Abstract
Background
So far, only limited studies exist that evaluate patients with brain metastases (BM) from GI cancer and associated primary cancers who were treated by Gamma Knife Radiosurgery (GKRS) and concomitant immunotherapy (IT) or targeted therapy (TT).
Methods
Survival after GKRS was compared to the general and specific Graded Prognostic Assessment (GPA) and Score Index for Radiosurgery (SIR). Further, the influence of age, sex, Karnofsky Performance Status Scale (KPS), extracranial metastases (ECM) status at BM diagnosis, number of BM, the Recursive Partitioning Analysis (RPA) classes, GKRS1 treatment mode and concomitant treatment with IT or TT on the survival after GKRS was analyzed. Moreover, complication rates after concomitant GKRS and mainly TT treatment are reported.
Results
Multivariate Cox regression analysis revealed IT or TT at or after the first Gamma Knife Radiosurgery (GKRS1) treatment as the only significant predictor for overall survival after GKRS1, even after adjusting for sex, KPS group, age group, number of BM at GKRS1, RPA class, ECM status at BM diagnosis and GKRS treatment mode. Concomitant treatment with IT or TT did not increase the rate of adverse radiation effects. There was no significant difference in local BM progression after GKRS between patients who received IT or TT and patients without IT or TT.
Conclusion
Good local tumor control rates and low rates of side effects demonstrate the safety and efficacy of GKRS in patients with BM from GI cancers. The concomitant radiosurgical and targeted oncological treatment significantly improves the survival after GKRS without increasing the rate of adverse radiation effects. To provide local tumor control, radiosurgery remains of utmost importance in modern GI BM management.
Similar content being viewed by others
Introduction
The incidence of gastrointestinal (GI) cancer has increased over the past decades with 5.1 million newly diagnosed cases and 3.6 million deaths worldwide in 2020 [1]. It is predicted that these numbers will further increase [1]. Furthermore, despite GI cancer rarely metastasizes into the brain, the incidence of brain metastases (BM) from GI cancer has increased as well [2, 3]. This might result from the recent advent of new systemic oncological therapies and the increased availability of neuroradiological diagnostics [3]. Still, the prognosis of GI patients rapidly deteriorates once BM occur [4,5,6,7].
Standard local treatment options for BM from GI tumors comprise microsurgery and stereotactic radiosurgery (SRS) [8, 9]. Recently, SRS has become one of the most essential treatment options in patients with BM due to its efficacy and safe applicability [10, 11]. Moreover, modern oncological treatment options have significantly improved the prognosis in patients with BM from other primary tumors [12,13,14,15,16]. However, in contrast to other primary tumors, it appears that less attention has been paid to patients with BM from GI cancer with only few data on the prognosis of these patient cohort under immunotherapy (IT) or targeted therapy (TT) [7, 17, 18]. Therefore, we aimed to evaluate the outcome after GKRS treatment and the effect of concurrent treatment with IT or TT in patients with BM from GI cancer.
Methods
Study population and data evaluation
We performed a retrospective analysis of 81 patients with BM from GI and associated cancers who had been treated by at least one GKRS for at least one BM at our department between 2012 and 2020 (Table 1). As previously described, we included BM patients with GI and associated cancers, since the response to radiosurgical treatment is comparable among BM from these primary tumors [3, 19,20,21,22]. At time of BM diagnosis, the Score Index for Radiosurgery (SIR), the general and specific Graded Prognostic Assessment (GPA) and RPA classes were evaluated [6, 23,24,25]. The primary study endpoint was defined as death, irrespective of the cause of death. In addition, a national death register comparison was performed for data completion. Patients who were lost to follow-up were excluded from the outcome analysis but included in the descriptive analysis.
Radiosurgical technique
The radiosurgical treatments were planned with GammaPlan® (Elekta, Stockholm, Sweden) and performed with Leksell Gamma Knife® Perfexion™ (Elekta, Stockholm, Sweden). Planning sequences were performed on a 1.5 or 3.0 Tesla magnetic resonance imaging (MRI) and always included Gadolinium contrast-enhanced T1-weighted MRI sequences. Planning was performed as we have described in detail before [12, 26]. GKRS parameters are summarized in Table 2. In 56/81 (69%) patients only one GKRS treatment was performed, with 14 patients being treated by a boost dose (reduced dose). Of the remaining 25 patients, 12/81 (15%) had multiple GKRS treatments due to new BM and 13/81 (16%) received dose-staged GKRS treatments as described before [12, 26]. At GKRS1, a median of 2 BM (range: 1–10 BM) were treated in each patient. Including all GKRS treatments per patient, 264 BM were treated in 116 GKRS procedures with a median of 2 BM (range: 1–25 BM) per patient.
Neuroradiological definitions
As standard, clinical and neuroradiological follow-up of all radiosurgically treated patients were performed every three months. We defined metastases as contrast-enhanced tumor lesions and tumor progression according to the response assessment in neuro-oncology (RANO) criteria [27]. Intralesional hemorrhage, radiation reaction and radiation necrosis were defined according to Heit et al. and Stockham et al. [28,29,30]. All follow-up MRIs of patients with potential postinterventional complications were reviewed by a senior neuroradiologist (W.M.), who was blinded to the clinical follow-up data.
Statistical analysis
Statistical analysis was performed with SPSS software version 26.0 (IBM, Armonk, NY, USA).
Categorical data were presented as counts and percentages, and continuous parameters as median and range. The chi-square, Mann-Whitney U, and Wilcoxon signed-rank tests were performed as statistically appropriate. Median survival after the first GKRS was estimated by the Kaplan-Meier method and compared using Mantel-Cox log-rank test. We performed univariate followed by multivariate Cox proportional hazard regression analyses to assess if sex, KPS group (< 80% vs. ≥80%), age group (≤ 65 vs. >65 years), number of BM at GKRS1 (single vs. multiple), RPA class, ECM status at BM diagnosis, GKRS1 treatment mode (boost, staged or regular high dose) and IT or TT status at or after GKRS1 predicted survival after GKRS1. A two-sided probability value of < 0.05 was considered as statistically significant for all performed tests.
Results
Overall survival and outcome
The median time between the diagnosis of GI cancer and BM was 23 months (range: 0-107 months). Only one patient (1/81, 1%) was truly lost to follow-up. From the remaining 80 patients (80/81, 99%) survival data were available. The vast majority of patients (76/81; 94%) had succumbed to their disease at time of last follow-up.
The median overall survival after the diagnosis of GI cancer, the diagnosis of the initial BM and the first GKRS treatment was 35.3 months (95% confidence interval (CI) = 22-48.5), 7.5 months (95% CI = 5.8–9.2) and 6.4 months (95% CI = 4.5–8.3), respectively.
After GKRS1, there were no differences in overall survival between female and male patients (p = 0.397, Fig. 1A). Patients with a KPS ≥ 80% showed a slightly longer median overall survival after GKRS1 compared to those with a KPS < 80% (p = 0.069, Fig. 1B).
We subsequently compared the median overall survival between patients ≤ 65 and > 65 years. The estimated median overall survival after GKRS1 in the ≤ 65 years group (6.4 months, 95% CI = 3.1–9.7) appeared to be similar compared to the > 65 years group (5.9 months, 95% CI = 2.8-9, p = 0.709).
The status of ECM at diagnosis of brain metastases did not affect the estimated median overall survival after GKRS1 (patients with ECM: 6.9 months, 95% CI = 5-8.7; patients without ECM: 3.4 months, 95% CI = 1.1–5.8, p = 0.820).
Patients with the largest BM ≤ 6 cm³ (6.9 months, 95% CI = 4.3–9.4) had an almost similar median overall survival compared to patients with at least one BM > 6 cm³ (5.3 months, 95% CI = 0.6–10, p = 0.711) at GKRS1. However, patients with a single BM at GKRS1 had a statistically significant longer median overall survival after GKRS1 compared to patients with multiple BM (p = 0.037, Fig. 1C).
According to the RPA classification system, 64/81 (79%) and 17/81 (21%) patients were rated as class II and III, respectively. There were no patients with RPA class I. We could not find any statistically significant differences in the median overall survival after GKRS1 between patients with RPA class II and patients with RPA class III (p = 0.087, Fig. 1D).
Notably, regarding the treatment dose at GKRS1, patients with regular high dose (7 months, 95% CI = 5.1–8.9), staged dose (11.4 months, 95% CI = 0-25.2) and boost dose (4.6 months, 95% CI = 4.2-5, p = 0.345) showed a similar median overall survival after GKRS1 compared to each other.
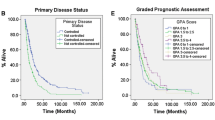
Finally, we compared the actual survival of our patients to the predicted survival by prognostic scores and could show that our patients had a significantly longer survival after GKRS1, compared to the general GPA (p < 0.0001), specific GPA (p < 0.001), RPA (p < 0.0001) and SIR (p < 0.008).
Kaplan-Meier curves showing the differences of the overall survival after GKRS1 between different treatment groups. A There is no difference in the median overall survival between female (8.8 months, 95% CI = 3.9-13.7) and male patients (5.7 months, 95% CI = 2.8-8.5; p=0.397). B Even though a trend towards a longer median overall survival in patients with a KPS ?80% (7.4 months, 95% CI = 6.3-8.4) compared to those with a KPS <80% (5.2 months, 95% CI = 2.2-8.3) could be observed, the results were statistically not significant (p=0.069). C Patients with a single BM (7.9 months, 95% CI = 3.3-12.5) at GKRS1 had a statistically significant longer median overall survival after GKRS1 compared to patients with multiple BM (4.4 months, 95% CI = 1.8-7.1, p=0.037). D A trend towards a longer median overall survival in patients with RPA class II (7.2 months, 95% CI = 5.4-9.1) compared to patients with RPA class III (2.2 months, 95% CI = 0-6.6) could be observed, however, the results were statistically not significant (p=0.087). BM brain metastasis, CI Confidence Interval, GKRS Gamma Knife Radiosurgery, KPS Karnofsky performance status scale, RPA Recursive partitioning analysis
Outcome and survival after GKRS1 in relation to IT or TT
Overall, 61/71 (86%) patients received chemotherapy before or at GKRS1 treatment. Data on the treatment with IT or TT at (± 30 days) and after (> 30 days) GKRS1 treatment, were available from 71 patients (71/81, 88%).
In regard to the concomitant oncological treatment at or after GKRS1, 21/71 (30%) patients received IT or TT (Table 3) and 50/71 (70%) patients did not receive any IT or TT. All patients (21/21, 100%) from the IT or TT group also received chemotherapy before or at GKRS1. Both groups showed similar baseline characteristics, except for age with significantly younger patients in the “IT or TT group” (p = 0.011, Table 1).
We could show that patients with IT or TT at or after GKRS1 had a significantly longer survival after GKRS1 compared to patients without IT or TT at or after GKRS1 (p = 0.005, Fig. 2A).
Kaplan-Meier curves showing the overall survival after GKRS1 between different groups in relation to IT or TT. A Patients with IT or TT at or after GKRS1 had a significantly longer survival after GKRS1 (21/70 or 30%, 11.2 months, 95% CI = 3.1–19.4) compared to patients without IT or TT at or after GKRS1 (50/71 or 70%, 3.7 months, 95% CI = 0.8–6.6, p = 0.005). B Among patients with KPS ≥ 80%, patients with IT or TT (13.4 months, 95% CI = 8.4–18.5) showed a significantly longer median overall survival after GKRS1 compared to those without IT or TT (6.3 months, 95% CI = 3-9.7, p = 0.012). C In the group of patients ≤ 65 years, those with IT or TT at or after GKRS1 (11.2 months, 95% CI = 4.3–18.2) showed a significantly longer median overall survival compared to patients without IT or TT (2.6 months, 95% CI = 0.3–4.9, p = 0.017). D Analyzing patients with multiple BM at GKRS1, we could show that those with IT or TT at or after GKRS1 (7.3 months, 95% CI = 0-14.6) showed a significantly longer median overall survival after GKRS1 compared to patients without IT or TT (2.3 months, 95% CI = 1.2–3.4, p = 0.009). E Among patients with ECM at diagnosis of BM, those with IT or TT at or after GKRS1 (11.2 months, 95% CI = 2.8–19.7) showed a significantly longer median overall survival after GKRS1 compared to patients without IT or TT (4.4 months, 95% CI = 0.7–8.1, p = 0.002). F Among female patients, those with IT or TT at or after GKRS1 (19.1 months, 95% CI = 11.1–27) showed a significantly longer median overall survival after GKRS1 compared to patients without IT or TT (2.7 months, 95% CI = 0-7.2, p = 0.003). BM brain metastasis, CI Confidence Interval, ECM extracranial metastases, GKRS Gamma Knife Radiosurgery, IT immunotherapy, KPS Karnofsky performance status scale, TT targeted therapy
Even after excluding patients with a KPS of < 80%, we could show that the survival after GKRS1 remained significantly longer in patients with IT or TT compared to patients without IT or TT (p = 0.012, Fig. 2B).
In addition, a sub-analysis only among younger patients ≤ 65 years revealed that, patients treated with IT or TT had a statistically significant longer estimated median overall survival after GKRS1 compared to patients without IT or TT (p = 0.017, Fig. 2C).
In the older patient group > 65 years, those with IT or TT (11.6 months, 95% CI = 0-23.5) did not show any statistically significant differences in the estimated median overall survival after GKRS1 compared to patients without IT or TT (5.3 months, 95% CI = 1.9–8.7, p = 0.158).
Even among patients with multiple BM at GKRS1, the estimated median overall survival after GKRS1 remained significantly longer in patients with IT or TT compared to those without IT or TT (p = 0.009, Fig. 2D).
Same results applied to the positive ECM status at diagnosis of BM, as patients with IT or TT had a longer median overall survival after GKRS1 compared to those without IT or TT (p = 0.002, Fig. 2E).
By analyzing the median overall survival separately in the female and male group, we could also show that female patients with IT or TT showed a statistically significant longer estimated median overall survival after GKRS1 compared to female patients without IT or TT (p = 0.003, Fig. 2F). In the male group, patients with IT or TT (7.3 months, 95% CI = 6-8.5) did not show any statistically significant differences in estimated median overall survival after GKRS1 compared to patients without IT or TT (4.4 months, 95% CI = 2-6.9, p = 0.091).
Consequently, we calculated univariate Cox regression analyses evaluating sex, KPS group, age group, number of BM at GKRS1, RPA class, ECM status at BM diagnosis, GKRS1 treatment mode (boost, staged or regular high dose) and IT or TT status at or after GKRS1 as survival predictors among the total sample. Univariate Cox regressions showed that IT or TT at or after GKRS1 had a significant influence on the OS (HR: 2.227, 95% CI 1.261–3.935, p = 0.006).
Next, for the 71 patients of whom data on IT or TT were available, we included all covariates into a multivariate Cox regression model (sex, KPS group, age group, number of BM at GKRS1, RPA class, ECM status at BM diagnosis, GKRS1 treatment mode (boost, staged or regular high dose) and IT or TT status at or after GKRS1). Consequently, only IT or TT at or after GKRS1 was revealed as an independent predictor for a longer survival after GKRS1 (HR: 2.221, 95% CI 1.257–3.925, p = 0.006).
Tumor control and Complications after GKRS in relation to IT or TT
For the assessment of post-radiosurgical complications, clinical and radiological follow-up data from 58 patients (58/81, 72%) were available. One patient from the IT or TT group and 15 patients without IT or TT died before the first clinical follow-up. After excluding all patients without an available neuroradiological follow-up, we evaluated the follow-up MR images of 58 patients.
The Kaplan-Meier estimated mean time until local progression was 20 months (95% CI = 17.4–22.6). There was no significant difference of the Kaplan-Meier estimated mean time until local progression between patients who received IT or TT (18.7 months, 95% CI = 14.6–22.9) and patients without IT or TT (21.3 months, 95% CI = 18.7–23.9, p = 0.637).
There were no statistically significant differences in regard to the progression rate (p = 0.169), hemorrhage rate (p = 0.352) and radiation reaction or necrosis (p = 1.000) between patients with IT or TT and those without IT or TT.
Radiation reaction and necrosis after GKRS1 were observed in 6/58 (10%) and 1/58 (2%) patient, respectively. Intralesional hemorrhage was diagnosed in one patient (2%). Progression after GKRS1 was observed in 7/58 (12%). One patient (2%) with progression was subsequently treated by whole brain radiation therapy (WBRT) and further 3 patients (5%) underwent microsurgical resection. The remaining 3 patients had no further BM treatment.
Discussion
Prognostic factors for survival after radiosurgery among GI BM patients
In recent years, the treatment of BM patients with IT or TT and concomitant radiosurgery has improved the survival after radiosurgery in patients with BM from non-small cell lung cancer or melanoma [12,13,14,15,16]. Some studies on non-small cell lung cancer or melanoma even suggest that the radiosurgical treatment with concurrent IT has a positive synergetic effect on the systemic progression of primary malignancies via the abscopal effect [31]. Still, most of GI cancer patients are in a palliative care setting at the time of BM diagnosis [7]. In this study, we report the outcome of radiosurgically treated BM cancer patients from GI and associated primary cancers. As others have done before, we included BM patients with pancreatic and liver cancer as well, since the response to radiosurgical treatment is comparable among BM from these primary tumors [3, 19,20,21,22, 32].
In a first step, we could show that the actual overall survival after GKRS was significantly longer among all our patients compared to the predicted overall survival according to calculated prognostic scores [6, 23,24,25]. Nonetheless, previously published scores do not consider contemporary systemic oncological treatment options [6, 23,24,25, 33]. Subsequently, we aimed to analyze which factors affect the overall survival after radiosurgery in our patient cohort.
We could observe a trend towards a longer median overall survival after GKRS1 in patients with a KPS ≥ 80% and with RPA class II. These results were statistically not significant, which is in contrast to some former studies and might be caused by our sample size [5, 7, 19, 32, 34, 35]. It could also indicate that factors such as KPS, age, ECM status at GKRS1 do not represent the most important predictors on the overall survival after GKRS.
In contrast, a significantly longer overall survival after GKRS1 was observed in patients treated with concomitant IT or TT compared to patients without IT or TT. Of note, the vast majority of these patients received TT treatment and only a limited number of patients in our study was treated with IT. However, this clear benefit in survival after GKRS of patients treated with concomitant GKRS and mainly targeted therapy remained evident even in the sub-group analyses of patients with a KPS ≥ 80%, of patients aged ≤ 65 years and among female patients alone. Moreover, IT or mainly TT treatment at or after GKRS1 was revealed as the only independent predictor for a longer survival after GKRS1 among GI BM patients in a multivariate analysis that included sex, KPS score, age, number of BM, RPA class, ECM status, GKRS treatment mode and IT or TT treatment status. Our results are in accordance with previously published studies, which demonstrated a clear benefit of IT or TT on the survival after radiosurgery among GI and other primary cancers [12, 36, 37].
Previous studies have hypothesized that sex hormones may also have an effect on tumor aggressiveness, with the male sex being associated with a poorer survival [34, 38, 39]. Thus, the impact of the concomitant radiosurgical and IT or TT treatment on the outcome in male patients should be investigated in a larger sample.
Radiosurgery in the modern management of BM from GI cancers
Radiosurgery has established itself as the primary local treatment option for BM patients, generally achieving excellent tumor control and low complication rates [10, 11]. Compared to BM from lung or breast cancer or melanoma, BM from GI cancers are rare [4,5,6,7]. Consequently, data on the radiosurgical treatment of BM and the effect of IT or TT on the outcome after GKRS in GI cancer patients are scarce [17, 18, 37, 40]. However, the incidence rates of GI cancer and subsequently BM from GI cancer are rising [1, 3, 5,6,7, 41].
The treatment of BM from GI cancers remains a challenge since by the time BM are diagnosed, the overall prognosis is generally poor, they are often difficult to control and regularly react with persistent peritumoral edema after radiosurgical treatment [19, 21]. Consequently, the modern management of patients with BM from GI cancers requires a multimodal approach [41].
According to the treatment strategy at our department, patients with single or oligometastases with the largest tumor volume ≤ 6 cm³ received a regular high dose treatment [21]. In cases of multiple metastases (> 5 BM) a slightly reduced boost dose was mainly administered. Previous studies showed that the presence of multiple BM is a significant predictor among GI cancer patients [33, 37]. However, it is not per se a prognostic factor in modern BM management among other primary tumors [7, 42]. In our present study, the number of BM was not an independent predictor of survival after GKRS in the multivariate analysis.
Moreover, two-fraction dose-staged treatment was performed to enable the treatment of large or high-risk BM, as published before [26]. Of note, our analyses showed that there were no statistically significant differences in the outcome of patients treated with different radiosurgical strategies. Thus, we conclude that with the advancement of radiosurgical technique and oncological treatment options, the number of BM will become of less importance also among GI cancer patients and that even patients with more than 5 BM or large high-risk BM benefit from radiosurgical treatment [12, 26].
Of note, the vast majority of patients after GKRS were diagnosed with stable / regressive BM. Thus, good local tumor control rates and acceptable low rates of radiation reaction / necrosis demonstrate the safety and efficacy of GKRS in patients with BM from GI cancers under concomitant IT or TT.
However, there was no difference in BM progression between patients with and without IT or TT. This observation is supported by other data demonstrating that tumor histology, whole brain irradiation, targeted therapies, and antineoplastic therapies did not improve local tumor control in GI BM patients [19].
In summary, the combined radiosurgical and targeted oncological treatment has the potential to prolong the overall survival of BM patients from GI cancers. However, since systemic therapies, even IT or TT, are limited in their access through the blood-brain barrier and thus, may not sufficiently prevent BM progression, local treatment options, such as radiosurgery, remain of utmost importance in modern BM management [32, 33, 37, 41, 43, 44].
Limitations
We are aware that this study has some limitations which need to be addressed. First, this study was limited by its retrospective character, with data presented only by a single institute with a limited number of patients including different types of GI and associated primary cancers. Thus, the information on the type of systemic oncological treatment was missing in some cases. Moreover, in the group of IT or TT treatment, the vast majority of patients received TT treatment and only a limited number of patients was treated with IT.
Second, although there is no statistically significant difference in most of the baseline characteristics between patients with and without IT or TT, patients with IT or TT were significantly younger than patients without IT or TT. Therefore, age could have been a major factor in choosing candidates for systemic treatment. However, our multivariate analysis including age revealed IT and TT as an independent predictor for a longer survival after GKRS1.
Conclusions
Good local tumor control rates and low rates of side effects demonstrate the safety and efficacy of GKRS in patients with BM from GI cancers. The concomitant radiosurgical and targeted oncological treatment significantly improves the survival after GKRS without increasing the rate of adverse radiation effects. To provide local tumor control, radiosurgery remains of utmost importance in modern GI BM management.
Data Availability
Research data will not be shared.
Abbreviations
- BM:
-
Brain Metastases
- CI:
-
Confidence Interval
- ECM:
-
Extracranial Metastases
- GI:
-
Gastrointestinal
- GKRS:
-
Gamma Knife Radiosurgery
- GKRS1:
-
First Gamma Knife Radiosurgery
- GPA:
-
Graded Prognostic Assessment
- HR:
-
Hazard Ratio
- IT:
-
Immunotherapy
- KPS:
-
Karnofsky Performance Status Scale
- MRI:
-
Magnetic Resonance Imaging
- NSCLC:
-
Non-Small Cell Lung Cancer
- RPA:
-
Recursive Partitioning Analysis
- RANO:
-
Response Assessment in Neuro-Oncology
- SIR:
-
Score Index for Radiosurgery
- SRS:
-
Stereotactic Radiosurgery
- TT:
-
Targeted Therapy
- WBRT:
-
Whole Brain Radiation Therapy
References
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021.
Bartelt S, Momm F, Weissenberger C, Lutterbach J. Patients with brain metastases from gastrointestinal tract cancer treated with whole brain radiation therapy: prognostic factors and survival. World J Gastroenterol. 2004;10(22):3345–8.
Go PH, Klaassen Z, Meadows MC, Chamberlain RS. Gastrointestinal cancer and brain Metastasis: a rare and ominous sign. Cancer. 2011;117(16):3630–40.
Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Survival in patients with brain metastases: Summary Report on the updated diagnosis-specific graded Prognostic Assessment and Definition of the eligibility quotient. J Clin Oncol. 2020;38(32):3773–84.
Esmaeilzadeh M, Majlesara A, Faridar A, Hafezi M, Hong B, Esmaeilnia-Shirvani H, et al. Brain Metastasis from gastrointestinal cancers: a systematic review. Int J Clin Pract. 2014;68(7):890–9.
Sperduto PW, Fang P, Li J, Breen W, Brown PD, Cagney D, et al. Estimating survival in patients with gastrointestinal cancers and brain metastases: an update of the graded prognostic assessment for gastrointestinal cancers (GI-GPA). Clin Transl Radiat Oncol. 2019;18:39–45.
Sperduto PW, Fang P, Li J, Breen W, Brown PD, Cagney D, et al. Survival and prognostic factors in patients with gastrointestinal cancers and brain metastases: have we made progress? Transl Res. 2019;208:63–72.
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–91.
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29(2):134–41.
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–44.
Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of Radiosurgery alone vs Radiosurgery with Whole Brain Radiation Therapy on cognitive function in patients with 1 to 3 brain metastases: a Randomized Clinical Trial. JAMA. 2016;316(4):401–9.
Gatterbauer B, Hirschmann D, Eberherr N, Untersteiner H, Cho A, Shaltout A, et al. Toxicity and efficacy of Gamma Knife radiosurgery for brain metastases in Melanoma patients treated with immunotherapy or targeted therapy-A retrospective cohort study. Cancer Med. 2020;9(11):4026–36.
Shepard MJ, Xu Z, Donahue J, Eluvathingal Muttikkal TJ, Cordeiro D, Hansen L et al. Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell Lung cancer to the brain: a matched cohort study. J Neurosurg. 2019:1–8.
Chen L, Douglass J, Kleinberg L, Ye X, Marciscano AE, Forde PM, et al. Concurrent Immune checkpoint inhibitors and stereotactic radiosurgery for Brain metastases in Non-small Cell Lung Cancer, Melanoma, and renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2018;100(4):916–25.
Foster CC, Sher DJ, Rusthoven CG, Verma V, Spiotto MT, Weichselbaum RR, et al. Overall survival according to immunotherapy and radiation treatment for metastatic non-small-cell Lung cancer: a National Cancer database analysis. Radiat Oncol. 2019;14(1):18.
Di Lorenzo R, Ahluwalia MS. Targeted therapy of brain metastases: latest evidence and clinical implications. Ther Adv Med Oncol. 2017;9(12):781–96.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–20.
Trifiletti DM, Patel N, Lee CC, Romano AM, Sheehan JP. Stereotactic radiosurgery in the treatment of brain metastases from gastrointestinal primaries. J Neurooncol. 2015;124(3):439–46.
Aizer AA, Lamba N, Ahluwalia MS, Aldape K, Boire A, Brastianos PK, et al. Brain metastases: a Society for Neuro-Oncology (SNO) consensus review on current management and future directions. Neuro Oncol. 2022;24(10):1613–46.
Skeie BS, Enger PO, Ganz JC, Skeie GO, Parr E, Hatteland S, et al. Gamma knife Surgery of colorectal brain metastases: a high prescription dose of 25 gy may improve growth control. World Neurosurg. 2013;79(3–4):525–36.
Da Silva AN, Nagayama K, Schlesinger DJ, Sheehan JP. Gamma Knife Surgery for brain metastases from gastrointestinal cancer. J Neurosurg. 2009;111(3):423–30.
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–51.
Weltman E, Salvajoli JV, Brandt RA, de Morais Hanriot R, Prisco FE, Cruz JC, et al. Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys. 2000;46(5):1155–61.
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70(2):510–4.
Cho A, Medvedeva K, Kranawetter B, Untersteiner H, Hirschmann D, Lepilina O et al. How to dose-stage large or high-risk brain metastases: an alternative two-fraction radiosurgical treatment approach. J Neurosurg. 2022:1–10.
Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270–8.
Stockham AL, Tievsky AL, Koyfman SA, Reddy CA, Suh JH, Vogelbaum MA, et al. Conventional MRI does not reliably distinguish radiation necrosis from Tumor recurrence after stereotactic radiosurgery. J Neurooncol. 2012;109(1):149–58.
Heit JJ, Iv M, Wintermark M. Imaging of intracranial Hemorrhage. J Stroke. 2017;19(1):11–27.
Bradley WG. Jr. MR appearance of Hemorrhage in the brain. Radiology. 1993;189(1):15–26.
Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503–10.
Singh R, Bowden G, Mathieu D, Perlow HK, Palmer JD, Elhamdani S et al. Local Control and Survival Outcomes After Stereotactic Radiosurgery for Brain Metastases From Gastrointestinal Primaries: An International Multicenter Analysis. Neurosurgery. 2023.
Yamamoto M, Serizawa T, Sato Y, Higuchi Y, Kawabe T, Kasuya H, et al. Stereotactic Radiosurgery results for patients with brain metastases from gastrointestinal Cancer: a retrospective cohort study of 802 patients with GI-GPA Validity Test. Adv Radiat Oncol. 2021;6(6):100721.
Radkiewicz C, Johansson ALV, Dickman PW, Lambe M, Edgren G. Sex differences in cancer risk and survival: a Swedish cohort study. Eur J Cancer. 2017;84:130–40.
Stankiewicz M, Tomasik B, Blamek S. A new prognostic score for predicting survival in patients treated with robotic stereotactic radiotherapy for brain metastases. Sci Rep. 2021;11(1):20347.
Cho A, Untersteiner H, Hirschmann D, Shaltout A, Gobl P, Dorfer C et al. Gamma Knife Radiosurgery for Brain metastases in Non-small Cell Lung Cancer patients treated with immunotherapy or targeted therapy. Cancers (Basel). 2020;12(12).
Gu L, Qing S, Zhang HJ. A new prognostic model for brain metastases of specific primary tumors with stereotactic radiotherapy. Cancer Radiother. 2023;27(3):183–8.
Adami HO, Holmberg L, Persson I. Female sex hormones and cancer survival. Lancet. 1994;344(8924):760–1.
Shahabi S, He S, Kopf M, Mariani M, Petrini J, Scambia G, et al. Free testosterone drives cancer aggressiveness: evidence from US population studies. PLoS ONE. 2013;8(4):e61955.
Skeie BS, Eide GE, Flatebo M, Heggdal JI, Larsen E, Bragstad S, et al. Quality of life is maintained using Gamma Knife radiosurgery: a prospective study of a brain metastases patient cohort. J Neurosurg. 2017;126(3):708–25.
Mjahed RB, Astaras C, Roth A, Koessler T. Where are we now and where might we be Headed in understanding and managing Brain metastases in Colorectal Cancer patients? Curr Treat Options Oncol. 2022;23(7):980–1000.
Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–95.
Hartgerink D, van der Heijden B, De Ruysscher D, Postma A, Ackermans L, Hoeben A, et al. Stereotactic radiosurgery in the management of patients with brain metastases of Non-small Cell Lung Cancer: indications, decision tools and future directions. Front Oncol. 2018;8:154.
Page BR, Wang EC, White L, McTyre E, Peiffer A, Alistar A, et al. Gamma Knife radiosurgery for brain metastases from gastrointestinal primary. J Med Imaging Radiat Oncol. 2017;61(4):522–7.
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, F.K. and J.M.F.; Methodology, F.K. and J.M.F.; Acquisition of data, F.K., A.C., A.S., H.U., B.K., D.H., P.G., W.M. B.G. and J.M.F; Formal Analysis, F.K. and J.M.F.; Writing – Original Draft Preparation, F.K., J.M.F.; Writing – Review & Editing, F.K., A.C., A.S., H.U., B.K., D.H., P.G., W.M., B.G., K.R., C.D. and J.M.F.; Analysis and interpretation of data, F.K., J.M.F.; Visualization, F.K.; Supervision, J.M.F.; Project Administration, J.M.F. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the local ethics committee of the Medical University of Vienna (EK 1788/2020, 04.08.2020). For this type of study, formal consent is not required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khalaveh, F., Cho, A., Shaltout, A. et al. Concomitant radiosurgical and targeted oncological treatment improves the outcome of patients with brain metastases from gastrointestinal cancer. Radiat Oncol 18, 197 (2023). https://doi.org/10.1186/s13014-023-02383-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-023-02383-5