Abstract
Background
Few dosimetric comparisons have been published between linear accelerator (LA)-based systems and CyberKnife (CK)-based robotic radiosurgery systems for cardiac radio-ablation in ventricular tachycardia. This study aimed to compare the dosimetry of noninvasive cardiac radio-ablation deliverable on LA with that on CK.
Methods
Thirteen patients who underwent noninvasive cardiac radio-ablation by LA were included. The prescribed dose was 25 Gy in 1 fraction, and the average planning target volume was 49.8 ± 31.0 cm3 (range, 14.4–93.7 cm3). CK plans were generated for comparison.
Results
Both the CK and LA plans accomplished appropriate dose coverage and normal tissue sparing. Compared with the LA plans, the CK plans achieved significantly lower gradient indices (3.12 ± 0.71 vs. 3.48 ± 0.55, p = 0.031) and gradient measures (1.00 ± 0.29 cm vs. 1.17 ± 0.29 cm, p < 0.001). They had similar equivalent conformity indices (CK vs. LA: 0.84 ± 0.08 vs. 0.87 ± 0.07, p = 0.093) and maximum doses 2 cm from the planning target volume (PTV) in any direction (CK vs. LA: 50.8 ± 9.9% vs. 53.1 ± 5.3%, p = 0.423). The dosimetric advantages of CK were more prominent in patients with a PTV of ≤ 50 cm3 or a spherical PTV. In patients with a PTV of > 50 cm3 or a non-spherical PTV, the LA and CK plans were similar regarding dosimetric parameters. CK plans involved more beams (232.2 ± 110.8 beams vs. 10.0 ± 1.7 arcs) and longer treatment times (119.2 ± 43.3 min vs. 22.4 ± 1.6 min, p = 0.007).
Conclusions
Both CK and LA are ideal modalities for noninvasive cardiac radio-ablation. Upfront treatment should be considered based on clinical intent.
Similar content being viewed by others
Background
Noninvasive cardiac radio-ablation by stereotactic ablative radiotherapy represents an alternative treatment modality for ventricular tachycardia (VT). It effectively reduced the VT burden and improved the quality of life for patients who are refractory to medical therapy or catheter ablation or who cannot tolerate it [1, 2]. Currently, the linear accelerator (LA)-based system and the CyberKnife (CK) robotic radiosurgery system are used for noninvasive cardiac radio-ablation. Notably, several studies on the LA-based system from the USA, Asia, and Europe applied coplanar and non-coplanar volumetric-modulated arc radiotherapy (VMAT) to generate a highly conformal ablative radiation dose to the VT substrate [3,4,5,6,7,8,9]. Meanwhile, studies on the CK robotic radiosurgery system from the USA and Europe used a six-dimensional robotic arm, multiple non-coplanar small beams, and a continuous image-tracking system to deliver ablative radiation doses accurately [10,11,12,13].
Noninvasive cardiac radio-ablation for VT is a new and unique challenge for clinicians and radiation physicists. One case report presented the dosimetric difference between the VT substrate’s LA- and CK-based systems at the anterior basal heart [14]. The report concluded that CK was superior to the LA-based planning system for a steeper dose gradient and better real-time target tracking. The RAVENTA benchmark study included three patients’ plans using LA- and CK-based systems from five academic centers [15]. They demonstrated that VMAT plans had steeper dose gradients in the high-dose region, while the CK plans had smaller low-dose regions. They concluded that plans from both systems were considered deliverable based on the internal guidelines and protocols for SBRT and noninvasive cardiac radio-ablation [16].
Few dosimetric comparisons have been published between the LA-based and CK-based robotic radiosurgery systems for cardiac radio-ablation. Therefore, this study aimed to compare the dosimetry of noninvasive cardiac radio-ablation deliverables on CK with LA systems and discuss related clinical issues.
Methods
Study design and patients
This study was approved by the National Taiwan University Hospital Research Ethics Committee (approval number: 201804026RINC). All procedures in this study followed the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from all patients. Thirteen patients who underwent noninvasive cardiac radio-ablation for VT by an LA-based system using approved VMAT plans at our institution were included between March 2018 and March 2022.
Treatment protocol
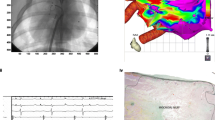
Stereotactic body radiation therapy (SBRT) simulations, substrate identification, and contour delineation have been previously described [5, 17]. All patients in this study underwent standard SBRT simulation while immobilized in an individualized vacuum bag (BodyFIX; Elekta, Stockholm, Sweden), which limited diaphragmatic motion through external abdominal compression. Axial images (1-mm slices) were obtained. Cardiac magnetic resonance imaging, diagnostic dual-energy computed tomography (CT), and three-dimensional electroanatomic maps defined the treatment target volume. The average planning target volume (PTV) was 49.8 ± 31.0 cm3 (range, 14.4–93.7 cm3), and the prescribed dose was 25 Gy in 1 fraction. The targets were heterogeneous concerning shape and location; involved complex-shaped target volumes at the left ventricular (LV) basal, middle, or apical areas; and were surrounded by non-identical critical structures, as shown in Fig. 1. The bull’s eye view displayed the target area in the 17-segment mode proposed by the American Heart Association [18, 19].
Demonstrations of the 13 target volumes. Complex-shaped target volumes are shown at the left ventricle (LV)’s basal, middle, and apical areas. a Axial view of the planning target volume (PTV) as a green-colored wash area. b Three-dimensional representation of the PTV (green), LV (pink), and right ventricle (RV, yellow) on left anterior oblique (LAO) views. c Bull’s eye view displaying the green target area in the 17-segment American Heart Association model
Treatment planning
The clinical LA VMAT plans were generated initially using the treatment planning system (TPS) (Eclipse, version 15.5; Varian Medical Systems, Palo Alto, CA, USA), with 6- or 10-MV flattening filter-free photon beams, multiple coplanar and non-coplanar partial arcs, and the VMAT technique. The dose calculation algorithm was anisotropic analytical algorithm (AAA). The prescribed isodose was adequate to cover at least 95% of the target. The dose constraints for single-fraction SBRT for organs at risk (OARs) were adopted from the American Association of Physicists in Medicine Task Group 101 (TG101) as follows: skin, 26 Gy; rib, 30 Gy; trachea/large bronchus, 20 Gy; spinal cord, 14 Gy; stomach, 12.4 Gy; the esophagus, 15.4 Gy; lungs (volume of at least 1500 cm3), < 7 Gy; liver (volume of at least 700 cm3), < 9.1 Gy [20, 21].
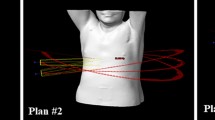
The planning CT dataset and pre-drawn structures in the Eclipse TPS were transferred to the CyberKnife M6 TPS (Precision version 3.3.1.2; Accuray, Sunnyvale, CA, USA) to generate CK plans for comparison. Experienced medical physicists performed planning in the CK TPS based on the planning directive without prior knowledge of the quality of the LA clinical plan. Specifically, these research plans were generated as if they were for clinical use rather than demonstrating superiority over the LA plan. For the IRIS mode, the treatments were planned with VOLO optimizer utilizing variable aperture IRIS which uses different sizes of the collimator to generate hundreds of non-isocentric beams in one path to spare all the critical organs near the target and fulfill the criteria as above, and the dose calculation algorithm was Ray-tracing with contour correction; for the InCise MLC mode frequently used to treat large and irregularly shaped targets with the advantage of shorter delivery time, the dose calculation algorithm was finite size pencil beam (FSPB). The 6-MV flattening filter-free beams were delivered from non-coplanar angles to improve the conformity of the radiation dose and reduce radiation damage to healthy tissues. An example of noninvasive cardiac radio-ablation using the CK- or LA-based systems with associated isodose curves is shown in Fig. 2.
Example of noninvasive cardiac radio-ablation using the linear accelerator (LA) and CyberKnife (CK)-based radiosurgery systems. Patient No. 10 was a 61-year-old man with recurrent sustained ventricular tachycardia (VT) related to left apical ventricular hypertrophy refractory to antiarrhythmic medication and an implantable cardiac defibrillator. Electro-anatomical mapping revealed that the substrate originated from the apical junction of the right coronary commissure (RCC) and left coronary commissure (LCC). He underwent multiple sessions of CARTO-guided cardiac ablation of the apical junction of the LCC-RCC and the right ventricular outflow tract posterior septal area. He developed recurrent VT, and noninvasive cardiac radio-ablation was performed using an LA-based 6-MV flattening filter-free photon beam and stereotactic volumetric modulated arc radiotherapy technique to the posterior-septal wall at 25 Gy in 1 fraction. The VT burden was reduced after noninvasive cardiac radio-ablation, and the patient was followed up at the clinic. The CK plan was generated for dosimetric comparison. The homogeneity indices, conformity indices, gradient indices, gradient measures, and maximum doses at 2 cm from the planning target volume (PTV) of the LA and CK plans are shown in Table 1. The beam arrangement is shown in the upper panels of the figure. a Four coplanar arcs and three non-coplanar arcs are presented in the LA plan. b A total of 253 non-coplanar beams are presented in the CK plan. The isodose curves are shown in the lower panel for the LA plan (c) and the CK plan (d). Green areas indicate the PTV. The orange, red, and blue lines represent the isodose curves at 2875, 2500, and 1250 cGy, respectively
Plan comparison
All plans were planned for at least 95% coverage of the target volume with the prescribed dose. As previously described, the plans had to fulfill the predefined dose constraints for OARs. When there was a trade-off between OAR dosimetry and target coverage, dose constraints to the esophagus and stomach were prioritized over the PTV coverage. For a fair comparison between LA and CK, the CK plan’s coverage was normalized to that of the LA plan. For example, if the CK and LA plans of the same patient had coverage of 95.0% and 95.5%, the CK plan was normalized so that 95.5% of the PTV received the prescription dose, the same as in the counterpart LA plan.
Since DVH calculation on different TPS possessed potential uncertainties, dose distributions of CK plans were imported into Eclipse TPS for plan comparison [15, 22]. The quality indices used for plan comparison were the homogeneity index (HI), Paddick conformity index (CI), gradient index (GI), gradient measure (GM), and maximum dose 2 cm from the PTV in any direction (D2cm), regarding the standardization of terminology in stereotactic radiosurgery [23]. The HI was calculated as the maximum target dose divided by the prescription dose. The CI was calculated as TVPIV2/(TV × PIV), where TV represented the target volume; prescription isodose volume (PIV) represented the volume that received the prescription dose, whether within or outside the targets; and TV PIV represented TV covered by the PIV. A perfect plan was defined to have a CI score of 1, and a plan CI of > 0.85 was considered ideal. GI was calculated as PV50%/PIV, where PV50% represented the volume covered by 50% of the prescription dose. The GM defined the average distance between the 12.5-Gy equivalent spherical volume and the 25-Gy equivalent spherical volume. A smaller GI or GM value indicated a steeper dose gradient. D2cm was used to assess the intermediate-to-low dose spillage outside the PTV.
The maximal doses to the spinal cord, stomach, esophagus, trachea/large bronchus, and coronary arteries (including the left anterior descending artery, right coronary artery, and left circumflex artery) were recorded for OAR dosimetry. The TG101 study also suggested that the heart volume receiving at least 16 Gy should be maintained at < 15 cm3 [19]. However, this was not possible during cardiac radio-ablation owing to the nature of the prescription dose and the target locations. In addition to the abovementioned parameters, the total volume of the heart receiving > 16 Gy, excluding the PTV (heart minus PTV), was also compared.
The number of beams and monitor units required to deliver the prescribed dose in the LA or CK plans was evaluated. The estimated treatment time for LA included both the treatment delivery time and a 15-min length for the cone beam CT (CBCT) imaging registration; according to the institutional SBRT protocol, at least three sets of CBCT scans (the first CBCT was for bony and soft tissue registration, the second was to confirm the correction before coplanar arcs irradiation, and the third was before the start of non-coplanar beams) were scheduled for each patient. The CK TPS reported the beam delivery time, which included both the beam-on time and time for location tracking.
For subgroup analysis, the targets were classified into large or small volumes, spherical or non-spherical, according to their volumes and shapes in three dimensions. Large targets were as PTV > 50 cm3 [24]. Spherical targets were shapes in three dimensions, being more or less round, with smooth borders, and recognizable decreasing areas in three dimensions from the central plane to the periphery [25].
Statistical analyses
Two-tailed paired-sample t-tests were conducted to evaluate the statistical significance of the differences between the two modalities for the plan quality indices. All statistical analyses were performed using Statistical Package for Social Sciences for Windows (SPSS version 22.0; IBM, Armonk, NY, USA). Statistical significance was set at p ≤ 0.05.
Results
The clinical goal of 95% PTV coverage was optimally achieved, except in patient No. 12, who had a large PTV of 93.7 cm3 located at the apical lateral wall of the LV adjacent to the stomach. The PTV coverage was traded off in both the LA and CK systems to reach the dose constraint of the stomach. The plan quality parameters are listed in Table 1 and Fig. 3. The HI ratio was significantly higher in the CK plans than in the LA plans (1.34 ± 0.06 vs. 1.24 ± 0.03, p < 0.001), reflecting that a higher maximal dose within target volumes was usually tolerable for CK-based radiosurgery plans. The CK and LA plans achieved acceptable CI ratios, with no significant difference (CK vs. LA: 0.84 ± 0.08 vs. 0.87 ± 0.07, respectively, p = 0.093).
Dosimetric comparison between linear accelerator (LA) and CyberKnife (CK) plans. The conformity indices (a), gradient indices (b), gradient measures (c), and maximum doses 2 cm from the planning target volume in any direction (d) of the LA and CK plans are demonstrated. p-values for statistical comparisons are obtained using a paired Student’s t-test
The CK plans achieved significantly lower GI ratios (3.12 ± 0.71 vs. 3.48 ± 0.55, p = 0.031) and GM values (1.00 ± 0.29 cm vs. 1.17 ± 0.29 cm, p < 0.001) than the LA plans, indicating that the CK plans provided a steeper dose gradient benefit. The D2cm was comparable between the LA and CK plans (CK vs. LA: 50.8 ± 9.9% vs. 53.1 ± 5.3%, respectively, p = 0.423), indicating that in both systems, the radiation dose could be decreased to approximately half values at 2 cm from the PTV.
The dosimetric parameters of the OARs are listed in Table 2. The maximal doses to the esophagus, stomach, spinal cord, trachea, large bronchus, and coronary artery were similar between the CK and LA plans. The volumes of the normal heart receiving ≥ 16 Gy, the normal heart’s mean dose, and the normal lung receiving ≥ 7 Gy were similar between the CK and LA plans. These results suggested that the OARs were well protected with the two systems.
The treatment delivery parameters are listed in Table 3. An average of 232.2 non-coplanar beams in the CK system and a median of 10 arcs (six coplanar arcs and four non-coplanar arcs) in the LA system were designed for one fully qualified plan. Compared with the LA plans, the CK plans had significantly longer treatment times (22.4 ± 1.6 min vs. 119.2 ± 43.3 min, p = 0.007).
The dosimetric comparison between large (PTV > 50 cm3, n = 6) and small (PTV ≤ 50 cm3, n = 7) target volumes for subgroup analyses is shown in Table 4. The dosimetric advantages of CK providing steeper dose gradients were prominent in patients with small target volumes, as evidenced by the lower GI ratios (2.78 ± 0.36 vs. 3.38 ± 0.46, p = 0.028), GM values (0.76 ± 0.13 cm vs. 0.93 ± 0.15 cm, p = 0.007), and D2cm values (42.9 ± 4.1% vs. 51.0 ± 5.8%, p = 0.027). Meanwhile, the dosimetric advantages of CK were less evident in patients with large target volumes, as evidenced by the lower GM values (1.28 ± 0.10 cm vs. 1.45 ± 0.07 cm, p < 0.001) but similar GI ratios (CK vs. LA: 3.52 ± 0.83 vs. 3.60 ± 0.66, respectively, p = 0.619) and D2cm values (CK vs. LA: 59.9 ± 5.4% vs. 55.5 ± 3.8%, respectively, p = 0.273). There was no difference in the estimated treatment time between large and small target volumes in both the LA system (small vs. large: 21.7 ± 1.8 vs. 23.2 ± 0.8 min, respectively, p = 0.072) and the CK system (small vs. large: 120.2 ± 18.1 vs. 118.2 ± 37.4 min, p = 0.907).
Additional analysis was conducted for dosimetric parameters by stratifying the PTV shape into spherical (n = 6) or non-spherical (n = 7). The benefits of CK plans for treating spherical targets were prominent, as manifested by lower GI ratios (2.63 ± 0.23 vs. 3.35 ± 0.50, p = 0.029), GM ratios (0.73 ± 0.12 vs. 0.93 ± 0.17, p = 0.006), and D2cm values (42.9 ± 4.4% vs. 51.5 ± 6.2%, p = 0.046). When treating non-spherical PTVs, the LA plans achieved equivalent dosimetric advantages, as supported by similar GI ratios (CK vs. LA: 3.51 ± 0.76 vs. 3.59 ± 0.60, respectively, p = 0.531) and D2cm values (CK vs. LA: 57.5 ± 8.0% vs. 54.4 ± 4.4%, respectively, p = 0.395).
Discussion
There have been few dosimetric comparisons between the LA-based and CK-based robotic radiosurgery systems for cardiac radio-ablation. This study found that the LA and CK systems are suitable for noninvasive cardiac radio-ablation, with adequate dose coverage and appropriate normal tissue sparing. CK plans provided dosimetric advantages of steeper dose gradients in patients with small target volumes (PTV ≤ 50 cm3) or spherical PTVs. Meanwhile, CK plans required longer treatment times.
The present study demonstrated complex-shaped target volumes at the LV basal, middle, or apical areas surrounded by non-identical critical structures, indicating noninvasive cardiac radio-ablation heterogeneity and dosimetric challenges. The dose distribution of homogeneity and gradient measures were significantly more favorable in the CK plans than in the LA plans, especially in patients with small target volumes and spherical PTVs. This suggested a much sharper and rapid dose fall-off of the margin from target volumes and a more centralized uniform radiation dose within target volumes in CK plans. These results align with the published case report establishing the dosimetric benefit of using the CK system to treat non-ischemic basal VT with a small and spherical PTV (31.8 cm3) [14].
The RAVENTA trial demonstrated three benchmark cases with PTVs of 36.5 cc, 62.4 cc, and 52.8 cc [15]. The present study parallels their study regarding dose distribution and conformity values. The RAVENTA trial showed that the dose distributions were more axial in VMAT plans and more spherical in CK plans, and a trend toward higher CI ratios in the LA-based VMAT plans (LA 0.87 vs. CK 0.79), which following our distribution shown in Fig. 2 and the CI ratio results (LA 0.87 vs. CK 0.84). The GI advantage of CK plans in the present study was merely seen in the RAVENTA trial. In their series, the LA-based VMAT plans had steeper dose gradients in the high-dose region, while the CK plans had smaller low-dose regions. This may partly be related to their series’ target volumes and cardiac substructures constraint. The dosimetric advantages of CK were less evident in our series in patients with PTV > 50 cm3 showing similar GI ratios (CK 3.52 vs. LA 3.60). Moreover, the RAVENTA trial adopted dose constraints to cardiac substructures, including the aorta, the left coronary arteries, the superior vena cava, and the left atrium, which are not implemented in our study. The trade-offs between the gradient dose fall-off and OAR sparing varied from plan to plan, depending on individual planners, different institutions, and varied clinical situations [26, 27]. In our series, we observed that LA plans exhibited dosimetric superiority in thin and flat targets in the apical LV lateral wall, as shown in patients No. 6 and No. 13. This implies that treatment systems should be considered based on PTV volumes, locations, and shapes.
Literature has shown that for stereotactic radiotherapy, different dose calculation approaches like AAA, ray-tracing, or pencil beam algorithms may have differences regarding dose distributions, with the largest differences in the lung encompassing high tissue heterogeneities[28]. AAA algorithm accounts for changes in electron transport and volume scatter with improved calculation results, especially in regions of high heterogeneities [29], thus may be more reliable in circumstances where VT targets overlapping lung regions, while both the AAA algorithm and the pencil beam algorithm are accurate enough for cases where VT targets reside within the heart, where almost no heterogeneities are present. In the RAVENTA benchmark study, the dosimetric accuracy was tested during patient-specific quality assurance using an electronic portal imaging device (EPID) for VMAT plans and PTW PinPoint or SRS array for CK plans; all fulfilled the criteria without major discrepancy. Therefore, plans were considered deliverable by both CK and LA-based systems [15].
In the present study, significantly longer treatment times were shown in the CK system. This finding aligns with previous dosimetric comparisons between CK-based robotic radiosurgery and LA-based systems under variable circumstances [30]. Importantly, this finding has pertinent implications for patients whose medical conditions do not allow long irradiation periods, especially those experiencing recurrent VT that leads to life-threatening electrical storms.
Dose limits to heart substructures are largely unknown and have only been recently reported, though not yet correlated to toxicity; however, our institution did not set dosimetry constraints for the coronary arteries. In the present study, as VT substrate could be very close to or overlapping the coronary arteries, the maximal dose of the coronary arteries was as high as 105%-114% of the prescribed dose. The RAVENTA trial has set the major violation for left coronary arteries to be 20 Gy[15]. So far, in our institution, there has been no evidence of short-term toxicities in the coronary arteries. However, patients are followed up after study completion to obtain further long-term safety data. Long-term data will define the full safety profile of cardiac radio-ablation for toxicities on healthy intra-cardiac structures.
Motion management varies between the LA and CK treatment strategies. The internal target volume (ITV) was delineated using four-dimensional CT in both systems to cover respiratory and cardiac movements. Abdominal compression was employed to minimize respiratory motion during the LA-based treatments, which usually required short treatment time with acceptable patient compliance. The advantage of time efficiency in LA-based systems may have biological benefits to treatment outcomes [31] and is also more suitable to life-threatening VT patients experiencing electrical storms. For CK plans with longer treatment times, the CK-based system adopted the synchrony fiducial tracking system (Accuray, Sunnyvale, CA, USA) in which the distal dipole of the right ventricular lead of the implanted cardiac defibrillator is used as a fiducial, allowing real-time tracking of both respiratory and cardiac movements during the long treatment session [12, 13]. Furthermore, the CK-based InCise MLC mode has been used to treat large and irregularly shaped targets with the advantage of shorter delivery time [32], was adopted for patient 12’s treatment plan, and resulted in a relatively acceptable treatment time of 46 min.
The present study had some limitations. First, it only included 13 patients with heterogeneous PTV sizes, shapes, and locations. The small target volumes (PTV ≤ 50 cm3) and spherical PTVs were mainly located at the basal segments. However, the large target volumes (PTV > 50 cm3) and non-spherical PTVs were mostly located at the apical segments. The small number of patients and heterogeneous PTV volumes, shapes, and locations may limit the comparison of dosimetric advantages between the two systems. Second, the study did not investigate the correlation between dosimetry and clinical outcomes. Further prospective studies with a larger patient size may be needed to validate whether the dosimetric difference affects the clinical efficacy of reducing the VT burden.
Conclusions
In conclusion, the LA and CK systems are ideal modalities for noninvasive cardiac radio-ablation, with adequate dose coverage and appropriate normal tissue sparing. Moreover, the CK plans provide dosimetric advantages of steeper dose gradients in patients with small target volumes (PTV ≤ 50 cm3) and spherical PTVs, although they also require longer treatment times. LA plans may be beneficial in treating large PTVs (> 50 cm3) or non-spherical PTVs located at the apical LV lateral wall, with the advantage of time efficiency. Therefore, upfront treatment should be considered based on the clinical intent.
Availability of data and materials
The data supporting this study’s findings were obtained under license from the National Taiwan University Hospital Healthcare System database and are not publicly available. Data are available from the corresponding author upon request.
Abbreviations
- CI:
-
Conformity index
- CK:
-
CyberKnife
- CT:
-
Computed tomography
- D2cm :
-
Maximum dose 2 cm from the planning target volume in any direction
- GI:
-
Gradient index
- GM:
-
Gradient measure
- HI:
-
Homogeneity index
- ITV:
-
Internal target volume
- LA:
-
Linear accelerator
- LV:
-
Left ventricular
- OARs:
-
Organs at risk
- PIV:
-
Prescription isodose volume
- PTV:
-
Planning target volume
- SBRT:
-
Stereotactic body radiation therapy
- SD:
-
Standard deviation
- TG101:
-
American Association of Physicists in Medicine Task Group 101
- TPS:
-
Treatment planning system
- TV:
-
Target volume
- VMAT:
-
Volumetric-modulated arc radiotherapy
- VT:
-
Ventricular tachycardia
References
Siedow M, Brownstein J, Prasad RN, Loccoh E, Harfi TT, Okabe T, Tong MS, Afzal MR, Williams T. Cardiac radioablation in the treatment of ventricular tachycardia. Clin Transl Radiat Oncol. 2021;31:71–9.
Jumeau R, Ozsahin M, Schwitter J, Elicin O, Reichlin T, Roten L, Andratschke N, Mayinger M, Saguner AM, Steffel J, et al. Stereotactic radiotherapy for the management of refractory ventricular tachycardia: promise and future directions. Front Cardiovasc Med. 2020;7:108.
Qian PC, Quadros K, Aguilar M, Wei C, Boeck M, Bredfeldt J, Cochet H, Blankstein R, Mak R, Sauer WH, et al. Substrate modification using stereotactic radioablation to treat refractory ventricular tachycardia in patients with ischemic cardiomyopathy. JACC Clin Electrophysiol. 2022;8:49–58.
Lee J, Bates M, Shepherd E, Riley S, Henshaw M, Metherall P, Daniel J, Blower A, Scoones D, Wilkinson M, et al. Cardiac stereotactic ablative radiotherapy for control of refractory ventricular tachycardia: initial UK multicentre experience. Open Heart. 2021;8:e001770.
Ho LT, Chen JL, Chan HM, Huang YC, Su MY, Kuo SH, Chang YC, Lin JL, Chen WJ, Lee WJ, et al. First Asian population study of stereotactic body radiation therapy for ventricular arrhythmias. Sci Rep. 2021;11:10360.
Chin R, Hayase J, Hu P, Cao M, Deng J, Ajijola O, Do D, Vaseghi M, Buch E, Khakpour H, et al. Non-invasive stereotactic body radiation therapy for refractory ventricular arrhythmias: an institutional experience. J Interv Card Electrophysiol. 2021;61:535–43.
Carbucicchio C, Jereczek-Fossa BA, Andreini D, Catto V, Piperno G, Conte E, Cattani F, Rondi E, Vigorito S, Piccolo C, et al. STRA-MI-VT (stereotactic radioablation by multimodal imaging for ventricular tachycardia): rationale and design of an Italian experimental prospective study. J Interv Card Electrophysiol. 2021;61:583–93.
Lloyd MS, Wight J, Schneider F, Hoskins M, Attia T, Escott C, Lerakis S, Higgins KA. Clinical experience of stereotactic body radiation for refractory ventricular tachycardia in advanced heart failure patients. Heart Rhythm. 2020;17:415–22.
Robinson CG, Samson PP, Moore KMS, Hugo GD, Knutson N, Mutic S, Goddu SM, Lang A, Cooper DH, Faddis M, et al. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation. 2019;139:313–21.
Gianni C, Rivera D, Burkhardt JD, Pollard B, Gardner E, Maguire P, Zei PC, Natale A, Al-Ahmad A. Stereotactic arrhythmia radioablation for refractory scar-related ventricular tachycardia. Heart Rhythm. 2020;17:1241–8.
Neuwirth R, Cvek J, Knybel L, Jiravsky O, Molenda L, Kodaj M, Fiala M, Peichl P, Feltl D, Januska J, et al. Stereotactic radiosurgery for ablation of ventricular tachycardia. Europace. 2019;21:1088–95.
Herrera Siklody C, Pruvot E, Pascale P, Kinj R, Jumeau R, Le Bloa M, Teres C, Domenichini G, Porretta AP, Ozsahin M, et al. Refractory ventricular tachycardia treated by a second session of stereotactic arrhythmia radioablation. Clin Transl Radiat Oncol. 2022;37:89–93.
Ninni S, Gallot-Lavallée T, Klein C, Longère B, Brigadeau F, Potelle C, Crop F, Rault E, Decoene C, Lacornerie T, et al. Stereotactic radioablation for ventricular tachycardia in the setting of electrical storm. Circ Arrhythm Electrophysiol. 2022;15:e010955.
Weidlich GA, Hacker F, Bellezza D, Maguire P, Gardner EA. Ventricular tachycardia: a treatment comparison study of the CyberKnife with conventional linear accelerators. Cureus. 2018;10:e3445.
Kluge A, Ehrbar S, Grehn M, Fleckenstein J, Baus WW, Siebert FA, Schweikard A, Andratschke N, Mayinger MC, Boda-Heggemann J, et al. Treatment planning for cardiac radioablation: multicenter multiplatform benchmarking for the radiosurgery for ventricular tachycardia (RAVENTA) trial. Int J Radiat Oncol Biol Phys. 2022;114:360–72.
Blanck O, Buergy D, Vens M, Eidinger L, Zaman A, Krug D, Rudic B, Boda-Heggemann J, Giordano FA, Boldt LH, et al. Radiosurgery for ventricular tachycardia: preclinical and clinical evidence and study design for a German multi-center multi-platform feasibility trial (RAVENTA). Clin Res Cardiol. 2020;109:1319–32.
Huang YS, Chen JL, Hsu FM, Huang JY, Ko WC, Chen YC, Jaw FS, Yen RF, Chang YC. Response assessment of stereotactic body radiation therapy using dynamic contrast-enhanced integrated MR-PET in non-small cell lung cancer patients. J Magn Reson Imaging. 2018;47:191–9.
Brownstein J, Afzal M, Okabe T, Harfi TT, Tong MS, Thomas E, Hugo G, Cuculich P, Robinson C, Williams TM. Method and atlas to enable targeting for cardiac radioablation employing the American heart association segmented model. Int J Radiat Oncol Biol Phys. 2021;111:178–85.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. Circulation. 2002;105:539–42.
Knutson NC, Samson PP, Hugo GD, Goddu SM, Reynoso FJ, Kavanaugh JA, Mutic S, Moore K, Hilliard J, Cuculich PS, et al. Radiation therapy workflow and dosimetric analysis from a phase 1/2 trial of noninvasive cardiac radioablation for ventricular tachycardia. Int J Radiat Oncol Biol Phys. 2019;104:1114–23.
Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L, et al. Stereotactic body radiation therapy: the report of AAPM task group 101. Med Phys. 2010;37:4078–101.
Pepin MD, Penoncello GP, Brom KM, Gustafson JM, Long KM, Rong Y, de Fong Los Santos LE, Shiraishi S. Assessment of dose-volume histogram precision for five clinical systems. Med Phys. 2022;49:6303–18.
Torrens M, Chung C, Chung HT, Hanssens P, Jaffray D, Kemeny A, Larson D, Levivier M, Lindquist C, Lippitz B, et al. Standardization of terminology in stereotactic radiosurgery: report from the standardization committee of the international leksell gamma knife society: special topic. J Neurosurg. 2014;121(Suppl):2–15.
Janowski E, Chen LN, Kim JS, Lei S, Suy S, Collins B, Lynch J, Dritschilo A, Collins S. Stereotactic body radiation therapy (SBRT) for prostate cancer in men with large prostates (≥ 50 cm3). Radiat Oncol. 2014;9:241.
Collins SP, Coppa ND, Zhang Y, Collins BT, McRae DA, Jean WC. CyberKnife radiosurgery in the treatment of complex skull base tumors: analysis of treatment planning parameters. Radiat Oncol. 2006;1:46.
Moustakis C, Blanck O, Ebrahimi Tazehmahalleh F, Kaheng Chan M, Ernst I, Krieger T, Duma M-N, Oechsner M, Ganswindt U, Heinz C, et al. Planning benchmark study for SBRT of early stage NSCLC. Strahlenther Onkol. 2017;193:780–90.
Villaggi E, Hernandez V, Fusella M, Moretti E, Russo S, Vaccara EML, Nardiello B, Esposito M, Saez J, Cilla S, et al. Plan quality improvement by DVH sharing and planner’s experience: Results of a SBRT multicentric planning study on prostate. Phys Med. 2019;62:73–82.
Petrovic B, Grządziel A, Ślosarek K. Quality assurance of TPS: comparison of dose calculation for stereotactic patients in Eclipse and iPlan RT dose. Rep Pract Oncol Radiother. 2009;14:5–10.
Akino Y, Das IJ, Cardenes HR, Desrosiers CM. Correlation between target volume and electron transport effects affecting heterogeneity corrections in stereotactic body radiotherapy for lung cancer. J Radiat Res. 2014;55:754–60.
Blamek S, Grządziel A, Miszczyk L. Robotic radiosurgery versus micro-multileaf collimator: a dosimetric comparison for large or critically located arteriovenous malformations. Radiat Oncol. 2013;8:205.
Fowler JF, Welsh JS, Howard SP. Loss of biological effect in prolonged fraction delivery. Int J Radiat Oncol Biol Phys. 2004;59:242–9.
Jang SY, Lalonde R, Ozhasoglu C, Burton S, Heron D, Huq MS. Dosimetric comparison between cone/Iris-based and InCise MLC-based CyberKnife plans for single and multiple brain metastases. J Appl Clin Med Phys. 2016;17:184–99.
Acknowledgements
We thank the staff of the Core Labs, the Department of Medical Research, National Taiwan University Hospital for their technical support.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan [Grant Number MOST 110-2314-B-002-138-MY3] and the National Taiwan University Hospital [Grant Number NTUH 111-S0025].
Author information
Authors and Affiliations
Contributions
JL-YC and C-YW were responsible for study design and plan, literature collection, data management and interpretation, statistical analysis, and manuscript writing. C-YW, L-TH, and L-YL were responsible for the statistical analysis and interpretation of the statistical findings. H-MC, H-YC, T-LY, and Y-SH contributed to the patient cohort and clinical data. JL-YC, Y-SH, S-HK, and W-JL were responsible for the management. C-YW, Y-SH, JL-YC, and W-JL planned, designed, and coordinated the study over the entire period and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the National Taiwan University Hospital Research Ethics Committee (Approval Number: 201804026RINC). All procedures in this study followed the ethical standards of the institutional and/or national research committee and the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from all patients.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, CY., Ho, LT., Lin, LY. et al. Noninvasive cardiac radioablation for ventricular tachycardia: dosimetric comparison between linear accelerator- and robotic CyberKnife-based radiosurgery systems. Radiat Oncol 18, 187 (2023). https://doi.org/10.1186/s13014-023-02370-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-023-02370-w