Abstract
Background
Radiotherapy (RT) in head and neck squamous cell cancer (HNSCC) often leads to sticky saliva and xerostomia (SSX). Dose sparing of salivary glands (SG) reduces occurrence of SSX but few studies investigated the relationship between RT dose to SG substructures and SSX. We therefore investigated this hypothesis, focusing on the parotid duct (PD).
Methods
Retrospective data was collected from 99 HNSCC patients treated at our center with (chemo-)radiotherapy (CRT). PD and other organs-at-risk (OAR) were (re-)contoured and DVHs were generated without re-planning. SSX was graded according to CTCAE v.4.03 and evaluated at acute, subacute, and two late timepoints.
Results
Most patients presented with loco-regionally advanced disease. In 47% of patients, up-front neck dissection preceded CRT. Weighted mean dose was 28.6 Gy for bilateral parotid glands (PG), and 32.0 Gy for PD. Acute SSX presented as grades 0 (35.3%), I (41.4%), II (21.2%) and III (2.0%). There was no association of OARs and SSX ≥ grade 2 in univariable logistic regression (LR). Multivariable LR showed statistically significant relationship of acute SSX with: PG weighted mean dose (OR 0.84, p = 0.004), contralateral PG mean dose (OR 1.14, p = 0.02) and contralateral PD planning OAR (PD PRV) mean dose (OR 1.84, p = 0.03).
Conclusions
There was an association of acute SSX with dose exposure of PD PRV in multivariable regression, only. Due to statistical uncertainties and the retrospective nature of this analysis, further studies are required to confirm or reject the hypothesis.
Highlights
A common strategy to prevent sticky saliva and xerostomia (SSX) in patients undergoing radiotherapy of the head and neck area is dose sparing of salivary gland parenchyma during treatment planning.
It is largely unknown whether dose exposure of other salivary gland structures such as salivary ducts might play a role in the development of SSX.
We performed a retrospective analysis of head and neck squamous cell carcinoma patients that were treated with curative-intent (chemo-)radiotherapy and investigated the association of parotid duct dose exposure and occurrence of SSX.
Some analyzed parameters suggest a connection between parotid duct dose exposure and SSX but further studies will be necessary to confirm or reject these results.
Similar content being viewed by others
Background
Radiotherapy is a mainstay in the treatment of head and neck squamous cell cancer (HNSCC) but is often accompanied by acute and chronic side effects [1]. One of the most prominent treatment sequelae is xerostomia with a significant impact on self-reported quality of life [2]. It has long been established that a relationship exists between the severity of xerostomia and the exposed volume of the parotid gland (PG) to various dose parameters, most prominently the mean dose to one or both glands [3, 4]. Consequently, sparing the PG of irradiation has been associated in several retrospective reports and prospective trials with a reduced occurrence and severity of xerostomia [5,6,7]. In an attempt to advance this model beyond simple volume parameters, several groups have since tried to establish more refined approaches considering anatomical subcompartments or imaging features [8,9,10]. A recent example along these lines, integrating advances in the understanding of gland regeneration, is a prospective randomized trial employing stem-cell sparing radiotherapy by reducing the dose to the anatomically stem cell-rich regions (SCRR) of the PG. Apart from mean dose to the ipsilateral PG, results suggested a statistically significant relationship between dose to contralateral SCRR, however, the primary endpoint, parotid saliva production, was unaltered between the two arms [11]. Another important structure in the vicinity of the PG is the parotid duct (PD), which is responsible for channeling the saliva from the gland to the oral cavity. Given this function as an anatomical bottleneck, it is conceivable that changes in the structure or function of the PD after radiotherapy, such as fibrosis and strictures, may contribute to blockage of the salivary flow and/or slow atrophy of the gland and subsequently to dry mouth (analogous to other tubular organs such as the ureter) [12]. A recent prospective study reported reduced xerostomia in 38 enrolled patients that underwent MRI-guided, PD-sparing radiotherapy, albeit in comparison with a historic patient cohort [13]. Without being aware of the latter work, the aim of our study was to investigate whether PD dose exposure may lead to a worsening in xerostomia after radiotherapy in a large, retrospective cohort with a long follow-up period.
Methods
Patients with the following criteria were included: AJCC/UICC stage (7th edition) HNSCC, intensity-modulated radiotherapy with curative intent (prescribed median dose to the PTV 72 Gy, with PTV D95% ≥ 95% of the prescribed dose ), no prior surgery of the parotid or the surrounding area. All patients had a minimum of 2 years of post-treatment follow-up and no HNSCC recurrence or a second HNSCC until their last follow-up.
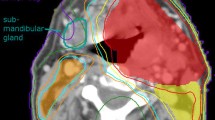
The PDs were not delineated and therefore not accounted for at the time of the generation and optimization of the radiotherapy plans. All analyzed structures were contoured by an experienced radiation oncologist on the original, contrast-enhanced planning computed tomographies of HNSCC patients (Fig. 1) treated between 2008 and 2016 in the treatment planning system Eclipse v.15.5 (Varian, Palo Alto, CA). Planning organ-at-risk volumes (PRV) were defined as the respective contour expanded with an isotropic margin of 5 mm (parts extending the body contour cropped). In case of paired organs (e.g. parotid gland, PD) weighted mean doses were calculated as the average of the mean structure doses, weighted by the respective structure volume. Cumulative dose-volume histograms (DVH) were used to determine dose to 0.03 cm (D0.03cc). For the purposes of this work, “ipsilateral” (IL) was defined as being situated on the side of the left-right axis with higher dose exposure with respect to a structure or organ-at-risk (OAR), and “contralateral“ (CL) being on the opposite side. This usually correlated with the lateralization of the center of gravity of the primary tumor and the high-dose target volume. Because the submandibular and sublingual glands were not actively protected in the radiotherapy plans during this time period and some had been removed with up-front neck dissection, no dosimetric evaluation of these structures was carried out.
Dose parameters were exported from the treatment planning system and matched with collected clinical data obtained from retrospective chart review. Sticky saliva / xerostomia (SSX) was graded according to the CTCAE v.4.03 (visual assessment of the physician and patient-reported without objective measurement of the salivary flow). Occurrence of toxicity over time was defined as follows: acute (during treatment), subacute (90 days post-treatment), late I (beginning from the 4th to 6th month post-treatment), late II (at the time of last follow-up, at least 2 years post-treatment). Statistical analysis was performed in JMP v.15.2 (SAS Institute, Cary, NC). Statistical tests used for univariate analysis were: Chi-squared test, univariable and multivariable logistic regression. Statistical significance was defined as a p-value ≤ 0.05 on two-tailed tests. Odds ratios (OR) apply to change in regressor per unit of the independent variable, unless otherwise indicated.
Results
Patient and treatment characteristics
After screening, 99 patients were included with a median follow-up of 62 months (Table 1). A history of smoking was self-reported by 79% of patients, whereas any alcohol consumption in the past or present was affirmed in 67%. Median age was 61 years and approximately two thirds of patients were male. Staging revealed T2 or T3 tumors in the majority of cases with metastatic involvement of regional lymph nodes. 47% of patients received an up-front neck dissection, followed by radiotherapy with a total median dose of 72, 66 and 54 Gy prescribed to the primary tumor, potential regions of pathological extracapsular extension, and elective treatment volume, respectively. Patients without neck dissection received 72 Gy to the primary tumor and involved lymph nodes, and 54 Gy to the elective volume, in accordance with international consensus guidelines. Fraction dose was 2 Gy, applied daily, five times per week. More than 90% of patients were treated with volumetric modulated arc therapy and a similar percentage received concomitant systemic therapy.
Dose parameters
When assessing dose exposure to OARs, weighted mean dose for bilateral PG was 28.6 Gy, and 32.0 Gy for PD. Dose to the left and right PG was slightly lower with 27.6 Gy and 28.0 Gy, respectively (Table 2). Similarly, there was no marked side difference in dose to 0.3 cc (D0.03cc) and mean dose to the PD and the PRV around the PD. In contrast, PD and PG on the ipsilateral side showed higher mean doses than the overall bilateral weighted mean dose and D0.03cc, indicating adequate sparing of the contralateral PG and PD. Although the latter might have been caused mainly due to the geometrical distance from the target volume as well as the optimization algorithm which prioritizes the further protection of one PG (which is usually the contralateral PG) in case the dose constraint for both PG combined cannot be met. This difference was also more prominent for the D0.03cc of the PD (IL: 31.6 Gy, CL: 43.3 Gy) and the surrounding PRV (IL: 29.4 Gy, CL: 55.9 Gy).
Toxicity
Acute SSX presented as grades 0 (35.3%), 1 (41.4%), 2 (21.2%) and 3 (2.0%). Symptoms showed improvement over time with a decrease in grade 2 and 3 SSX at subacute and late time points (Table 3). When correlating the assessed dose parameters for each organ with acute SSX grade ≥ 2 in univariate logistic regression models, there was no statistically significant association with evaluated OARs. Similarly, no such association could be demonstrated for the subacute and late timepoints. Multivariable logistic regression demonstrated an association of bilateral PG weighted mean dose with SSX at the acute time point (OR 0.84 [95%CI 0.73–0.95], p = 0.004) but with an OR < 1, signaling that higher doses were inversely correlated with SSX symptoms. Additionally, contralateral PG mean dose (OR 1.14 [95%CI 1.02–1.30], p = 0.02) and contralateral PD PRV mean dose (OR 1.84 [95%CI 1.07–3.58], p = 0.03) were also statistically significant parameters in this model (Table 4). No statistically significant results were found for the other time points (Tables S1, S2). However, when performing backwards elimination on the multivariable model, the following parameters reached statistical significance: contralateral PD PRV, mean dose (p < 0.01); bilateral PD PRV weighted mean dose (p = 0.02); contralateral PD, D0.03 cc (p = 0.02); bilateral PD PRV weighted mean dose (p = 0.02) (Table S2). Finally, we assessed whether patients having received neck dissection (with removal of the submandibular gland) differed in SSX from those treated with radiotherapy, but multivariable models with (Table S3) and without backwards exclusion (Table S4) did not yield conclusive results.
Discussion
Acute and chronic xerostomia are among the most relevant and frequent toxicities accompanying radiotherapy of the head and neck region, have a potentially long-lasting impact on quality of life and are in large part caused by PG dysfunction. Most of the literature to date has focused on dose exposure to the PG itself to predict and prevent SSX and evidence from several clinical trials supports this approach. It is, however, conceivable that other dose parameters or anatomical structures may also be relevant in the development of xerostomia, such as oral cavity or submandibular, sublingual, tubarial and minor salivary glands. In this retrospective study we investigated the hypothesis that irradiation of the PD may contribute to the emergence of acute and/or chronic xerostomia in association with radiotherapy.
A recent publication by Steenbakkers et al. reported the results of stem cell-sparing radiotherapy [11]. They delineated a part of the PG rich in stem cells which is anatomically in close proximity to the PD. After treatment planning with active sparing of this region, mean doses of 11.4 Gy were reported in contrast to 17.1 Gy in the group of patients without sparing. Xerostomia was assessed at 6, 12 and 24 months. The primary endpoint (> 75% reduction in parotid gland saliva production compared with pretreatment production) and secondary endpoints (several aspects of xerostomia 12 months after treatment) were not met, but the authors describe that the SCRR was more predictive of the development of parotid gland function − related xerostomia endpoints than dose to the entire parotid gland in multivariable analysis.
Another study approached the putative association of PD dose and xerostomia from a different angle [13]: The authors prospectively enrolled 38 patients, delineated the PDs using MRI sialography and actively spared the PD on both sides during treatment planning. However, the quality of sialography and planning CT image fusion was not reported, or whether only the PD or also its afferent branches in the PG were contoured. Xerostomia was assessed 6 and 12 months after radiotherapy and compared to a historic cohort of 89 patients. Patients received total doses of 70 Gy (n = 10) or 60 Gy (in a de-intensified arm, n = 28). The study reported mean PD doses of 11.6 Gy and 11.3 Gy in these two arms, respectively (data for the historical cohort not provided). A statistically significant relationship was reported between mean PD dose and xerostomia at 12 months (OR = 1.62 [1.06–2.49], p = 0.03) in nested logistic regression models. In comparison, the retrospective nature of our study without any re-planning to spare the PD allowed us to investigate the dose-response relationship in plans reflecting the current widely-used standard of care.
In our data, we could demonstrate an association of SSX with dose parameters to OARs in multivariable models at the acute timepoint. Surprisingly, and in contrast to the published literature, multivariable logistic regression demonstrated a statistically-significant decrease in SSX with increasing dose to the bilateral PG. When evaluating only the contralateral gland, however, the effect was reversed and in line with what is commonly assumed from a radiobiological and clinical perspective. Hence, we interpret this result as a false-positive, which could possibly be caused by the planning procedure: our department routinely mandates a dose constraint of mean dose < 26 Gy for both PGs combined. However, if this constraint cannot be reached, a stricter constraint (mean dose < 20 Gy) is applied to only one gland (which is usually the contralateral side) to compensate for the loss of the other PG. This could be an explanation for the apparently improved SSX with increasing dose to the other (i.e., ipsilateral) gland and the reversed effect for the contralateral one.
Concerning the PD, univariable analysis demonstrated no statistically significant relationships between dose parameters and any of the assessed endpoints. However, when using multivariable logistic regression, the mean dose to the contralateral PD PRV and the weighted mean dose to the bilateral PD PRV became statistically significant, but only at the acute timepoint. Backward elimination also demonstrated these two parameters as statistically significant, as well as D0.03cc to the contralateral PD (p = 0.02), however, only at the late I time point. The inconsistent statistical behavior between (uni- and multivariable) analyses and timepoints make a definitive interpretation of these results difficult and may indicate false positivity due to multiple testing, but do not categorically contradict the hypothesis of an association between SSX and dose to the PD.
Strengths of our study include a relatively large cohort of patients that were all treated in accordance with our institutional therapeutic guidelines with intensity-modulated radiotherapy. The analysis is, however, limited by its retrospective nature with physician-assessed toxicity without objective measurements of salivary function. Additionally, time intervals between toxicity evaluations were not identical between patients, especially at late II time points, which might have introduced variability. We did not include dosimetric parameters of other OARs in our analysis to reduce the already extensive number of parameters in a limited patient cohort with a significant subset having undergone uni- / bi-lateral up-front neck dissection with removal of submandibular glands. Lastly, we only delineated the parts of the parotid duct outside of the gland because additional imaging (e.g., sialography) to identify interlobular ducts was not available.
Conclusions
In summary, we could not find a meaningful correlation between dose exposure of the PD and xerostomia after radiotherapy. However, our results are not in contradiction with the parallel study by Fried et al. [13]. Further evaluation in properly designed prospective trials would be required for confirmation and better characterization of the observed effect.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- CL:
-
Contralateral
- CRT:
-
(chemo–)radiotherapy
- CT:
-
Computed tomography
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- HNSCC:
-
Head and neck squamous cell carcinoma
- IL:
-
Ipsilateral
- IMRT:
-
Intensity–modulated radiotherapy
- LR:
-
Logistic regression
- MRI:
-
Magnetic resonance imaging
- OAR:
-
Organs at risk
- OR:
-
Odds ratio
- PD:
-
Parotid duct
- PG:
-
Parotid gland
- PRV:
-
Planning organ at risk volume
- PTV:
-
Planning target volume
- RT:
-
Radiotherapy
- SCRR:
-
Stem cell–rich regions
- SG:
-
Salivary glands
- SSX:
-
Sticky saliva and xerostomia
- UICC:
-
Union for International Cancer Control
References
Trotti A, Pajak TF, Gwede CK, Paulus R, Cooper J, Forastiere A, et al. TAME: development of a new method for summarising adverse events of cancer treatment by the Radiation Therapy Oncology Group. Lancet Oncol. 2007;8:613–24. https://doi.org/10.1016/S1470-2045(07)70144-4.
Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–6. https://doi.org/10.1200/JCO.2007.14.6647.
Deasy JO, Moiseenko V, Marks L, Chao KSC, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76:S58–63. https://doi.org/10.1016/j.ijrobp.2009.06.090.
Beetz I, Schilstra C, van der Schaaf A, van den Heuvel ER, Doornaert P, van Luijk P, et al. NTCP models for patient-rated xerostomia and sticky saliva after treatment with intensity modulated radiotherapy for head and neck cancer: the role of dosimetric and clinical factors. Radiother Oncol. 2012;105:101–6. https://doi.org/10.1016/j.radonc.2012.03.004.
Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–36. https://doi.org/10.1016/S1470-2045(10)70290-4.
Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:695–704. https://doi.org/10.1016/s0360-3016(01)01512-7.
van Rij CM, Oughlane-Heemsbergen WD, Ackerstaff AH, Lamers EA, Balm AJM, Rasch CRN. Parotid gland sparing IMRT for head and neck cancer improves xerostomia related quality of life. Radiat Oncol. 2008;3:41. https://doi.org/10.1186/1748-717X-3-41.
Buettner F, Miah AB, Gulliford SL, Hall E, Harrington KJ, Webb S, et al. Novel approaches to improve the therapeutic index of head and neck radiotherapy: an analysis of data from the PARSPORT randomised phase III trial. Radiother Oncol. 2012;103:82–7. https://doi.org/10.1016/j.radonc.2012.02.006.
Sheikh K, Lee SH, Cheng Z, Lakshminarayanan P, Peng L, Han P, et al. Predicting acute radiation induced xerostomia in head and neck Cancer using MR and CT Radiomics of parotid and submandibular glands. Radiat Oncol. 2019;14:131. https://doi.org/10.1186/s13014-019-1339-4.
van Dijk LV, Langendijk JA, Zhai T-T, Vedelaar TA, Noordzij W, Steenbakkers RJHM, et al. Delta-radiomics features during radiotherapy improve the prediction of late xerostomia. Sci Rep. 2019;9:12483. https://doi.org/10.1038/s41598-019-48184-3.
Steenbakkers RJHM, van Rijn-Dekker MI, Stokman MA, Kierkels RGJ, van der Schaaf A, van den Hoek JGM, et al. Parotid gland stem cell sparing Radiation Therapy for patients with Head and Neck Cancer: a double-blind randomized controlled trial. Int J Radiat Oncol Biol Phys. 2022;112:306–16. https://doi.org/10.1016/j.ijrobp.2021.09.023.
Boyd GH, Miao R, Jee KW, Sethi R, Mullen JT, Haynes AB, et al. Association between Radiation Dose and Ureteral strictures in the treatment of Retroperitoneal Sarcoma. Int J Radiation Oncology*Biology*Physics. 2018;102:e205. https://doi.org/10.1016/j.ijrobp.2018.07.724.
Fried DV, Zhu T, Das SK, Shen C, Marks LB, Tan X, et al. Prospective assessment of sparing the parotid ducts via MRI sialography for reducing patient reported xerostomia. Radiother Oncol. 2022;172:42–9. https://doi.org/10.1016/j.radonc.2022.05.001.
Acknowledgements
Not applicable.
Funding
This study was conducted without external sources of funding.
Author information
Authors and Affiliations
Contributions
OE, DHS and DMA conceived and designed the project. DUA, JL and OE collected and prepared the data. OE and DHS performed the analyses. DHS wrote the manuscript with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All data collection and analysis was done with approval of the Bernese cantonal ethics committee (Project ID: 2019 − 00701) in accordance with national law and the Declaration of Helsinki. Because it was not possible to collect study-specific informed consent forms from patients who were either deceased or lost to follow-up, patients with any written or documented oral rejection of the use of personal or health-related data were not included in the study. All patients diagnosed after the implementation of the Swiss Human Research Act in 2014 were required to have a signed written informed consent to be eligible for the study.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Schanne, D.H., Alder, D.U., Lippmann, J. et al. Effect of dose to parotid ducts on Sticky Saliva and Xerostomia in radiotherapy of head and neck squamous cell carcinoma. Radiat Oncol 19, 104 (2024). https://doi.org/10.1186/s13014-024-02495-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-024-02495-6