Abstract
Background
Although intravenous tranexamic acid administration (ivTXA) has prevailed in clinical antifibrinolytic treatment, whether it increases thromboembolic risks has remained controversial. As a potent alternative to ivTXA, topical use of TXA (tTXA) has been successfully applied to attenuate blood loss in various surgical fields while minimizing systemic exposure to TXA. This meta-analysis was conducted to gather scientific evidence for tTXA efficacy on reducing postoperative drainage, blood loss, and the length of hospital stay in spine surgeries.
Objectives
To examine whether topical use of TXA (tTXA) reduces postoperative drainage output and duration, hidden blood loss, hemoglobin level drop, hospital stay, and adverse event rate, we reviewed both randomized and non-randomized controlled trials that assessed the aforementioned efficacies of tTXA compared with placebo in patients undergoing cervical, thoracic, or lumbar spinal surgeries.
Methods
An exhaustive literature search was conducted in MEDLINE and EMBASE databases from January 2000 through March 2020. Measurable outcomes were pooled using Review Manager (RevMan) version 5.0 in a meta-analysis.
Results
Significantly reduced postoperative drainage output (weighted mean difference [WMD]= − 160.62 ml, 95% confidence interval (95% CI) [− 203.41, − 117.83]; p < .00001) and duration (WMD= − 0.75 days, 95% CI [− 1.09, − 0.40]; p < .0001), perioperative hidden blood loss (WMD= − 91.18ml, 95% CI [− 121.42, − 60.94]; p < .00001), and length of hospital stay (WMD= − 1.32 days, 95% CI [− 1.90, − 0.74]; p < .00001) were observed in tTXA group. Pooled effect for Hb level drop with tTXA vs placebo crossed the equivalent line by a mere 0.05 g/dL, with the predominant distribution of 95% confidence interval (CI) favoring tTXA use.
Conclusions
With the most comprehensive literature inclusion up to the present, this meta-analysis suggests that tTXA use in spinal surgeries significantly reduces postoperative drainage, hidden blood loss, and hospital stay duration. The pooled effect also suggests that tTXA appears more effective than placebo in preserving postoperative Hb level, which needs further validation by future studies.
Similar content being viewed by others
Background
Spinal surgeries are commonly associated with massive perioperative blood losses, both visible in the surgical field and hidden into dead space, which leads to excessive fibrinolysis within the wound as a result of acute consumptive coagulopathy [1]. As a well-accepted antifibrinolytic agent, tranexamic acid (TXA) has been conventionally administered through the intravenous route and has achieved satisfactory outcomes in minimizing total blood loss [2,3,4]. However, accumulating evidence has questioned the safety of intravenous use of TXA (ivTXA), as the treatment has been reported to cause postoperative seizures and systemic thrombogenicity [5, 6]. A 3% incidence rate of thromboembolic events has been reported after high-dose ivTXA during spinal fusion surgery [7], while ivTXA dose ≥ 100 mg/kg has been identified as a risk factor for developing postoperative TXA-related seizures and strokes [8].
As a potent alternative to ivTXA, topical TXA (tTXA) inhibits the fibrinolytic process in situ while minimizing systemic exposure to ivTXA. tTXA treatment has been successfully applied to attenuate visible and hidden blood losses in hip and knee arthroplasty [9,10,11,12]. Sporadic randomized and non-randomized controlled trials regarding tTXA use have been recently reported in spinal surgeries. However, high-quality evidence from strictly performed meta-analyses on tTXA efficacy and safety is still lacking due to the limited scope of literature inclusion and underpowered analysis [13, 14].
Therefore, we conducted this meta-analysis with all available research to gather scientific evidence for the differences between tTXA versus placebo in postoperative drainage, hidden blood loss, hemoglobin level drop, hospital stay, and adverse event rate. To the best of our knowledge, this study was conducted with the most comprehensive study inclusion up to the present, and we have followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to help improve the reporting quality of our study.
Materials and methods
Literature search
To identify the published articles on spine surgery and tTXA delivery, an exhaustive literature search of EMBASE and MEDLINE, both manual and computer-assisted, was conducted according to the predetermined search strategies (Appendix 1; Appendix 2). Registered clinical trials on the use of tTXA in spinal surgeries were searched on the same day, using the US National Institutes of Health database (www.ClinicalTrials.gov), the World Health Organization International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/), and the International Standard Randomized Controlled Trial Number ISRCTN registry (https://www.isrctn.com). The following search terms were used in all databases: (1) local/topical tranexamic acid (including all relevant synonyms/usages, i.e., topical TXA, tTXA, local TXA); AND (2) spine surgery OR spinal surgery. Prospective randomized controlled trials (RCTs), non-RCT studies including quasi-randomized trials (qi-RCTs), retrospective cohort studies, and case-control studies that compared tTXA infiltration with no tTXA administration at wound closure in spinal surgeries were distinguished from January 2000 through March 2020. There was no restriction on the reporting language of the article. Reference lists in studies, reviews, and previous meta-analyses were checked to identify any initially omitted studies. Particular attention was paid to duplicate reports; when studies were published as an abstract and an original article, only the latter was considered.
Study selection
To be selected, studies should meet the following inclusion criteria: (1) patients underwent cervical, thoracic, or lumbar spinal surgeries irrespective of the anterior or posterior approach; (2) topical administration of TXA (tTXA) was compared with no tTXA administration at wound closure; and (3) studies have evaluated the efficacy or safety of tTXA using at least one of the following endpoints: (a) output and duration of postoperative drainage, (b) hidden blood loss (HBL), (c) hemoglobin (Hb) level changes from baseline, (d) hospital stay, or (e) numbers of postoperative adverse events, including wound infections and thrombosis events. Once the studies met the eligible criteria, they would be included even published in gray literature.
Methodology quality assessment
Two reviewers independently scanned the quality of the eligible studies; a third reviewer would solve discrepancies. We assessed the study quality using the Newcastle-Ottawa Scale described by Wells et al. [15], in which a study was judged on three broad perspectives: selection of the study groups, comparability of the groups, and ascertainment of either the exposure or outcome of interest. Studies with 7 points or higher were considered high-quality research and were included in the meta-analysis.
Risk of bias assessment
Two reviewers independently assessed the risk of bias of the enrolled studies. We assessed the risk of bias for randomized trials using the criteria specified in the Cochrane Handbook for Systematic Reviews of Interventions [16]. For non-randomized studies, we used the ROBINS-I tool criteria for assessing the risk of bias [17]. A third reviewer solved discrepancies between the reviewers.
Data extraction
Predefined data from individual trials were extracted independently by two authors. The data extracted included both study characteristics and measuring outcomes. The name of the first author, country, year of publication, design type, tTXA delivery methods, surgical procedure, and quality assessment were recorded as study characteristics, whereas output and duration of postoperative drainage, postoperative Hb level change, perioperative hidden blood loss, length of hospital stay, and the number of postoperative adverse events were extracted as the measuring outcomes.
Statistical analysis
Statistical analyses were conducted using Review Manager (RevMan) version 5.3 (The Cochrane Library, Oxford, UK). The mean and standard deviations were pooled to a mean difference (MD) and 95% confidence interval (CI) for continuous outcomes. When median and interquartile range (IQR) were reported rather than mean and SD, the data were converted to the desired format according to the Cochrane Handbook for Systematic Reviews of Interventions [16]. Computation of Hb change from baseline and combination of subgroup measurements were also conducted following the Handbook's instructions [16]. Odds ratios (OR) and 95% confidence interval (CI) were calculated for dichotomous outcomes.
The quantity of heterogeneity was assessed using I2 statistics. When there was no statistical evidence of substantial heterogeneity (I2 ≤ 50%), a fixed-effect model was adopted; otherwise, a random-effect model was chosen.
Strength of evidence assessment
Two reviewers independently assessed the required 5 domains (study limitations, consistency, directness, precision, and reporting bias) for each major outcome, with discrepancies solved by a third reviewer. The overall strength of evidence (SOE) was then established by incorporating the 5 domains into an overall grade, which was denoted as high, moderate, low, or insufficient. The SOE assessment procedures were strictly conducted according to the series paper by the US Agency for Healthcare Research and Quality (AHRQ) and the Evidence-based Practice Center (EPC) program [18] and presented as per the suggested approach in the EPC update [19].
Results
Literature search and study selection
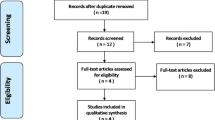
Figure 1 shows the flow chart for identifying eligible studies. The search strategy yielded a total of 270 articles and 1 registered clinical trial from the electronic databases (MEDLINE = 114, EMBASE = 156, US National Institutes of Health database = 1). Two studies were identified through checking the reference lists in previous reviews and meta-analyses. After excluding 208 studies as literature appearing in more than 1 database, 65 studies remained. After screening by titles and abstracts, 15 studies remained potentially relevant. After full-text review, 2 studies were excluded because of the lack of an appropriate control group with either a placebo or no tTXA, or outcome measures that were not specified in the meta-analysis. Finally, 9 RCTs [20,21,22,23,24,25,26,27,28] and 4 non-RCT studies [29,30,31,32] involving 882 patients were included in the final analysis, with individual sample sizes ranging from 29 to 100 patients. Among them, 11 studies were published in English, 1 study in Chinese, and 1 study in Persian. The included studies were determined as above 7 points according to the 9-star Newcastle-Ottawa Scale. The characteristics of included studies are presented in Table 1.
Postoperative drainage output was used to generate the funnel plot analysis of publication bias (Fig. 2). The asymmetric characteristic of the resultant plot indicated the presence of publication bias.
Risk of bias in included studies
The risk of bias in included studies was summarized in Figs. 3 and 4. All randomized studies were at low risk of selection bias, with the randomization and allocation process being clearly reported. All non-randomized studies were at a high risk of selection bias, as patients in Ren (2017), Zhinan (2017), Liang (2020), and Weera (2018) [29,30,31,32] were most likely selected for different study treatments based on clinical factors. The blinding process was not followed in 1 randomized study [24] due to some patients requesting more information to understand their diagnosis and treatment fully.
Postoperative drainage output
Twelve studies (n=782) were included in the meta-analysis for postoperative drainage output. Pooled results indicated that tTXA application was more effective than the placebo in reducing postoperative drainage (weighted mean difference [WMD]= − 160.62 ml, 95% confidence interval (95% CI) [− 203.41, − 117.83]; p< .00001) (Fig. 5).
Postoperative drainage duration
A total of 6 studies (n=411) provided data on drainage retention duration. Pooled results indicated that tTXA application was more effective than placebo in reducing the duration of postoperative drainage (WMD= − 0.75 days, 95% CI [− 1.09, − 0.40]; p< .0001) (Fig. 6).
Hidden blood loss (HBL)
Three studies (n=241) were included in the meta-analysis for perioperative HBL. Pooled results revealed a significant reduction in HBL with the application of tTXA compared with placebo (WMD= − 91.18 ml, 95% CI [− 121.42, − 60.94]; p< .00001) (Fig. 7).
Hemoglobin (Hb) level drop
Data were extracted from 4 studies (n=210) for the meta-analysis of postoperative Hb level drop. The pooled 95% CI resided predominantly on the left side of the equivalent line and crossed the line by a mere 0.05 g/dL, indicating that tTXA application caused less Hb drop compared with the placebo in most cases (WMD= − 0.65 g/dL, 95% CI [− 1.36, 0.05]; p=0.07) (Fig. 8).
Length of hospital stay (LOH)
Data on hospital stay were available in 10 studies with 680 patients. Pooled results indicated a statistically significant reduction in LOH with tTXA treatment compared with placebo (WMD= − 1.32 days, 95% CI [− 1.90, − 0.74]; p< .00001) (Fig. 9).
Adverse event (AE) rate
This meta-analysis did not show a significantly increased risk for postoperative adverse events with tTXA treatment. Nine articles (n=579) were included in the meta-analysis. Nine adverse events were reported in the included studies, with 1 case of myocardial infarction and 4 cases of wound infection in the tTXA group, and 4 cases of wound infection in the placebo group. The pooled result indicated event rates of 5 of 298 versus 4 of 281 for tTXA administration compared with placebo treatment, with OR 1.52 (0.40, 5.82), p = .54 (Fig. 10).
Strength of evidence (SOE)
Outcomes were categorized into major outcomes, patient-centered outcomes, and adverse events. The strength of evidence (SOE) for each outcome was illustrated in Table 2. Except for hemoglobin (Hb) level drop, all other major outcomes were determined to be of moderate-high evidence quality. SOE for Hb level drop was denoted as insufficient, since no conclusion can be drawn from the existing findings. The patient-centered outcome was denoted as having moderate SOE, with consistent and precise effect yielded from medium-risk studies. However, with sparse events reported in small-sized studies, SOE for adverse events was denoted as insufficient in this study.
Discussion
Topical application of TXA was first reported to effectively reduce blood loss in spine surgery in 2003 [20]. The researchers also reported significantly lower concentrations of plasmin/α2-antiplasmin (PAP) and D-dimer in drained blood by tTXA treatment compared with placebo, indicating that tTXA contributes to inhibiting blood loss by preventing excessive fibrinolysis. The suppression of fibrinolytic activity by tTXA has spurred an investigation into its use in spinal surgeries. With the most comprehensive inclusion of qualified studies, this study aimed to pool all the current evidence for the efficacy of tTXA in reducing postoperative drainage, attenuating HBL, shortening hospital stay, and the safety of its use in spinal surgeries by meta-analysis.
Our results revealed that tTXA application led to a significant reduction in postoperative drainage output and duration. Prolonged postoperative drainage may aggravate wound contamination and compromise postoperative rehabilitation, which poses a great challenge for patient recovery after surgery. To alleviate such concerns, a team of spine surgeons have conducted consecutive clinical trials, including a retrospective study in 2018 [32] and a prospective study in 2019 [26]. Both studies reached consistent conclusions that tTXA infiltration on decorticated laminae surface could significantly reduce postoperative drainage. With enriched evidence from the largest number of included studies so far, our analysis further validated that TXA use effectively reduced both the output and duration of postoperative drainage in spinal surgeries.
While the visible postoperative drainage has gained extensive research interests, the invisible hidden blood loss has long been overlooked, largely due to its residual in dead spaces and extravasation into tissues. The concept of HBL was first put forth in 2000 [33]. According to previous studies, both ours and others’, quantities of HBL in spine surgeries are substantial. Smorgick et al. reported that HBL accounted for 39–42% of TBL in primary/revision posterior spinal fusion surgeries [34], and a retrospective study conducted at our center reported the percentage as high as 47% [35]. HBL has also been reported as significantly associated with increased postoperative complications and length of hospital stay (LOH) [36]. Therefore, we preliminarily investigated the efficacy of tTXA application on HBL management in this meta-analysis. According to our pooled results, the application of tTXA at wound closure significantly reduced HBL in spinal surgeries. The underlying mechanism may be that tTXA directly blocked the lysine binding sites of plasminogen and retarded fibrinolysis, thereby stabilizing the blood clot and reducing HBL within the wound. However, studies regarding HBL management by tTXA application have been scarce. This meta-analysis has included all the relevant publications and provided essential evidence for the positive effect of tTXA on HBL control. Larger scale randomized controlled trials are still required to further investigate the effect of tTXA application as a potent measure of reducing perioperative HBL.
It is reasonable to predict that decreased visible drainage and invisible HBL amounts should collaboratively lead to lesser Hb level drop. In our pooled analysis, we observed an obvious tendency of tTXA as more efficient than placebo in preserving Hb level, despite the fact that the 95% CI of Hb change crossed the equivalent line by a mere 0.05 g/dL. This could be due to the fact that data sources of Hb level changes were different from those of postoperative drainage and HBL. Considering the predominant distribution of the interval favoring tTXA use, we deduced that the statistical insignificance should not impede the clinical significance of tTXA application in conserving blood. With existing data from only four included studies [22, 24, 25, 31], further well-designed clinical trials are expected to provide more validated evidence over this issue.
Our pooled results also indicated that tTXA use significantly decreased the length of hospital stay (LOH). Reasons for the early discharge from the hospital are multiple, including shortened drainage maintenance, less postoperative bleeding, lower incidence of anemia, and the resulting better conditions that lead to earlier functional exercises. Such improvements suggest that tTXA treatment not only optimizes the enhanced recovery after surgery (ERAS) scheme in spinal surgeries, but also benefits reducing hospital costs [37, 38]. Yet, it should be noted that since the included trials were not designed with LOH as the primary outcome, more powerful evidence from well-designed trials is still needed in the future to confirm the efficacy of tTXA in reducing hospital stay.
It should also be noted that our pooled analysis revealed no statistically significant differences for adverse event rates between the two groups. tTXA use provided a maximal concentration of TXA at the bleeding site with minimal systemic exposure of TXA, and therefore attenuated the potential risks for thromboembolic complication and neurotoxicity [39, 40]. Additionally, strict sterilization was followed for tTXA delivery in each trial, which has constrained the risks of wound complications. However, it could also be due to the fact that the studies included in our analysis were designed to assess efficacy rather than adverse events. Therefore, great care should still be taken regarding the safety profile of tTXA, and more studies specifically screening for tTXA-related adverse events are still expected.
Our study has several substantial strengths compared with the existing meta-analyses regarding tTXA use in spinal surgeries [13, 14]. Firstly, this meta-analysis was conducted with the most comprehensive study inclusion up to the present. Secondly, postoperative drainage duration and hidden blood loss were pooled as measuring outcomes for the first time. Thirdly, this study has shed light on the combined use of intravenous TXA administration (ivTXA) and tTXA application in spinal surgeries. Our previous study has confirmed the efficacy of ivTXA in reducing perioperative blood losses, leaving its safety undetermined [41]. Combination use of both TXA regimens may reduce ivTXA dosages while benefiting local hemostasis, thereby attenuating potential risks of thromboembolic events. To address this issue, tTXA and ivTXA applications are compared in our ongoing prospective TARGETS trials, with further evidence for combining tTXA to ivTXA use in spinal surgeries to be expected [42].
However, this study has several limitations that need to be addressed. Different topical routes were adopted in the included studies, including wound irrigation at the conclusion of operation, applying TXA-soaked sponges, or injection via drainage plus drain-clamping. Varied delivery methods may introduce heterogeneity into pooled analyses. Furthermore, the enrolled ages, diagnoses, surgical procedures, fusion levels, and TXA dosages varied from one study to another, which may also introduce considerable bias in the analyses.
Conclusions
With the most comprehensive literature inclusion up to the present, this meta-analysis suggests that tTXA use in spinal surgeries significantly reduces postoperative drainage, hidden blood loss, and hospital stay duration. The pooled effect also suggests that tTXA appears more effective than placebo in preserving postoperative Hb level, which needs further validation by future studies. However, considerable biases may be introduced due to the diagnoses, surgical procedures, fusion levels, tTXA delivery routes, and dosages varying from one study to another. Therefore, larger prospective trials with specifically designed outcomes are still required to define the optimal delivery method and to confirm the safety of tTXA use in spinal surgeries.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ivTXA:
-
Intravenous tranexamic acid administration
- tTXA:
-
Topical use of TXA
- RevMan:
-
Review Manager
- CI:
-
Confidence interval
- RCTs:
-
Randomized controlled trials
- qi-RCTs:
-
Quasi-randomized trials
- HBL:
-
Hidden blood losses
- MD:
-
Mean difference
- IQR:
-
Interquartile range
- LOH:
-
Length of hospital stay
- PAP:
-
Plasmin/α2-antiplasmin
- ERAS:
-
Enhanced recovery after surgery
References
Ipema HJ, Tanzi MG. Use of topical tranexamic acid or aminocaproic acid to prevent bleeding after major surgical procedures. Ann Pharmacother. 2012;46(1):97–107. https://doi.org/10.1345/aph.1Q383.
Xie J, Lenke LG, Li T, et al. Preliminary investigation of high-dose tranexamic acid for controlling intraoperative blood loss in patients undergoing spine correction surgery. Spine J. 2015;15(4):647–54. https://doi.org/10.1016/j.spinee.2014.11.023.
Myles PS, Smith JA, Forbes A, et al. Tranexamic acid in patients undergoing coronary-artery surgery. New Engl J Med. 2017;376(2):136–48. https://doi.org/10.1056/NEJMoa1606424.
Mebel D, Akagami R, Flexman AM. Use of tranexamic acid is associated with reduced blood product transfusion in complex skull base neurosurgical procedures. Anesthesia Analgesia. 2016;122(2):503–8. https://doi.org/10.1213/ANE.0000000000001065.
Zhou Z-F, Zhang F-J, Huo Y-F, et al. Intraoperative tranexamic acid is associated with postoperative stroke in patients undergoing cardiac surgery. PLoS ONE. 2017;12(5):e0177011. https://doi.org/10.1371/journal.pone.0177011.
Fuah KW, Lim CTS, Pang DCL, Wong JS. Seizure induced by tranexamic acid in a patient with chronic kidney disease on maintenance dialysis. Saudi J Kidney Dis Transpl. 2018;29(1):207–9. https://doi.org/10.4103/1319-2442.225177.
Lin JD, Lenke LG, Shillingford JN, et al. Safety of a high-dose tranexamic acid protocol in complex adult spinal deformity: analysis of 100 consecutive cases. Spine Deform. 2018;6(2):189–94. https://doi.org/10.1016/j.jspd.2017.08.007.
Kalavrouziotis D, Voisine P, Mohammadi S, Dionne S, Dagenais F. High-dose tranexamic acid is an independent predictor of early seizure after cardiopulmonary bypass. Ann Thoracic Surg. 2012;93(1):148–54. https://doi.org/10.1016/j.athoracsur.2011.07.085.
Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29(8):1528–31. https://doi.org/10.1016/j.arth.2014.03.011.
Chang C-H, Chang Y, Chen DW, Ueng SWN, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472(5):1552–7. https://doi.org/10.1007/s11999-013-3446-0.
Gilbody J, Dhotar HS, Perruccio AV, Davey JR. Topical tranexamic acid reduces transfusion rates in total hip and knee arthroplasty. J Arthroplasty. 2014;29(4):681–4. https://doi.org/10.1016/j.arth.2013.09.005.
Gao F, Sun W, Guo W, Li Z, Wang W, Cheng L. Topical application of tranexamic acid plus diluted epinephrine reduces postoperative hidden blood loss in total hip arthroplasty. J Arthroplasty. 2015;30(12):2196–200. https://doi.org/10.1016/j.arth.2015.06.005.
Yerneni K, Burke JF, Tuchman A, et al. Topical tranexamic acid in spinal surgery: a systematic review and meta-analysis. J Clin Neurosci. 2019;61:114–9. https://doi.org/10.1016/j.jocn.2018.10.121.
Fatima N, Barra ME, Roberts RJ, et al. Advances in surgical hemostasis: a comprehensive review and meta-analysis on topical tranexamic acid in spinal deformity surgery. Neurosurg Rev. 2020;21(6):1–13. https://doi.org/10.1007/s10143-020-01236-z.
Wells GA, Tugwell P, O'Connell D, Welch V, Peterson J. Cited by – ScienceOpen; 2015.
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collab. 2011. Available at: www.cochrane-handbook.org. Accessed 23 Feb 2017.
Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919.
Owens DK, Lohr KN, Atkins D, et al. AHRQ Series Paper 5: Grading the strength of a body of evidence when comparing medical interventions—Agency for Healthcare Research and Quality and the Effective Health-Care Program. J Clin Epidemiol. 2010;63(5):513–23. https://doi.org/10.1016/j.jclinepi.2009.03.009.
Berkman ND, Lohr KN, Ansari MT, et al. Grading the strength of a body of evidence when assessing health care interventions: an EPC update. J Clin Epidemiol. 2015;68(11):1312–24. https://doi.org/10.1016/j.jclinepi.2014.11.023.
Krohn CD, Sørensen R, Lange JE, Riise R, Bjørnsen S, Brosstad F. Tranexamic acid given into the wound reduces postoperative blood loss by half in major orthopaedic surgery. Eur J Surg Suppl. 2003;588:57–61.
Saberi H, Miri SM, Namdar MP. The effects of topically applied tranexamic acid on reduction of postlaminectomy hemorrhage. Tehran Univ Med J. 2010;68(9):527-33.
Liang J, Liu H, Huang X, et al. Using tranexamic acid soaked absorbable gelatin sponge following complex posterior lumbar spine surgery: a randomized control trial. Clin Neurol Neurosurg. 2016;147:110–4. https://doi.org/10.1016/j.clineuro.2016.06.001.
Xu D, Zhuang Q, Li Z, Ren Z, Chen X, Li S. A randomized controlled trial on the effects of collagen sponge and topical tranexamic acid in posterior spinal fusion surgeries. J Orthop Surg Res. 2017;12(1):166. https://doi.org/10.1186/s13018-017-0672-2.
Mu X, Wei J, Wang C, et al. Intravenous administration of tranexamic acid significantly reduces visible and hidden blood loss compared with its topical administration for double-segment posterior lumbar interbody fusion: a single-center, placebo-controlled, randomized trial. World Neurosurg. 2019;122:e821–7. https://doi.org/10.1016/j.wneu.2018.10.154.
Effectiveness study of the drug tranexamic acid to reduce post-surgery blood loss in spinal surgery.FullTextViewClinicalTrials.govn.d. https://clinicaltrials.gov/ct2/show/NCT02063035 (Accessed April 24, 2018).
Sudprasert W, Tanaviriyachai T, Choovongkomol K, Jongkittanakul S, Piyapromdee U. A randomized controlled trial of topical application of tranexamic acid in patients with thoracolumbar spine trauma undergoing long-segment instrumented posterior spinal fusion. Asian Spine J. 2019;13(1):146–54. https://doi.org/10.31616/asj.2018.0125.
Xu D, Chen X, Li Z, Ren Z, Zhuang Q, Li S. Tranexamic acid reduce hidden blood loss in posterior lumbar interbody fusion (PLIF) surgery. Medicine (Baltimore). 2020;99(11):e19552. https://doi.org/10.1097/MD.0000000000019552.
ZHENG H, PENG J, REN Y-Q, ZHANG P, YAN H. Effect of local application of tranexamic acid on coagulation and fibrinolysis in patients undergoing spinal surgery. Med J Chinese People's Liberation Army. 2019;44(5):405–11. https://doi.org/10.11855/j.issn.0577-7402.2019.05.08.
Ren Z, Li S, Sheng L, et al. Topical use of tranexamic acid can effectively decrease hidden blood loss during posterior lumbar spinal fusion surgery: a retrospective study. Medicine (Baltimore). 2017;96(42):e8233. https://doi.org/10.1097/MD.0000000000008233.
Ren Z, Li S, Sheng L, et al. Efficacy and safety of topical use of tranexamic acid in reducing blood loss during primary lumbar spinal surgery: a retrospective case control study. Spine. 2017;42(23):1779–84. https://doi.org/10.1097/BRS.0000000000002231.
Liang JQ, Rong TH, Liu HZ, et al. Topical injection of tranexamic acid via a drain plus drain-clamping to reduce blood loss in degenerative lumbar scoliosis surgery. Orthopaedic Surg. 2020;12(1):67–73. https://doi.org/10.1111/os.12583.
Sudprasert W, Tanaviriyachai T, Choovongkomol K, Jongkittanakul S, Piyapromdee U. Topical tranexamic acid reduces postoperative blood loss in posterior spinal fusion with instrumentation: a retrospective clinical study of patients with thoracolumbar spinal injury. J Med Assoc Thailand. 2018;101(3):15.
Sehat K, Evans R, Newman J. How much blood is really lost in total knee arthroplasty?. Correct blood loss management should take hidden loss into account. Knee. 2000;7(3):151–5. https://doi.org/10.1016/s0968-0160(00)00047-8.
Smorgick Y, Baker KC, Bachison CC, Herkowitz HN, Montgomery DM, Fischgrund JS. Hidden blood loss during posterior spine fusion surgery. Spine J. 2013;13(8):877–81. https://doi.org/10.1016/j.spinee.2013.02.008.
Ren Z, Li S, Hui S, Zhuang Q, Chen X, Xu D. Letter concerning “Hidden blood loss during posterior spine fusion surgery” by Yossi et al. Spine J. 2015;15(9):2113–4. https://doi.org/10.1016/j.spinee.2015.05.016.
BFN, HK. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br. 2006;88-B(8):1053–9. https://doi.org/10.1302/0301-620X.88B8.17534.
Staartjes VE, de Wispelaere MP, Schröder ML. Improving recovery after elective degenerative spine surgery: 5-year experience with an enhanced recovery after surgery (ERAS) protocol. Neurosurg Focus. 2019;46(4):E7. https://doi.org/10.3171/2019.1.FOCUS18646.
Dietz N, Sharma M, Adams S, et al. Enhanced Recovery After Surgery (ERAS) for spine surgery: a systematic review. World Neurosurg. 2019;130:415–26. https://doi.org/10.1016/j.wneu.2019.06.181.
Lecker I, Wang D-S, Whissell PD, Avramescu S, Mazer CD, Orser BA. Tranexamic acid-associated seizures: causes and treatment. Ann Neurol. 2016;79(1):18–26. https://doi.org/10.1002/ana.24558.
Odabaş AR, Cetinkaya R, Selçuk Y, Kaya H, Coşkun U. Tranexamic-acid-induced acute renal cortical necrosis in a patient with haemophilia A. Nephrol Dial Transplant. 2001;16(1):189–90. https://doi.org/10.1093/ndt/16.1.189.
Hui S, Xu D, Ren Z, et al. Can tranexamic acid conserve blood and save operative time in spinal surgeries? A meta-analysis. Spine J. 2018;18(8):1325–37. https://doi.org/10.1016/j.spinee.2017.11.017.
Hui S, Tao L, Mahmood F, et al. Tranexamic Acid in Reducing Gross Hemorrhage and Transfusions of Spine Surgeries (TARGETS): study protocol for a prospective, randomized, double-blind, non-inferiority trial. Trials. 2019;20(1):125. https://doi.org/10.1186/s13063-019-3231-9.
Acknowledgements
We want to thank Dr. Kayla Brown, for her precious time and valuable instructions in revising this manuscript.
Funding
None.
Author information
Authors and Affiliations
Consortia
Contributions
SH and QZ made contributions to the conception; QZ and JZ made contributions to the design of the work; SH, YP, and LT made contributions to the acquisition and analysis; YY and SW made contributions to the interpretation of data; SH and YP have drafted the work; and QZ and YD revised it. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any authors.
Consent for publication
This article does not contain any studies with human participants performed by any authors.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1. MEDLINE Search Strategy
-
1.
exp Antifibrinolytic Agents/
-
2.
(anti-fibrinolytic* or antifibrinolytic* or antifibrinolysin* or anti-fibrinolysin* or antiplasmin* or anti-plasmin* or ((plasmin or fibrinolysis) adj3 inhibitor*)).ab,ti.
-
3.
exp Tranexamic Acid/
-
4.
(tranexamic or Cyclohexanecarboxylic Acid* or Methylamine* or trans-4-aminomethyl-cyclohexanecarboxylic acid* or t-amcha or amca or kabi 2161 or transamin* or exacyl or amchafibrin or spotof or cyklokapron or ugurol oramino methylcyclohexane carboxylate or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anvito or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or tranex or TXA).ab,ti.
-
5.
1 or 2 or 3 or 4
-
6.
exp Administration, Topical/
-
7.
(topical* or (local* adj3 appl*) or irrigat* or spray*).ab,ti.
-
8.
6 or 7
-
9.
5 and 8
-
10.
exp Spine/
-
11.
exp Spinal Fusion/
-
12.
10 or 11
-
13.
9 and 12
-
14.
randomized controlled trial.pt.
-
15.
controlled clinical trial.pt.
-
16.
randomized.ab.
-
17.
placebo.ab.
-
18.
clinical trials as topic.sh.
-
19.
randomly.ab.
-
20.
trial.ti.
-
21.
exp Cohort Studies/
-
22.
(cohort* or prospective* or retrospective*).mp.
-
23.
14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
-
24.
exp animals/ not humans.sh.
-
25.
23 not 24
-
26.
13 and 25
Appendix 2. EMBASE Search Strategy
1. exp Antifibrinolytic Agents/
2. (anti-fibrinolytic* or antifibrinolytic* or antifibrinolysin* or anti-fibrinolysin* or antiplasmin* or anti-plasmin* or ((plasmin or fibrinolysis) adj3 inhibitor*)).ab,ti.
3. exp Tranexamic Acid/
4. (tranexamic or Cyclohexanecarboxylic Acid* or Methylamine* or amcha or trans-4-aminomethyl-cyclohexanecarboxylic acid* or t-amcha or amca or kabi 2161 or transamin* or exacyl or amchafibrin or anvito or spotof or cyklokapron or ugurol oramino methylcyclohexane carboxylate or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anvito or cl?65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or exacyl or frenolyse or hexacapron or hexakapron or tranex or TXA).ab,ti.
5. 1 or 2 or 3 or 4
6. exp Administration, Topical/
7. (topical* or (local* adj3 appl*) or irrigat* or spray*).ti,ab.
8. 6 or 7
9. 5 and 8
10. exp Spine/
11. exp Spinal Fusion/
12. 10 or 11
13. 9 and 12
16. randomized controlled trial/
17. single-blind procedure/
18. random*.mp.
19. placebo*.mp.
20. (double* adj blind*).mp.
21. (singl* adj blind*).mp.
22. exp Cohort Studies/
23. (cohort* or prospective* or retrospective*).mp.
24. 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25. exp animals/ not humans.sh.
26. 24 not 25
27. 13 and 26
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hui, S., Peng, Y., Tao, L. et al. Tranexamic acid given into wound reduces postoperative drainage, blood loss, and hospital stay in spinal surgeries: a meta-analysis. J Orthop Surg Res 16, 401 (2021). https://doi.org/10.1186/s13018-021-02548-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-021-02548-6