Abstract
Background
Accurate prediction of response to neoadjuvant chemoradiotherapy (nCRT) is very important for treatment plan decision in locally advanced rectal cancer (LARC). The aim of this study was to investigate whether self-attention mechanism based multi-sequence fusion strategy applied to multiparametric magnetic resonance imaging (MRI) based deep learning or hand-crafted radiomics model construction can improve prediction of response to nCRT in LARC.
Methods
This retrospective analysis enrolled 422 consecutive patients with LARC who received nCRT before surgery at two hospitals. All patients underwent multiparametric MRI scans with three imaging sequences. Tumor regression grade (TRG) was used to assess the response of nCRT based on the resected specimen. Patients were separated into 2 groups: poor responders (TRG 2, 3) versus good responders (TRG 0, 1). A self-attention mechanism, namely channel attention, was applied to fuse the three sequence information for deep learning and radiomics models construction. For comparison, other two models without channel attention were also constructed. All models were developed in the same hospital and validated in the other hospital.
Results
The deep learning model with channel attention mechanism achieved area under the curves (AUCs) of 0.898 in the internal validation cohort and 0.873 in the external validation cohort, which was the best performed model in all cohorts. More importantly, both the deep learning and radiomics model that applied channel attention mechanism performed better than those without channel attention mechanism.
Conclusions
The self-attention mechanism based multi-sequence fusion strategy can improve prediction of response to nCRT in LARC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision (TME) is widely recommended as the standard treatment strategy for locally advanced rectal cancer (LARC) [1, 2]. Response to nCRT is a favorable indicator of good prognosis. Approximately 50-60% of patients with LARC are downstaged and about 20% show pathological complete response after nCRT [1, 3, 4]. However, compared with good responders to nCRT, poor responders tend to have higher local recurrence and recurrence-free survival rate [3]. These poor responders may benefit little from nCRT, but still experience toxic effects of treatment such as diarrhea, nausea, hematological infection, and fever [5, 6]. Therefore, pretreatment prediction of the response to nCRT is of great importance for devising appropriate personalized treatment plans.
Magnetic resonance imaging (MRI) is the most important imaging approach for assessment of treatment response in LARC patients [7]. Pretreatment MRI features, such as tumor volume, tumor height, depth of tumor penetration, and absence of extramural venous invasion, are reported to be associated with a better response to therapy [8, 9]. However, visual assessment of MRI features is limited by subjectivities and relies on experiences of radiologists [10]. Thus, pretreatment accurate identification of nCRT poor responders still remains challenge other than by pathologic evaluation after completing neoadjuvant therapy.
Recently, radiomics and deep learning has drawn great attractions due to the superiority of quantifying imaging phenotype that associated with the underlying tumor pathological character from MRI or other imaging modalities beyond visual interpretation [11]. A growing number of studies aimed at prediction of treatment response have supported more effective performance basing on multiparametric MRI [12,13,14,15]. To predict poor responders, Zhou et al. developed a radiomics model based on pretreatment apparent diffusion coefficient (ADC) map, T1 weighted- (T1w), T1 contrast-enhanced (T1c) and T2 weighted (T2w) MRI with an area under the receiver-operating characteristic (ROC) curve (AUC) value of 0.773 [16]. However, all above studies just combined multiparametric MRI in a very simple strategy, which concatenated radiomic features or deep learning features according to different imaging sequences for further feature selection or fully connected network.
The self-attention mechanism has been widely used in various medical imaging analyses such as segmentation [17], classification [18] and survival prediction [19]. Without any explicit supervision, self-attention mechanism can learn to focus on important features via training end-to-end together with the original convolutional neural networks (CNN) backbone. Squeeze-and-Excitation (SE) [20] network is a prominent approach that focuses on channel attention, which natural fit for multi-parametric fusion. Therefore, the present multicenter study aims at investigating whether SE-based channel attention mechanism is better than feature concatenation on the task of treatment response prediction in LARC.
Methods
Patients
This retrospective multicenter study was approved by the Ethics Committee of the Six Affiliated Hospital of Sun Yat-sen University (SAHSYU) and that of the Zhejiang Cancer Hospital (ZCH). The requirement for informed patient consent was waived. This study included eligible patients who were diagnosed with LARC by multiparametric MRI examination and received standard chemoradiotherapy treatment between February 2012 and May 2018, as shown in Fig. 1. Patients who met the following exclusion criteria were removed from the analysis: (i) lack of pathologic treatment response evaluation after treatment; (ii) lack of MRI sequence including T2w, T1c and ADC; (iii) insufficient MRI quality due to bowel peristalsis-related artifacts; (iv) lack of clinical information including sex, age, clinical T stage (cT-stage), clinical N stage (cN-stage) and carcinoembryonic antigen (CEA; cutoff ≥ 5 ng/ml, < 5 ng/ml) blood level.
Neoadjuvant chemoradiotherapy
All enrolled patients underwent preoperative treatment with five cycles of infusional fluorouracil (leucovorin 400 mg/m2 intravenously followed by fluorouracil 400 mg/m2 intravenously and fluorouracil 2.4 g/m2 by 48-h continuous intravenous infusion) and concurrent radiation treatment. Radiotherapy was delivered at 1.8 to 2.0 Gy daily Monday through Friday for a total of 23 to 28 fractions over 5 to 6 weeks and a total dose of 46.0 to 50.4 Gy.
Assessment of response to nCRT
The pathologic treatment response after nCRT was evaluated based on TME resection specimens, according to the four-tier American Joint Committee on Cancer (AJCC) Cancer Staging tumor regression grade (TRG) systems [21]. The four TRG groups were as follows: TRG 0, no residual tumor cells; TRG 1, single tumor cell or small group of tumor cells; TRG 2, residual cancer with desmoplastic response; TRG 3, minimal evidence of tumor response. According to the AJCC TRG systems, patients were separated into 2 groups: poor responders (TRG 2, 3) versus good responders (TRG 0, 1).
MRI Acquisition and Tumor Segmentation
All patients from Six Affiliated Hospital of Sun Yat-sen University underwent T2w, T1c and ADC MRI scans before the nCRT with 1.5 Tesla MRI (Optima MR 360, GE Medical Systems, USA) using an eight-element body array coil with fixed image protocols. All patients from Zhejiang Cancer Hospital underwent T2w, T1c and ADC scans before the nCRT with 3.0 Tesla MRI (Verio 3.0T MR, Siemens Medical Systems, Germany). Parameters for MRI acquisition were shown in supplementary Table S1. Before further analysis, all axial slices were normalized into 1 mm × 1 mm pixel spacing. The region of interest (ROI) was manually delineated around the tumor outline via the itk-SNAP software (www.itksnap.org) on the axial slice with the largest lesion cross-section of T2w images by one gastrointestinal radiologist (reader 1) with 10 years of experience, and was then copied onto the corresponding slice of T1c and ADC images. In addition, the segmentation was examined by another gastrointestinal radiologist (reader 2) with 30 years of experience. If reader 2 found any discrepancies between the segmentation and the actual tumour outline, he provided feedback to reader 1 for consultation. The segmentation was then adjusted in consultation with reader 1. The main concern is to outline the tumour area avoiding gas in the intestinal lumen, fat or other structures around the lesion area, etc. This is done to ensure that the outlined area corresponds to the tumour.
Experimental design
In the present study, two kinds of channel attention fusion approaches were conducted for comparison, which consist of a hand-crafted radiomic features-based fusion and an image-based fusion. Radiomic features were firstly extracted from the ROIs of T2w, T1c and ADC images using PyRadiomics version 2.2.0 [22]. A total of 372 radiomics features were extracted from each imaging sequence in this study, of which 93 were extracted from original images and 279 were extracted from three scale Laplacian of Gaussian (LoG) transformed images. Among the 93 features extracted from original images, 18 were first-order statistics and 75 were textural features originated from Gray Level Cooccurence Matrix (n = 24), Gray Level Run Length Matrix (n = 16), Gray Level Size Zone Matrix (n = 16), Gray Level Dependence Matrix (n = 14) and Neighbouring Gray Tone Differnece Matrix (n = 5). Then ROIs of the three imaging sequences were cropped by an 80 × 80 bounding rectangle as a three-channel input for image-based fusion.
To realize radiomic features-based channel fusion (hereinafter referred to as CFRS.), a simple self-attention module was designed using 2D convolution kernel as shown in Fig. 2. For each patient, n features were extracted from each modality of MRI, generating a feature matrix of size 3 × n as the input for the self-attention module. The self-attention module consists of three convolutional layers. The first convolutional layer has 32 filters of size 3 × 1, followed with 32 filters of size 1 × 1 in the second convolutional layer. The third convolutional layer has one filter of size 1 × 1, followed with fully connected layer consisting of 3 elements activated with sigmoid function. Then the output of the fully connected layer was set as 3 self-attention weighting coefficients of the three channels, resulting a n-dimensional channel-fused radiomic feature. At last, the fused feature was sent to a fully connected layer consisting of 1 element activated with sigmoid function to predict the probability of poor response.
Experimental design of the present study. SAHSYU, Six Affiliated Hospital of Sun Yat-sen University; ZCH, Zhejiang Cancer Hospital; ADC, apparent diffusion coefficient; T1c, T1 contrast-enhanced; T2w, T2 weighted; BN, batch normalization; Conv, convolution; FC, fully- connected; SE, Squeeze-and-Excitation.
To realize image-based channel fusion (hereinafter referred to as CFDL), SE module [20] was used as shown in Fig. 2. Then a shallow CNN model consisting of SE module and 3 convolutional layers followed by 2 fully connected layers was constructed for poor responder prediction. The detailed parameters of this model were also shown in Fig. 2.
The comparative performance tests were conducted for the model with/without channel fusion. Concretely, a traditional radiomics model (hereinafter referred to as RS) was built by ridge regression using the three-sequence concatenated features, and a deep learning model without SE module (hereinafter referred to as DL) using three-sequence concatenated images was also built for comparison. There 70% of patients in SAHSYU were randomly selected for fitting the model parameters, and the rest 30% of patients in SAHSYU were used for internal validation. The loss function of ridge regression can be expressed as blow:
Where \({y}_{i}\) is the label of patient \(i\), \(m\) is the number of patients, \({x}_{i}^{T}\) is the feature vector of the patient\(i\), \(w\) is the coefficient of the feature, and λ is the regularization parameter. To determine the optimal value of the regularization parameter \(\lambda\), we performed 5-fold cross-validation in the training cohort and tested values of \(\lambda \in\)(0.001, 1) with a step size of 0.001 at the same time. The \(\lambda\) value that maximized the average AUC of the cross-validation was selected as the optimal regularization parameter.
Gradient (Grad)-CAM [23], a flexible method can interpret arbitrary layers of a CNN without the need of any architecture modifications, was used to identify classification-relevant CNN features in the present study. Heatmap generated by Grad-CAM was used for individually visualizing the critical discriminating image regions.
Statistics
R software (version 3.6.2) was used for all statistical analyses. All tests were 2-sided, and P values < 0.05 indicated statistical significance. The difference of clinical variables between two groups was compared by using the Mann-Whitney rank-sum test or adopting the chi-square (χ2) test. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and AUC of different models were calculated. Delong test was performed to compare the difference of predictive performance for two arbitrary models. Multivariate logistic regression analysis was performed to assess if the result predicted by each of the four constructed models was an independent predictive factor when considering the other three constructed models and clinical variables such as sex, age, clinical T stage, clinical N stage and CEA blood level.
Results
Patients
A total of 422 patients were finally included in this study. The clinical characteristics of these patients were shown in Table 1. The dataset with a sample size of 193 randomly separated from the SAHSYU was used as the training cohort, and dataset from the ZCH were used as the external validation cohort. The rate of poor responders was 55.1% (147/267) and 72.3% (112/155) in the SAHSYU and ZCH cohorts, respectively. The proportion of poor responders varies a lot (p < 0.001) in the two cohorts. CEA and clinical T stage were also significantly (p < 0.05) different between the two cohorts. Besides clinical T stage in ZCH, the other variables did not show significant difference between poor responders and good responders in each of the two cohorts.
Model construction and validation
The RS model constructed by three-sequence feature concatenation and ridge regression achieved an AUC of 0.773, 0.757 and 0.806 in the training cohort, internal cohort and external cohort, respectively. The coefficients of the RS model were shown in Fig. S1. The CFRS model achieved an AUC of 0.836, 0.812 and 0.832 in the training cohort, internal cohort and external cohort, respectively. However, compared with the RS model, the CFRS model did not show statistically significant improvement (Delong test P: 0.313 [internal validation cohort], 0.372 [external validation cohort]).
The DL model constructed without SE module achieved an AUC of 0.858, 0.835 and 0.837 in the training cohort, internal cohort and external cohort, respectively. The CFDL model achieved an AUC of 0.907, 0.898 and 0.873 in the training cohort, internal cohort and external cohort, respectively. However, compared with the DL model, the CFDL model did not show statistically significant improvement (Delong test P: 0.134 [internal validation cohort], 0.219 [external validation cohort]).
Compared with the RS model, the CFDL model showed a significant better performance in both the training and internal validation cohorts, while that was not significant in the external validation cohort. The distribution of probability values of patients predicted as poor responders was shown in Fig. 3(the left column), the ROC curves of the four models were depicted in Fig. 3(the middle column), and the p-values for Delong test were also recorded in Fig. 3(the right column). The values of sensitivity, specificity, positive PPV, NPV and accuracy were listed in Table 2. We also investigated if the combination of CFDL and CFRS can yield better results. The results showed that the combination of the 2 didn’t perform better. In the internal validation cohort, the combined model achieved better accuracy compared with CFDL (83.9% VS. 77.8%). However, in the external validation cohort, combined model achieved very close accuracy compared with CFDL (85.8% VS. 86.5). The detailed results were shown in the Fig. S2. Multivariate logistic regression analysis indicated that the result predicted by CFDL was the only highly significant (p < 0.05) predictive factor in all three cohorts, as shown in Fig. 4.
The performance of four models in the training, internal validation and external validation cohorts. The distribution of probability values of patients predicted as poor responders (the left column); the receiver operating characteristic curves (the middle column), p-values for Delong test (the right column)
Visualization
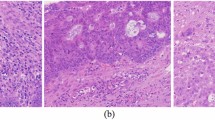
Two typical sample cases analyzed by the CFDL model were visualized in Fig. 5, of which a true poor responder was predicted to be a poor responder with a probability of 69.5% and a true good responder had a lower predicted probability of being a poor responder. The red area of the Grad-CAM heatmap indicated high probability of being poor response. Therefore, the Grad-CAM heatmaps also indicated that the most tumor area of a true poor responder had poor response and only partial area of a good responder had poor response.
Grad-CAM heatmap of the last max pooling layer and the first SE-block of the CFDL model in two typical samples. (a), visualization of a poor responder. (b), visualization of a good responder. The heatmaps in the second row of (a) and (b) belong to max pooling layer, and the heatmaps in the third row of (a) and (b) belong to SE-block.
Discussion
Prediction of response to nCRT in LARC has always been a hotspot of clinical research [11, 24, 25]. In the past decade, radiomics and deep learning have promoted the study of imaging markers for response prediction. This study is an extension of the research field of response prediction in LARC. Two hand-crafted radiomics models and two end-to-end deep learning models were established and validated in an independent external cohort. The results showed both the radiomics and deep learning were promising methods. By comparison, deep learning radiomics performed better than hand-crafted radiomics in the present study.
Recently, Giannini et al. collected pretreatment Positron Emission Tomography and MRI data from 52 LARC patients, and built a radiomics signature combining the two modalities to predict poor responders with an AUC of 0.86. However, this radiomics signature was not validated in another group of patients [26]. Petresc et al. collected pretreatment T2w MRI data from 67 single-center LARC patients, and built a radiomics signature using 44 patients’ MRI and validated this signature using the other 23 patients with an AUC of 0.80 [27]. Shayesteh et al. also conducted a single-center radiomics study with a sample size of 98 and estabished an ensemble learning model to combine four radiomics signature built by four machine learning algorithms. This ensemble learning model achieved an AUC of 0.95 in internal validation dataset [28]. Therefore, this multicenter study was designed to perform both internal and external validation for the developed models. The results showed the four developed models had stable predictive ability in all cohorts.
Many studies have demonstrated that radiomics signature or deep learning radiomics from joint multiparametric MRI performed better than that from the single modality [29,30,31]. Because different imaging sequences reflect different aspects of tumor biology including tumor intensity, cellularity and vascularization, the combination of multiparametric MRI might improve prediction [32]. Li et al. demonstrated their multi-modal radiomics model that combined Computed Tomography and MRI (T1c, T2w and ADC) features was associated with better performance than any individual sequence [33]. Our previous study also confirmed this conclusion, but with limitation that the therapeutic regimens of this cohort were not very consistent [16]. Based on the above study, the data collection criteria of the present study were strictly determined. Multiparametric MRI was used for modeling, and the parameters for MRI acquisition were consistent within each cohort. The results indicated that either radiomics models or deep learning models had good generalization performance, even though the MRI device version and field strength were both different between the two centers.
Multiparametric MRI and attention mechanism opened up the opportunity to fuse the different sequences to further improve the accuracy over current modeling algorithms. Because of attention mechanism having a great feature selection ability, it has been successfully used in image segmentation [17] and classification [18]. SE network, a lean but efficient self-attention model, that adaptively recalibrated channel-wise feature responses by explicitly modeling interdependencies between channels encouraged the aggregation of multi-channel information in the presence of useless or redundant information from multiple channels. Moreover, SE-based channel-attention module can be easily migrated to other models and does not change the original model structure. In view of the aforementioned advantages, the SE-based channel-attention should be appropriate for deep learning model construction using multiparametric MRI for treatment response prediction, but there are few similar studies published. The present study developed two image-based deep learning models that called DL and CFDL, and confirmed the hypothesis that SE-based channel attention mechanism can improve the predictive ability compared with attention-free model. Inspired by image-based SE module, the present study designed a simple radiomics features based self-attention fusion algorithm called CFRS. The results support that attention mechanism applied to radiomics study can also improve the predictive ability compared with feature concatenation based model.
Most radiomics studies always built a radiomic nomogram with both radiomic signature and clinical variables, expecting a more predictive model than single radiomic signature. The present study analyzed five clinical variables in two independent cohorts, and found that none of these clinical variables was statistically significant predictive in univariate analysis. This result came as no surprise, because many previous clinical studies have analyzed these variables but no robust predictive factors have been identified [34,35,36]. In multivariate analysis, there still no significant clinical variable was found, whereas, CFDL was a stable predictive factor. This may indicate biomarker derived from using advanced intelligent image analysis method is promising to be a complementary method for treatment response prediction.
This study had some limitations. The first limitation involved the limited sample size and retrospective data collection. Accordingly, the developed model should be validated in larger well-designed prospective studies, which would also enable the collection of more patient and tumor-specific clinical information for developing a more stable and more accurate model. Second, the ROIs were delineated in one single slice, which might not be representative of the entire tumor. Third, more complex and advanced self-attention methods should be adopted, which may further improve the prediction performance. Fourth, the two cohorts differ in terms of outcome and we cannot exclude the presence of a bias. Fifth, the MRI devices were quite different and a more detailed exploration of the role of the technology in the deep learning radiomics versus hand-crafted radiomics performance would be necessary. Sixth, we did not explore the opposite direction, by switching the two hospitals in building training/validation and testing models or by a joint model, obtained shuffling the two set of MRIs and then splitting a training and a testing set.
In summary, this study demonstrated attention mechanism based multi-sequence fusion method was effective for nCRT response prediction in LARC, and improved prediction performance than hand-crafted radiomics method.
Data Availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- AUC:
-
Area Under the Curve
- CEA:
-
Carcinoembryonic Antigen
- CFDL:
-
Channel Fusion Deep Learning
- CFRS:
-
Channel Fusion Radiomics
- CNN:
-
Convolutional Neural Networks
- DL:
-
Deep Learning
- LARC:
-
Locally Advanced Rectal Cancer
- nCRT:
-
Neoadjuvant Chemoradiotherapy
- ROC:
-
Receiver Operating Characteristic
- ROI:
-
Region of Interest
- RS:
-
Radiomics
- SAHSYU:
-
Six Affiliated Hospital of Sun Yat-sen University
- SE:
-
Squeeze-and-Excitation
- TME:
-
Total Mesorectal Excision
- TRG:
-
Tumor Rregression Grade
- ZCH:
-
Zhejiang Cancer Hospital
References
Benson AB, Venook AP, Al-Hawary MM, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(7):874–901.
Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22–iv40.
Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30(15):1770.
Fokas E, Ströbel P, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy as a prognostic factor and individual-level surrogate for disease-free survival in rectal cancer. J Natl Cancer Inst. 2017;109(12):djx095.
Bosset J-F, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23.
Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the german CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13(7):679–87.
Lambregts DM, Maas M, Boellaard TN, et al. Long-term imaging characteristics of clinical complete responders during watch-and-wait for rectal cancer—an evaluation of over 1500 MRIs. Eur Radiol. 2020;30(1):272–80.
Stanley K, Tait D, Chau I, Brown G. MRI predictive factors for tumor response in rectal cancer following neoadjuvant chemoradiation therapy-implications for induction chemotherapy? Int J Radiat Oncol Biol Phys. 2013;87(3):505–11.
Chang GJ, You YN, Park IJ, et al. Pre-treatment high-resolution rectal MRI and treatment response to neoadjuvant chemoradiation. Dis Colon Rectum. 2012;55(4):371.
Fusco R, Petrillo M, Granata V, et al. Magnetic resonance imaging evaluation in neoadjuvant therapy of locally advanced rectal cancer: a systematic review. Radiol and Oncol. 2017;51(3):252–62.
Bera K, Braman N, Gupta A, Velcheti V, Madabhushi A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat Rev Clin Oncol 2021:1–15.
Nie K, Shi L, Chen Q, et al. Rectal cancer: assessment of neoadjuvant chemoradiation outcome based on radiomics of multiparametric MRI. Clin Cancer Res. 2016;22(21):5256–64.
Liu Z, Zhang X-Y, Shi Y-J, et al. Radiomics analysis for evaluation of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Clin Cancer Res. 2017;23(23):7253–62.
Zhang X-Y, Wang L, Zhu H-T, et al. Predicting rectal cancer response to neoadjuvant chemoradiotherapy using deep learning of diffusion kurtosis MRI. Radiology. 2020;296(1):56–64.
Jin C, Yu H, Ke J, et al. Predicting treatment response from longitudinal images using multi-task deep learning. Nat Commun. 2021;12(1):1–11.
Zhou X, Yi Y, Liu Z, et al. Radiomics-based pretherapeutic prediction of non-response to neoadjuvant therapy in locally advanced rectal cancer. Ann Surg Oncol. 2019;26(6):1676–84.
Schlemper J, Oktay O, Schaap M, et al. Attention gated networks: learning to leverage salient regions in medical images. Med Image Anal. 2019;53:197–207.
Li Z, Yuan L, Xu H, Cheng R, Wen X. Deep multi-instance learning with induced self-attention for medical image classification. 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM): IEEE; 2020. p. 446–450.
Yao J, Zhu X, Jonnagaddala J, Hawkins N, Huang J. Whole slide images based cancer survival prediction using attention guided deep multiple instance learning networks. Med Image Anal. 2020;65:101789.
Hu J, Shen L, Sun G. Squeeze-and-excitation networks. Proceedings of the IEEE conference on computer vision and pattern recognition; 2018. p. 7132–7141.
Trakarnsanga A, Gönen M, Shia J, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. 2014;106(10):dju248.
Van Griethuysen JJ, Fedorov A, Parmar C, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77(21):e104–7.
Selvaraju RR, Cogswell M, Das A, Vedantam R, Parikh D, Batra D. Grad-cam: Visual explanations from deep networks via gradient-based localization. Proceedings of the IEEE international conference on computer vision; 2017. p. 618–626.
Kuremsky JG, Tepper JE, McLeod HL. Biomarkers for response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2009;74(3):673–88.
Jung G, Hernández-Illán E, Moreira L, Balaguer F, Goel A. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol. 2020;17(2):111–30.
Giannini V, Mazzetti S, Bertotto I, et al. Predicting locally advanced rectal cancer response to neoadjuvant therapy with 18F-FDG PET and MRI radiomics features. Eur J Nucl Med Mol Imaging. 2019;46(4):878–88.
Petresc B, Lebovici A, Caraiani C, Feier DS, Graur F, Buruian MM. Pre-treatment T2-WI based radiomics features for prediction of locally advanced rectal cancer non-response to neoadjuvant chemoradiotherapy: a preliminary study. Cancers. 2020;12(7):1894.
Shayesteh SP, Alikhassi A, Farhan F, et al. Prediction of response to neoadjuvant chemoradiotherapy by MRI-based machine learning texture analysis in rectal cancer patients. J Gastrointest Cancer. 2020;51(2):601–9.
Yu J, Zhang Q, Wang M et al. Comprehensive analysis of tumor mutation burden and immune microenvironment in gastric cancer. Biosci Rep 2021;41(2).
Zhang S, Yu M, Chen D, Li P, Tang B, Li J. Role of MRI–based radiomics in locally advanced rectal cancer. Oncol Rep. 2022;47(2):1–10.
Liu Z, Li Z, Qu J, et al. Radiomics of multiparametric MRI for pretreatment prediction of pathologic complete response to neoadjuvant chemotherapy in breast cancer: a multicenter study. Clin Cancer Res. 2019;25(12):3538–47.
Cui Y, Yang X, Shi Z, et al. Radiomics analysis of multiparametric MRI for prediction of pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Eur Radiol. 2019;29(3):1211–20.
Li Z-Y, Wang X-D, Li M, et al. Multi-modal radiomics model to predict treatment response to neoadjuvant chemotherapy for locally advanced rectal cancer. World J Gastroenterol. 2020;26(19):2388.
Das P, Skibber JM, Rodriguez-Bigas MA, et al. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer. 2007;109(9):1750–5.
Fischer J, Eglinton TW, Richards SJ, Frizelle FA. Predicting pathological response to chemoradiotherapy for rectal cancer: a systematic review. Expert Rev Anticancer Ther. 2021;21(5):489–500.
Spolverato G, Pucciarelli S, Bertorelle R, De Rossi A, Nitti D. Predictive factors of the response of rectal cancer to neoadjuvant radiochemotherapy. Cancers. 2011;3(2):2176–94.
Acknowledgements
Supported by National Key Clinical Discipline.
Funding
The work was supported by the grant from the Major Science Research Project of Henan Province (221100310500), the grant from the Henan Province Key Research and Development and Promotion Projects (232102310009), the grant from the National Natural Science Foundation of China (82302298, 82001765 and 82201709), the grant from the Zhejiang Province Medical and Health Science and Technology Project (2022KY084), the Innovative Research Team (in Science and Technology) in University of Henan Province: 24IRTSTHN042, and the Open Project Program of the First Affiliated Hospital of Xinxiang Medical University (No. XZZX2022009).
Author information
Authors and Affiliations
Contributions
WR, YZ and WC conceived and designed this study. Data were collected by YF, GD, PL and LL. Data analysis were performed by XZ and YY. XZ and YY mainly wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This retrospective multicenter study was approved by the Ethics Committee of the Six Affiliated Hospital of Sun Yat-sen University (SAHSYU) and that of the Zhejiang Cancer Hospital (ZCH). The requirement for informed patient consent was waived.
Consent for publication
All authors have approved the manuscript and agree with submission to Radiation Oncology.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, X., Yu, Y., Feng, Y. et al. Attention mechanism based multi-sequence MRI fusion improves prediction of response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Radiat Oncol 18, 175 (2023). https://doi.org/10.1186/s13014-023-02352-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-023-02352-y