Abstract
Background
Neoadjuvant radiotherapy (NRT) for resectable retroperitoneal sarcoma (RPS) has been shown to be systematically feasible. Whether NRT has equivalent or better clinical effects compared to surgery alone for RPS patients remains controversial.
Methods
We performed a systematic literature search of PubMed, Web of Science, Embase, ASCO Abstracts, and Cochrane library databases for studies in humans with defined search terms. Articles were independently assessed by 2 reviewers, and only randomized controlled trials and cohort studies were included. The hazard ratios (HRs) of overall survival (OS), recurrence-free survival (RFS), and local recurrence (LR) were extracted from included studies. Heterogeneity among study-specific HRs was assessed by the Q statistic and I2 statistic. Overall HR was assessed by random-effects or fixed-effects models. Publication bias was tested by Begg’s tests, and the quality of each study was assessed with the Newcastle Ottawa Scale.
Results
A total of 12 eligible studies with 7778 resectable RPS patients were finally included in this study. The pooled analysis revealed the distinct advantages of NRT as compared to surgery alone, including longer OS (HR = 0.81, P < 0.001), longer RFS (HR = 0.58, P = 0.04), and lower LR (HR = 0.70, P = 0.03). No evidence of publication bias was observed.
Conclusion
NRT is likely to be beneficial for resectable RPS patients in terms of OS and RFS. However, more multicenter clinical trials are needed to confirm these findings.
Similar content being viewed by others
Introduction
Retroperitoneal sarcomas (RPS) are rare malignant tumors that occur in the retroperitoneum, accounting for 20% of all soft tissue sarcomas (STS) in adults [1]. They tend to fill the abdominal cavity and encapsulate or invade the surrounding organs when initially diagnosed due to insidious onset and atypical symptoms. Surgery remains the only potentially effective curative approach, and concomitant multi-visceral resection often is performed to achieve better local control and to prolong the overall survival (OS) [2,3,4]. However, the special anatomical structure of the retroperitoneum, heterogeneity of RPS with different biological behavior, and oncological risks according to subtypes render a homogeneous surgical treatment difficult. Currently, neoadjuvant radiotherapy (NRT) has been applied to the treatment of RPS and presents a number of conceptual advantages over traditional surgical treatment. Preoperative radiotherapy is able to define the treatment field with high accuracy, minimizing the toxicity of adjacent structures caused by tumor mass displacement [5, 6], while maximizing R0 resection rates and minimizing the risk of local recurrence (LR) or peritoneal seeding [7, 8]. However, data supporting neoadjuvant radiotherapy in RPS are limited, and justification for its use has been extrapolated from its established role in extremity STS [9, 10]. To date, only one completed trial randomly (EORTC-62092: STRASS) assigned 266 patients, comparing 3D conformal or intensity-modulated radiotherapy (50.4 Gy, in 28 daily fractions of 1.8 Gy) plus surgery with surgery alone [11]. In this trial, patients who received NRT did not have improved recurrence-free survival (RFS) but more frequent grade 3–4 adverse events than control patients. The results of other retrospective studies, including analyses of large national databases, that investigate the role of NRT are not consistent [12,13,14,15,16,17,18,19,20,21,22]. In addition. Chinese consensus guidelines for diagnosis and treatment of primary retroperitoneal soft tissue sarcoma (2019 edition) suggest that local radiotherapy is not recommended for every patient with resectable RPS (level B evidence, level 2 recommendation) [23]. In the absence of a high level of evidence, whether to incorporate NRT into the clinical treatment of RPS has been controversial. To address a gap in knowledge, we aimed to evaluate the impact of NRT on RPS via this meta-analysis.
Methods
Database and bibliography retrieval
PubMed, Web of Science, Embase, ASCO Abstracts, and Cochrane library databases were searched for eligible studies published between January 2000 and January 2020 following the PRISMA statement (preferred reporting items for systematic reviews and meta-analysis). The search terms were: Retroperitoneal neoplasms OR Retroperitoneal sarcomas OR Retroperitoneal soft tissue sarcomas; Neoadjuvant therapy OR Neoadjuvant radiotherapy OR Preoperative radiotherapy; Surgery OR Radiosurgery. In addition, we also identified eligible studies from previous related reviews.
Inclusion and exclusion criteria
Studies were included based on the following criteria: (1) it was a randomized clinical trial (RCT) or comparative study of NRT versus surgery for resectable RPS patients; (2) RPS confirmed by pathological biopsy; (3) at least one of the following information was reported: the hazard ratio (HR) and 95% confidence interval (CI) of LR, RFS, and OS, Kaplan–Meier curve and other valid data to calculate HR. The exclusion criteria included: (1) reviews, letters, editorials, or non-comparative studies; (2) HR and its 95% CI unable to be calculated based on available data; (3) the cases or the groups in the study were fewer than 20 and five respectively; (4) repeated reports by the same institution; (5) Newcastle Ottawa Scale (NOS) less than 6 [24]; (6) non-human studies. Finally, this meta-analysis was conducted by reported outcomes indications from included studies and not individual data.
Data extraction
Two authors (Li and Dong) independently extracted data from eligible literature. Disagreements between authors were resolved by consensus and invited senior scholars to interpret if the differences were still controversial after discussion. The information extracted included: first author, year of publication, patient source, intervention, number of patients, type of study, and outcome. Study quality was evaluated by NOS which includes nine criteria to assess both randomized and non-randomized comparative studies. A study was considered of high quality if it scored 7 points or higher.
Statistical analysis
Meta-analysis was performed by Review Manager version 5.4 (Cochrane Collaboration, London, UK). The heterogeneity was determined by χ2 based on Q statistic and I2 statistic. When heterogeneity is significant (P < 0.05, I2 > 50%), a random effect model was used to merge the pool HR and a sensitivity analysis was performed subsequently. Otherwise, a fixed effect model was used. Publication bias was assessed by StataCorp version 15.1 (College Station, TX 77845, USA) with Begg’s test.
Results
Search results and characteristics of eligible studies
A total of 816 relative references were identified from those databases, of which 285 were from PubMed, 386 were from Web of Science, 133 were from Embase, 8 were from Cochrane library, and the remaining four were from ASCO Abstracts. After selection according to the inclusion/exclusion criteria, 12 studies including one RCT and 11 retrospective cohort studies (RCSs) were eligible for meta-analysis (Fig. 1). Among them, five studies divided participants into the NRT group and surgery group with propensity score-matched (PSM). OS, RFS, and LR were used as outcome indicators in all included studies, and the quality scores with NOS were between seven and eight (see Additional file 1: Table S1). The characteristics of those included studies were shown in Table 1.
Meta-analysis of OS
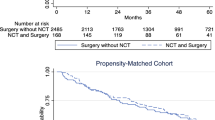
OS was reported in 10 of the 12 included studies and 7130 patients were included. 2081 in the NRT group and 5049 in the surgery group. The fixed-effect model was used (Fig. 2a), and the pooled HR showed that the NRT could significantly improve the OS of RPS compared to the surgery alone (HR = 0.81, P < 0.001). Subsequently, we conducted separate analyses of the studies with and without PSM, and the results revealed that there were no significant differences in the pooled analysis of studies with PSM (NRT vs surgery, HR = 0.82, P < 0.001; Fig. 2b) and without PSM (NRT vs surgery, HR = 0.78, P = 0.03; Fig. 2c).
Overall survival (OS). Forest plot and pooled analysis of hazard ratio for OS of all studies (a), studies with propensity score matched (PSM) (b), and studies without PSM (c). The area of symbols reflects the weight of studies, NRT = neoadjuvant radiotherapy, Sur = surgery, ††† = propensity score matched
Meta-analysis of RFS
The meta-analysis included 5 studies and 574 patients. There was significant statistical heterogeneity in these included studies. The random-effect model was used and a notable statistical difference was found in the RFS between the two groups (NRT vs surgery, HR = 0.58, P = 0.04; Fig. 3a).
Recurrence-free survival (RFS) and local recurrence (LR). Forest plot and pooled analysis of hazard ratio for RFS (a) and LR (b). Forest plot and sensitivity analysis of hazard ratio for RFS (c). The area of symbols reflects the weight of studies, NRT = neoadjuvant radiotherapy, Sur = surgery, ††† = propensity score matched
Meta-analysis of LR
Two eligible studies were included, including 168 patients in the NRT group and 306 patients in the surgery group. No significant statistical heterogeneity was found in the two studies (Fig. 3b). The result showed NRT group had lower LR than the surgery group (NRT vs surgery, HR = 0.70, P = 0.03).
Sensitivity analysis and publication bias
As mentioned above, there was notable heterogeneity in the analysis of RFS, thus we conducted a sensitivity analysis by excluding the study of high heterogeneity (Table 2). We found Bonvalot [11] et al. (2020) was the main source of heterogeneity. After excluding the heterogeneous factor, we used a fixed-effect model to conduct pooled analysis, and the result indicated RFS was also obviously improved in the NRT group (NRT vs surgery, HR = 0.50, P < 0.001; Fig. 3c).
Publication bias was evaluated by Begg’s test, and no significant statistical bias was found in the included studies (see Additional file 1: Fig. S1 and Table S2).
Discussion
High local recurrence rates (> 50%) and low overall survival rates (40%-60%) have been major challenges for clinicians in managing patients with RPS [25,26,27]. Radiotherapy is a potent approach to reduce postoperative recurrence, which has been proven in a variety of malignant tumors. Diamantis et al. [28] reported a systematic review and meta-analysis comparing perioperative radiotherapy with surgery alone demonstrating that both the OS and RFS could be elongated by radiotherapy (OR = 0.69, P = 0.005; OR = 0.19, P < 0.001).
According to the current experience of RPS management, postoperative radiotherapy could cause unwanted injury of sensitive abdominal organs, since those organs, especially the small bowel, easily falls into the space occupied by removed sarcoma mass and are exposed to high doses of irradiation [8]. Thus, preoperative radiotherapy was proposed as an alternative, but there is no clear evidence on whether preoperative radiotherapy could improve the prognostic outcomes of RPS patients.
Previously, we mentioned a randomized study comparing the curative effect of NRT versus surgery alone in patients with RPS [11]. It is the first large, international, randomized trial in primary, localized RPS that has been successfully completed. Although this trial is negative, with similar abdominal RFS (HR = 1.01; P = 0.95) and OS (HR = 1.16; P = 0.65) in both groups at 3 years of follow-up, it shows that key questions in rare cancer can be addressed through multi-institutional collaboration. The randomization offsets selection biases inherent in retrospective series such as the smaller tumors, in more favorable locations, easier to resect, and resected in academic centers. Therefore, this conclusion replaces the heterogeneous approach to NRT for RPS, whereby its use varied considerably based on investigator and institutional biases. In addition, adverse events were assessed in this trial, with more grade 3–4 adverse events in the NRT than surgery alone group (98/127 vs 1/128 for lymphopenia, 15/127 vs 10/128 for anemia, and 15/127 vs 5/128 for hypoalbuminemia), and more serious adverse events in NRT than surgery alone group (30/127 vs 13/128). However, a more prominent limitation in this trial is the controversial definition of abdominal recurrence, which leads to instability of the analysis results. In the post-hoc, exploratory of patients with liposarcoma histology, there was no significant difference in RFS between NRT and surgery alone [well-differentiated liposarcoma (HR = 0.69, 95%CI 0.33–1.46); dedifferentiated liposarcoma (HR = 0.92, 95%CI 0.53–1.61)]. However, in the first and second sensitivity analyses, NRT potential improved RFS compared to surgery alone (HR = 0.64, 95%CI 0.40–1.01; HR = 0.62, 95%CI 0.38–1.02). Therefore, more clinical trials should be performed to evaluate the efficacy of NRT in the treatment of resectable retroperitoneal liposarcoma.
In our meta-analysis, our pooled analysis revealed the distinct advantages of NRT versus surgery alone, including a longer OS, a longer RFS, and a lower LR. However, some limitations to be aware of when the results are considered. First, interventions in the treatment group in some studies were not limited to preoperative radiotherapy, several studies also have involved intraoperative radiation therapy and chemotherapy (Details are shown in Table 1). In order to minimize these confounding factors, we extracted the HR of NRT from the multivariate COX regression analysis (Details are shown in Additional file 1: Table S3). Second, strictly control of patient selection bias was difficult to achieve in the comparison of OS and RFS in all included studies [one RCT (8.3%) and eleven RCSs (91.7%)] due to inconsistencies in radiation dose, surgical margin, tumor grade, and pathological subtype of patients. Therefore, these inconsistent variables should be fully considered in optimal study design, the unification of radiotherapy dose and surgical margins is the prerequisite for contrasting the efficacy of NRT versus surgery for RPS patients, and directly determines the reliability of the pooled results. Investigators should at least acquire sufficient data for further analysis of pathological subtypes. Particularly for liposarcomas (well-differentiated liposarcoma and dedifferentiated liposarcoma) and leiomyosarcomas, as they are major components of RPS and exhibit significant biological heterogeneity. Although the previous RCT reported that NRT could not effectively improve the RFS of LMS compared with surgery alone (HR = 1.35, 95%CI: 0.55–3.32), the sample size in the study is too small to be convincing. (NRT vs Sur = 16 vs 22). Besides, it is worth noting that five studies (41.7%), including one RCT, used PSM, reducing data selection bias and decreasing the effect of confounding factors to some extent [11, 15, 17,18,19], and the pooled results of these PSM studies were consistent with those from non-PSM studies. Finally, there were only two studies in the subgroup analysis of LR. Although no major heterogeneity was found in this pooled analysis, it is still not sufficient to explain that NRT improves LR in RPS patients. Further subgroup analyses of RFS and OS by RPS subtype were also not performed due to a lack of data. It is noteworthy that several completed and ongoing studies could help refine which liposarcoma subtypes might benefit from radiotherapy, and we summarized the details in Table 3.
Some scholars do not support the use of NRT for RPS due to concerns regarding delayed surgery and RT-associated toxicity. A recent study showed that no difference was found in LR and OS associated with the timing of surgical resection after EBRT [29], which indirectly supported the conduct of the RCT to compare NRT with surgery alone in RPS. We believed that more RCTs should be conducted in the future to provide more clear evidence for the efficiency of NRT on RPS management.
Conclusion
Based on the above results, we believe that NRT is likely to be beneficial for resectable RPS patients in terms of OS and RFS. However, more multicenter clinical trials are needed to confirm these findings.
Availability of data and materials
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Abbreviations
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- LR:
-
Local recurrence
- NRT:
-
Neoadjuvant radiotherapy
- NOS:
-
Newcastle Ottawa scale
- OS:
-
Overall survival
- PRISMA:
-
Preferred reporting items for systematic reviews and mate-analysis
- PSM:
-
Propensity score-matched
- RCS:
-
Retrospective cohort study
- RCT:
-
Randomized clinical trial
- RESAR:
-
Retroperitoneal sarcoma register
- RFS:
-
Recurrence-free survival
- RPS:
-
Retroperitoneal sarcoma
- STS:
-
Soft tissue sarcoma
References
Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–45.
Gronchi A, Colombo C, Raut CP. Surgical management of localized soft tissue tumors. Cancer. 2014;120(17):2638–48.
Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann Surg. 2016;263(5):1002–9.
Pasquali S, Vohra R, Tsimopoulou I, Vijayan D, Gourevitch D, Desai A. outcomes following extended surgery for retroperitoneal sarcomas: results from a UK Referral Centre. Ann Surg Oncol. 2015;22(11):3550–6.
Caudle AS, Tepper JE, Calvo BF, Meyers MO, Goyal LK, Cance WG, et al. Complications associated with neoadjuvant radiotherapy in the multidisciplinary treatment of retroperitoneal sarcomas. Ann Surg Oncol. 2007;14(2):577–82.
Tzeng CW, Fiveash JB, Popple RA, Arnoletti JP, Russo SM, Urist MM, et al. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer. 2006;107(2):371–9.
Pawlik TM, Ahuja N, Herman JM. The role of radiation in retroperitoneal sarcomas: a surgical perspective. Curr Opin Oncol. 2007;19(4):359–66.
Pawlik TM, Pisters PW, Mikula L, Feig BW, Hunt KK, Cormier JN, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. 2006;13(4):508–17.
Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol Off J Am Soc Clin Oncol. 1998;16(1):197–203.
Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol Off J Am Soc Clin Oncol. 1996;14(3):859–68.
Bonvalot S, Gronchi A, Le Péchoux C, Swallow CJ, Strauss D, Meeus P, et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(10):1366–77.
Turner BT, Hampton L, Schiller D, Mack LA, Robertson-More C, Li H, et al. Neoadjuvant radiotherapy followed by surgery compared with surgery alone in the treatment of retroperitoneal sarcoma: a population-based comparison. Curr Oncol (Toronto, Ont). 2019;26(6):e766–72.
Bonvalot S, Rivoire M, Castaing M, Stoeckle E, Le Cesne A, Blay JY, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(1):31–7.
Bremjit PJ, Jones RL, Chai X, Kane G, Rodler ET, Loggers ET, et al. A contemporary large single-institution evaluation of resected retroperitoneal sarcoma. Ann Surg Oncol. 2014;21(7):2150–8.
Chouliaras K, Senehi R, Ethun CG, Poultsides G, Grignol V, Clarke CN, et al. Role of radiation therapy for retroperitoneal sarcomas: an eight-institution study from the US Sarcoma Collaborative. J Surg Oncol. 2019;120(7):1227–34.
Kelly KJ, Yoon SS, Kuk D, Qin LX, Dukleska K, Chang KK, et al. Comparison of perioperative radiation therapy and surgery versus surgery alone in 204 patients with primary retroperitoneal sarcoma: a retrospective 2-institution study. Ann Surg. 2015;262(1):156–62.
Ecker BL, Peters MG, McMillan MT, Sinnamon AJ, Zhang PJ, Fraker DL, et al. Preoperative radiotherapy in the management of retroperitoneal liposarcoma. Br J Surg. 2016;103(13):1839–46.
Nussbaum DP, Rushing CN, Lane WO, Cardona DM, Kirsch DG, Peterson BL, et al. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol. 2016;17(7):966–75.
Ma SJ, Oladeru OT, Farrugia MK, Shekher R, Iovoli AJ, Singh AK. Evaluation of preoperative chemotherapy or radiation and overall survival in patients with nonmetastatic, Resectable Retroperitoneal Sarcoma. JAMA Netw Open. 2020;3(11): e2025529.
Snow HA, Hitchen TX, Head J, Herschtal A, Bae S, Chander S, et al. Treatment of patients with primary retroperitoneal sarcoma: predictors of outcome from an Australian specialist sarcoma centre. ANZ J Surg. 2018;88(11):1151–7.
Berger NG, Silva JP, Mogal H, Clarke CN, Bedi M, Charlson J, et al. Overall survival after resection of retroperitoneal sarcoma at academic cancer centers versus community cancer centers: an analysis of the National Cancer Data Base. Surgery. 2018;163(2):318–23.
Lane WO, Cramer CK, Nussbaum DP, Speicher PJ, Gulack BC, Czito BG, et al. Analysis of perioperative radiation therapy in the surgical treatment of primary and recurrent retroperitoneal sarcoma. J Surg Oncol. 2015;112(4):352–8.
Chinese Medical Association, Cancer Society of Chinese Medical Association, Journal of Chinese Medical Association, Anorectal Physicians Branch of Chinese Medical Association, & Professional Committee on Retroperitoneal and Pelvic Floor Diseases, Chinese Research Hospital Association. Zhonghua zhong liu za zhi [Chinese journal of oncology], 2019;41(10):728–733.
Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Anaya DA, Lev DC, Pollock RE. The role of surgical margin status in retroperitoneal sarcoma. J Surg Oncol. 2008;98(8):607–10.
Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260(3):416–22.
Trans-Atlantic RPS Working Group. Management of primary retroperitoneal sarcoma (RPS) in the adult: a consensus approach from the Trans-Atlantic RPS Working Group. Ann Surg Oncol. 2015;22(1):256–63.
Diamantis A, Baloyiannis I, Magouliotis DE, Tolia M, Symeonidis D, Bompou E, et al. Perioperative radiotherapy versus surgery alone for retroperitoneal sarcomas: a systematic review and meta-analysis. Radiol Oncol. 2020;54(1):14–21.
Louie RJ, Wang K, Royce TJ, Beaty BT, Esther RJ, Tepper JE, et al. Does timing matter? surgical outcomes in high-grade sarcomas after neoadjuvant radiation therapy. J Surg Res. 2020;254:118–24.
Acknowledgements
Not applicable
Funding
This study was funded by Peking University International Hospital Research Grant (YN2019QN11) and Development Center for Medical Science & Technology, National Health Commission of the People’s Republic of China (WA2020RW29).
Author information
Authors and Affiliations
Contributions
XL: Study design, literature research, data analysis, manuscript preparation. RD: Study concepts, literature research, statistical analysis, manuscript preparation. MX: literature research and data analysis. LM: Study concepts and statistical analysis. CL: guarantor of integrity of the entire study, study concepts and design, and manuscript preparation. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have contributed to the creation of this manuscript for important intellectual content and read and approved the final manuscript.
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The quality of enrolled studies. Table S2. Begg’s test for publication bias. Table S3. Univariate/Multivariate Cox proportional HR for outcomes in patients (NRT vs surgery). Fig. S1. Begg’s test for overall survival (OS) of all included studies (a), studies with propensity score matched (PSM) (b), and studies without PSM (c). Begg’s test for recurrence-free survival (RFS) in all included studies (d).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, X., Dong, R., Xiao, M. et al. Neoadjuvant radiotherapy for resectable retroperitoneal sarcoma: a meta-analysis. Radiat Oncol 17, 215 (2022). https://doi.org/10.1186/s13014-022-02159-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-022-02159-3