells, and further damage the articular cartilage. Such changes weaken the ability of damaged cartilage tissue to withstand mechanical stimulation and may further aggravate the effects of mechanical stimulation leading to a vicious cycle of complete loss of cartilage tissue structure and function.
The experimental results showed that proliferative index values, mRNA, and cyclin D1 and collagen II protein expression increased gradually with increasing micro-strain stress, and peaked at 10% micro-strain stress and then decreased gradually. However, after pretreatment of cells with the Rock inhibitor Y-27632, the proliferative index values, mRNA, and cyclin D1 and collagen II protein expression decreased. The above results indicate that micro-strain stress affects the proliferation and functional marker expression of osteoarthritic chondrocytes, and 10% micro-strain stress might offer the best mechanical stimulation. Rock inhibitor Y-27632 inhibits this process.
After experiencing mechanical stimulation, the cells convert mechanical signals to chemical signals through specific signal transduction mechanisms resulting in changes in the biological function [23, 24]. In this series of signal transduction processes, the cytoskeleton plays a crucial role as the hub across the cell [25]. Cytoskeleton, a critical component of cells, is composed of a large number of actin filaments and is the internal framework of cells [26]. It consists of microtubules, microfilaments, and intermediate filaments, which are interlinked with protein-lipid molecules of the cytoplasmic side of the cell membrane to form the structural basis for cell movement, cell morphology, and transmembrane information transmission [27]. It was shown earlier that cytoskeletal rearrangements occur during periodic mechanical stress and space microgravity [28]. Phalloidin specifically binds to actin fibers in the cytoskeleton [29]. The results of this experiment confirmed that under appropriate micro-strain stress, the osteoarthritic chondrocyte cytoskeletal microfilaments changed in structure and arrangement. However, too much mechanical stimulation can suppress the above changes.
Studies have shown that Rho GTPases can regulate the structure and function of the cytoskeleton in several ways under biomechanical stimulation, thus playing a critical role in biomechanical signal transduction [30, 31]. Rock is the downstream signaling molecule of the Rho GTP family and plays a crucial role in the Rho signaling pathway [32]. Y-27632 has been widely used as an inhibitor of the Rho/ROCK signaling pathway [33,34,35]. Therefore, Rock specific inhibitor Y-27632 was selected in this study. Whether the Rho/Rock signaling pathway is involved in the regulation of osteoarthritic chondrocyte proliferation and functional expression of markers induced by micro-strain stress is still unclear. Western blot results showed that with increasing micro-strain stress, the Rock level increased gradually, reached a peak at 10% micro-strain, and decreased later. These results showed activation of the Rho/Rock signaling pathway in osteoarthritic chondrocytes by micro-strain stress, leading to cytoskeletal reorganization, and promoting the proliferation and functional expression of osteoarthritic chondrocyte markers.
In summary, adequate micro-strain stress can activate the Rho/Rock signaling pathway in osteoarthritic chondrocytes, which leads to the transmission of mechanical signals to the cytoskeleton. The above processes cause the cytoskeletal reorganization and transmit the mechanical signals, leading to the promotion of proliferation and functional expression of osteoarthritic chondrocyte markers.
Availability of data and materials
All data generated during this study are included in this manuscript.
Abbreviations
- H&E staining:
-
Hematoxylin and Eosin staining
- PBS:
-
Phosphate-buffered saline
- PI:
-
Propidium iodide
- NC:
-
Nitrocellulose
References
Charlier E, Deroyer C, Ciregia F, Malaise O, Neuvile S, Plener Z, et al. Chondrocyte dedifferentiation and ostesarthritis (OA). Biochem Pharmacol. 2019;165:49–65. https://doi.org/10.1016/j.bcp.2019.02.036.
Mesumeci G, Castrogiovanni P, Trovato FM, Weinberg AM, Al-Wasiyah MK, Alqahtani MH, et al. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci. 2015;16(9):20560–75. https://doi.org/10.3390/ijms160920560.
Luo P, Gao F, Niu D, Sun X, Song Q, Guo C, et al. The role of autophagy in chondrocyte metabolism and osteoarthritis: a comprehensive research review. Biomed Res Int. 2019;2019:5171602. https://doi.org/10.1155/2019/5171602.
Xu HG, Hu CJ, Wang H, Liu P, Yang XM, Zhang Y, et al. Effects of mechanical strain on ANK, ENPP1, and TGF-β1 expression in rat endplate chondrocytes in vitro. Mol Med Rep. 2011;4(5):831–5. https://doi.org/10.3892/mmr.2011.508.
Moukoko D, Pourquier D, Pithioux M, Chabrand P. Influence of cyclic bending loading on in vivo skeletal tissue regeneration from periosteal origin. Orthop Traumatol Surg Res. 2010;96(8):833–9. https://doi.org/10.1016/j.otsr.2010.07.006.
Ouyan X, Xie Y, Wang G. Mechanical stimulation promotes the proliferation and the cartilage phenotype of mesenchymal stem cells and chondrocytes co-cultured in vitro. Biomed Pharmacother. 2019;117:109146. https://doi.org/10.1016/j.biopha.2019.109146.
Chong PP, Panjavarnam P, Ahmad WNHW, Chan CK, Abbas AA, Merivan AM, et al. Mechanical compression controls the biosynthesis of human osteoarthritic chondrocytes in vitro. Clin Biomech (Bristol, Avon). 2020;79:105178. https://doi.org/10.1016/j.clinbiomech.2020.105178.
Sugio S, Nagasawa M, Kojima I, Ishizaki Y, Shibasaki K. Transient receptor potential vanilloid 2 activation by focal mechanical stimulation requires interaction with the actin cytoskeleton and enhances growth cone motility. FASEB J. 2017;31(4):1368–81. https://doi.org/10.1096/fj.201600686RR.
Yan Z, Wang P, Wu J, Feng X, Cai J, Zhai M, et al. Fluid shear stress improves morphology, cytoskeleton architecture, viability, and regulates cytokine expression in a time-dependent manner in MLO-Y4 cells. Cell Biol Int. 2018;42(10):1410–22. https://doi.org/10.1002/cbin.11032.
Artemenko Y, Axiotakis L Jr, Borleis J, Iglesias PA, Devreotes PN. Chemical and mechanical stimuli act on common signal transduction and cytoskeletal networks. Pro Natl Acad Sci U S A. 2016;113(47):E7500–9. https://doi.org/10.1073/pnas.1608767113.
Dalous J, Burghardt E, Muller-Taubenberger A, Bruckert F, Gerisch G, Bretschneider T. Reversal of cell polarity and actin-myosin cytoskeleton reorganization under mechanical and chemical stimulation. Biophys J. 2008;94(3):1063–74. https://doi.org/10.1529/biophysj.107.114702.
Samandari M, Abrinia K, Mokhtari-Dizaji M, Tamayol A. Ultrasound induced strain cytoskeleton rearrangement: an experimental and simulation study. J Biomech. 2017;60:29–47. https://doi.org/10.1016/j.jbiomech.2017.06.003.
Ohashi K, Fujiwara S, Mizuno K. Roles of the cytoskeleton, cell adhesion and rho signaling in mechanosensing and mechanotransduction. J Biochem. 2017;161(3):245–54. https://doi.org/10.1093/jb/mvw082.
Li F, Sun X, Zhao B, Ma J, Zhang Y, Li S, et al. Effects of cyclic tension stress on the apoptosis of osteoclasts in vitro. Exp Ther Med. 2015;9(5):1955–61. https://doi.org/10.3892/etm.2015.2338.
Zhao B, Zhao Z, Sun X, Zhang Y, Guo Y, Tian P, et al. Effects of micro strain stress on proliferation of endothelial progenitor cells in vitro by the MAPK-ERK1/2 signaling pathway. Biochem Biophs Res Commun. 2017;492(2):206–11. https://doi.org/10.1016/j.bbrc.2017.08.050.
Li S, Sun X, Ma X. Effects of cyclic tensile strain on oxidative stress and the function of Schwann cells. Biomed Res Int. 2018;2018:5746525. https://doi.org/10.1155/2018/5746525.
Hirano Y, Ishiguro N, Sokabe M, Takigawa M, Naruse K. Effects of tensile and compressive strains on response of a chondrocytic cell line embedded in type I collagen gel. J Biotechnol. 2008;133(2):245–52. https://doi.org/10.1016/j.jbiotec.2007.07.955.
Wiluse RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matirx Biol. 2014;39:25–32. https://doi.org/10.1016/j.matbio.2014.08.009.
Fahy N, Alini M, Stoddart MJ. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J Orthop Res. 2018;36(1):52–63. https://doi.org/10.1002/jor.23670.
Siegel MG, Lubowitz JH, Brand JC, Rossi MJ. Articular cartilage restoration requires cells, scaffolds, growth factors, and mechanical stimulation. Arthroscopy. 2021;37(5):1359–60. https://doi.org/10.1016/j.arthro.2021.03.014.
Xu HG, Zhang XH, Wang H, Liu P, Wang LT, Zuo CJ, et al. Intermittent cyclic mechanical tension-induced calcification and downregulation of ankh gene expression of end plate chondrocyte. Spine (Phila Pa 1976). 2012;37(14):1192–7. https://doi.org/10.1097/BRS.0b013e318244d989.
Thomopoulos S, Das R, Birman V, Smith L, Ku K, Elson EL, et al. Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression. Tissue Eng Part A. 2011;17(7–8):1039–53. https://doi.org/10.1089/ten.TEA.2009.0499.
Hughes-Fulford M. Signal transduction and mechanical stress. Sci STKE. 2004;249:RE12. https://doi.org/10.1126/stke.2492004re12.
Tidball JG. Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol (1985). 2005;98(5):1900–8. https://doi.org/10.1152/japplphysiol.01178.2004.
Zhang C, Wang F, Gao Z, Zhang P, Gao J, Wu X. Regulation of hippo signaling by mechanical signals and the cytoskeleton. DNA Cell Biol. 2020;39(2):159–66. https://doi.org/10.1089/dna.2019.5087.
Pollard TD, Goldman RD. Overview of the cytoskeleton from an evolutionary perspective. Cold Spring Hard Perspect Biol. 2018;10(7):a030288. https://doi.org/10.1101/cshperspect.a030288.
White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci. 2007;362(1484):1459–67. https://doi.org/10.1098/rstb.2007.2128.
Celik E, Abdulreda MH, Maiguel D, Li J, Moy VT. Rearrangement of microtube network under biochemical and mechanical stimulations. Methods. 2013;60(2):195–201. https://doi.org/10.1016/j.ymeth.2013.02.014.
DesMarais V, Eddy RJ, Sharma VP, Stone O, Condeelis JS. Optimizing leading edge F-actin labeling using multiple actin probes, fixation methods and imaging modalities. Biotechniques. 2019;66(3):113–9. https://doi.org/10.2144/btn-2018-0112.
Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–14. https://doi.org/10.1126/science.279.5350.509.
Wen SJ, Zhang W, Ni NN, Wu Q, Wang XP, Lin YK, et al. Expression of Rho GTPases family in melanoma cells and its influence on cytoskeleton and migration. Oncotarget. 2017;8(18):30112–22. https://doi.org/10.18632/oncotarget.15618.
Pleines I, Cherpokova D, Bender M. Rho GTPases and their downstream effectors in megakaryocyte biology. Platelets. 2019;30(1):9–16. https://doi.org/10.1080/09537104.2018.1478071.
Nakagawa K, Teramura T, Takehara T, Onodera Y, Hamanishi C, Akagi M, et al. Cyclic compression-induced p38 activation and subsequent MMP13 expression requires Rho/ROCK activity in bovine cartilage explants. Inflamm Res. 2021;61(10):1093–100. https://doi.org/10.1007/s00011-012-0500-4.
Zhang L, Jiang G, Zhao X, Gong Y. Dimethyloxalylglycine promotes bone marrow mesenchymal stem cell osteogenesis via Rho/ROCK signaling. Cell Physiol Biochem. 2016;39(4):1391–403. https://doi.org/10.1159/000447843.
Wang G, Woods A, Sabari S, Pagnotta L, Stanton LA, Beier F. RhoA/ROCK signaling suppresses hypertrophic chondrocyte differentiation. J Biol Chem. 2004;279(13):13205–14. https://doi.org/10.1074/jbc.M311427200.
Acknowledgements
We thank Sun Xiaolei, Guo yue and Zhang Yang for their help in this experiment.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81501061), Tianjin Science and Technology Committee (20JCZDJC00760).
Author information
Authors and Affiliations
Contributions
BZ and XM contributed to the conception and design of the study. BZ and JM contributed to the analysis and interpretation of the date. BZ and JH contributed to the drafting of manuscripts. BZ, JM and JH contributed to the carry out of the experiments. All the authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Ethical Committee of Tianjin Hospital approved this study. Following the Declaration of Helsinki guidelines, consents were obtained from either the patient or family member before enrollment in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, B., Ma, J., He, J. et al. Effect of micro-strain stress on in vitro proliferation and functional expression of human osteoarthritic chondrocytes. J Orthop Surg Res 17, 93 (2022). https://doi.org/10.1186/s13018-022-02987-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-022-02987-9