Abstract
Purpose
Pure mucinous breast cancer is a rare subtype of invasive breast cancer with favorable prognosis, in which the effect of postoperative radiotherapy remains unclear. We aimed to investigate the prognostic value of postoperative radiotherapy in women with localized pure mucinous breast cancer after lumpectomy.
Methods
We conducted a retrospective cohort study to compare the effectiveness of postoperative radiotherapy (RT) and omitting postoperative radiotherapy (non-RT) in patients with first primary T1-2N0M0 (T ≤ 3 cm) pure mucinous breast cancer who underwent lumpectomy between 1998 and 2015 using the Surveillance, Epidemiology, and End Results (SEER) database. Breast cancer-specific survival (BCSS) was compared between RT and non-RT groups using Kaplan–Meier method and Cox proportional hazards regression model. Propensity score matching (PSM) was carried out to balance cohort baselines. In addition, an exploratory analysis was performed to verify the effectiveness of RT in subgroup patients.
Results
Of 7832 eligible patients, 5352 (68.3%) underwent lumpectomy with postoperative RT, 2480 (31.7%) received lumpectomy without postoperative RT. The median follow-up duration was 92 months. The median age was 66 years in the RT group and 76 years in the non-RT group.The 15-year BCSS was 94.39% (95% CI, 93.08% to 95.35%) in the RT group versus 91.45%(95% CI, 88.93% to 93.42%) in the non-RT group (P < 0.001). The adjusted hazard ratio for BCSS was 0.64 (95% CI, 0.49 to 0.83; P = 0.001) for RT group versus non-RT group. After propensity score matching, similar results were yielded. Adjuvant RT reduced the 15-year risk of breast cancer death from 7.92% to 6.15% (P = 0.039). The adjusted hazard ratio for BCSS were 0.66 (95%CI, 0.47 to 0.92; P = 0.014) for RT group versus non-RT group. The benefit of RT was well consistent across subgroup patients.
Conclusion
Among women with T1-2N0M0 (tumor size ≤ 3 cm) pure mucinous breast cancer, the addition of RT after lumpectomy was significantly associated with a reduced incidence of breast cancer death compared with non-RT, and the magnitude of benefit may be modest. This suggests that postoperative RT is recommended in the treatment of localized pure mucinous breast cancer.
Similar content being viewed by others
Introduction
Mucinous breast carcinoma accounting for approximately 1 to 6% of all breast cancer is divided into two pathological subtypes: pure mucinous breast cancer and mix mucinous breast carcinoma [1]. Pure mucinous breast cancer exclusively consists of tumor tissue with extracellular mucin production over 90%, whereas mix mucinous breast cancer usually mixes infiltrating ductal epithelial component with mucinous areas covering from 50 to 90% [2]. The comparisons of biological features and clinical prognosis have been identified previously among pure mucinous breast cancer, mix mucinous breast carcinoma and invasive breast cancer of no special type [1, 3,4,5,6,7,8,9,10,11,12,13]. Pure mucinous breast cancer usually occurs in elderly patients, especially in postmenopausal women [8]. The tumor size of pure mucinous breast cancer ranges from less than 1 cm to more than 20 cm, with an average of 3 cm [14]. On account of fewer genetic mutations, pure mucinous breast cancer has a stabilized luminal A phenotype with higher expression of hormone receptor and a lower rate of positive human epidermal growth factors 2 (HER-2) [5, 15, 16]. A mechanical barrier made of abundant pools of extracellular mucus around cellular island restricts carcinoma cell invasion, leading to less axillary lymph node or distant metastases. Axillary node involvement, although rare, appears to be the worst prognostic factor followed by tumor size, age, progesterone receptor(PR), HER-2 status and nuclear grade [3, 17,18,19,20]. It has reported that the 5-year, 10-year disease-free survival (DFS) were up to 94% [1], 92% [9] for patients with node-negative pure mucinous breast cancer, respectively. Hence, pure mucinous breast cancer presents distinct clinicopathological characteristics with especially favorable prognosis.
At present, the recommendations of locoregional treatment for patients with operable pure mucinous breast cancer from the latest National Comprehensive Cancer Network are the same as that for patients with typical breast cancer [21]. However, it is difficult to evaluate the effect of local regional treatment on survival outcome in prospective cohort studies or randomized trials owing to the relatively low incidence rate and a limited follow-up prognosis of pure mucinous breast cancer. Guidelines on radiotherapy of pure mucinous breast cancer are extrapolated from evidence based on other common invasive breast cancer. Although scholars have done some retrospective studies, the effect of postoperative radiotherapy in patiens with pure mucinous breast cancer is uncertain so far. Previous study showed that adjuvant radiotherapy was an independent protective factor for both overall survival (OS) and BCSS in patients with pure mucinous breast cancer. However, this retrospective study was hetetogeneous in nature because inclusion criteria involved in advanced patients, and the cohort included mastectomy and lumpectomy [22]. A recent SEER research presented that postoperative radiotherapy following lumpectomy improved the 10-year BCSS rates from 94.5 to 97.6% in patients aged ≥ 65 years diagnosed with T1–2N0 and hormone receptor-positive pure mucinous breast cancer. Yet regrettably, patients aged < 65 years were not included in this study. Besides, patients with tumor size larger than 3 cm were more likely to receive endocrine therapy, which may confuse results [23]. Obviously, it is necessary to adequately assess individualized roles of postoperative RT in this special subtype of breast carcinoma. Hence, we proceeded to a large population-based study using SEER to investigate the effect of postoperative RT on BCSS in women undergoing lumpectomy with T1-2N0M0 (tumor size ≤ 3 cm) stage pure mucinous breast cancer.
Methods
Patients population
This retrospective study was performed utilizing SEER database (November 2018 submission) which released cancer data from 18 registries of national cancer institute and covered approximately 28% of the US population [24]. A case-listing session was derived from SEER*Stat version 8.3.5.
We selected all female cases of histological diagnosed first primary pure mucinous breast cancer with the International Classification of Diseases for Oncology, 3rd Revision (ICD-O-3) code 8480/3 from January 1998 through December 2015. Patients with T1-2N0M0 (tumor size less than 3 cm) stage were eligible. And patients were required to receive lumpectomy with or without postoperative RT. The exclusion criteria were listed as follows: diagnosed from death certificate or autopsy only; no active or complete follow-up data; died at the start of the follow-up; unknown T, N, M stage; with nodal positive disease or metastases disease at diagnosis; without operation or unknown surgery; without RT or unknown RT; non-postoperative radiotherapy; bilateral cancer or unknown laterality; unknown tumor size. The flowchart for patient selection was shown in Fig. 1.
Study covariates
According to administrations of lumpectomy and postoperative RT, a total of 7832 eligible patients under the inclusion criteria were stratified into RT group and non-RT group. We subsequently reviewed variable information of each case on patient baseline demographics, such as age at diagnoses, year of diagnoses, race, marital status at diagnoses. Then, tumor clinicopathological characteristics, including tumor laterality, tumor grade, T stage, tumor size, estrogen receptor (ER) and PR status, were extracted. Among them, the T stage was adjusted by the 6th American Joint Committee on Cancer (AJCC) TNM Staging System. Tumor grade was categorized into four levels on the biasis of the degree of differentiation: grade I, well differentiated; grade II, moderately differentiated; grade III, poorly differentiated; grade IV, undifferentiated or anaplastic. Borderline ER/PR status defined as having 1–10% positivity by immunohistochemistry were merged into positive ER/PR status [25, 26]. In this study, we did not evaluate HER-2 status because of lacking data before 2010.
Statistical analysis
Categoric variables were compared across treatment groups using the Pearson chi-squared test, and continuous variables were analyzed by two independent sample t-tests or Wilcoxon rank sum test. The primary endpoint of this study was BCSS. BCSS was defined as an interval from the data of pure mucinous breast cancer diagnosis to death as a result of breast cancer. Using Kaplan–Meier survival analysis, BCSS were estimated with log-rank tests in unmatched groups and matched groups. Hazard Ratio (HR) and 95% confidence interval (95%CI) were calculated by Cox proportional hazards model to estimate the effect of RT. The multivariable Cox proportional hazards regression analysis incorporated variables that were significant or approximately significant in univariate analyses. The proportional-hazards assumption was checked based on Schoenfeld residuals after fitting a Cox model. And all of the Cox models obeyed the proportional risk hypothesis. PSM was used to control confounding bias in the retrospective study. Propensity scores of being receipt of RT were calculated by using a multivariable logistic regression model. The independent variables are being those that were statistically significant for correlation with treatment modality. Patients treated with RT were matched 1:1 to patients managed without RT on propensity scores by using nearest neighbor matching algorithm. The threshold value of Caliper matching was set to 0.2. A standardized difference of less than 0.1 was considered an indifferent imbalance between comparison groups. Further, exploratory analysis and tests of interaction were undertaken to evaluate the effect of adjuvant RT among subgroups according to patient and tumor characteristics.
Statistical analyses were performed with SPSS, version 24.0 (SPSS Inc., Chicago, IL, USA) and STATA, version 15 (Stata Corp., College Station, TX, USA). Two-tailed P < 0.05 was considered statistically significant.
Results
Patient demographics and tumor characteristics
Comparisons of patient demographics and tumor characteristics between RT and non-RT group were summarized in Table 1. A total of 7832 eligible patients with pure mucinous breast cancer were identified in the cohort (mean [SD] age, 67.1 [13.3] years), of whom 5352 (68.3%) received lumpectomy and postoperative RT, 2480(31.7%) were treated with lumpectomy without RT. Among patients underwent lumpectomy, those who received RT were on average 9 years younger than those who did not (P < 0.001). The median age (interquartile range) was 66 years (56–74) in RT group and 76 years (65–83) in non-RT group. The main pathological feature of patients was hormone receptor positive (91.9%) and well differentiated (55.5%). The minority of patients received chemotherapy(7.8%). There was no significant difference between treatment groups in tumor size (P = 0.433). There was little change in the utilization of postoperative RT through the period between 1998 and 2015(Fig. 2). In order to eliminate the imbalance between groups that may affect results, PSM was subsequently conducted. After PSM between the RT group and non-RT group, 2149 pairs were generated. The distribution of covariates was well balanced between propensity-matched groups (Table 2).
Survival analyses of BCSS
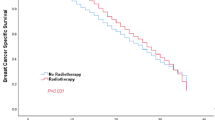
Overall, the median follow-up time was 92 months (interquartile range, 48 to142 months), and 239 breast cancer-special deaths were observed. The Kaplan–Meier survival estimate showed that 5-year, 10-year, 15-year BCSS rates were 99.01% (95% CI, 98.68% to 99.26%), 96.95% (95% CI, 96.29% to 97.49%), 94.39% (95% CI, 93.08% to 95.35%) for patients treated with RT respectively, whereas the corresponding were 97.38% (95% CI, 96.57–98.01%), 94.50% (95% CI, 93.08–95.64%), 91.45%(95% CI, 88.93–93.42%) for non-RT respectively. The difference between RT and non-RT curve was statistically significant (log-rank test, P < 0.001; Fig. 3A). The univariate Cox proportional hazards regression model showed the HR of BCSS for RT versus non-RT was 0.51 (95% CI, 0.39–0.66; P < 0.001). For the purpose of controlling the potential confounding factors in adjuvant RT effectiveness, the multivariable Cox proportional hazards regression analysis was further applied. After the prognostic analysis was adjusted for the following clinicopathological parameters: tumor size, tumor grade, PR status, age at diagnosis, race and married status, we observed postoperative RT was independently associated with better BCSS benefit (adjusted HR, 0.64; 95%CI, 0.49–0.83; P = 0.001). Moreover, the results also indicated that tumor size, age ≥ 70 years, negative PR expression and DSW (divorced, separated, widowed) marital status were risk predictors which independently associated with BCSS(Table 3).
In the propensity-matched cohort, the survival analysis of BCSS also showed a significant difference between the two groups (log-rank test, P = 0.039; Fig. 3B). The BCSS rate for RT group was marginally better than non-RT group. The 5-year BCSS was 98.85% (95%CI, 98.24–99.25%)in RT group and 94.93% (95%CI, 93.46–96.08%) in non-RT group. The 10-year BCSS was 95.96% (95%CI, 94.66–96.95%) in RT group and 94.93% (95%CI, 0.93.46–96.08%) in non-RT group.The 15-year BCSS rate was 93.82% (95%CI, 91.75–95.38%) in RT group and 92.02% (95%CI, 89.39–94.03%) in non-RT group. The univariate analyses also confirmed that the RT group indicated a significantly favorable prognosis (HR, 0.71; 95%CI, 0.51–0.98; P = 0.041; Table 4). After adjusted age, race, marital status and tumor size, the result of multivariable Cox analysis did not change substantially (adjusted HR, 0.66; 95%CI, 0.47–0.92; P = 0.014; Table 4).
The salutary effect of adjuvant RT on BCSS was further assessed in different subgroups among the matched population who underwent lumpectomy, and the HR interactions were tested (Fig. 4). The benefit of RT seemed to be significant in some patients. The HR was 0.64 (95%CI, 0.43–0.95) for patients aged 70 years and older, 0.44 (95%CI, 0.24–0.81) for married women, 0.44 (95%CI, 0.27–0.71) for patients with 1.1–2.0 cm tumor size, 0.63 (95%CI, 0.44–0.91) for patients with positive ER disease, 0.60 (95%CI, 0.40–0.90) for patients with positive PR tumor, 0.31 (95%CI, 0.10–0.96) for patients diagnosed during 2010–2015. However, as we can see from the Fig. 4, there were no statistically significance in global test for interaction (P > 0.05).
Discussion
Among women with early-stage breast cancer receiving lumpectomy, the addition of RT is a standardized treatment based not only on its benefit in reducing ipsilateral breast cancer recurrence, but also on its ability to significantly improve BCSS [27, 28]. In this large population-based study, by using matched approach among patients who received lumpectomy with T1-2N0M0 (T ≤ 3 cm) pure mucinous breast cancer, our result clearly indicated that adjuvant irradiation following lumpectomy was significantly associated with BCSS benefit. The cumulative 15-year BCSS rate was 94.39% for women with pure mucinous breast cancer received adjuvant RT after lumpectomy, and 91.45% for patients treated with lumpectomy alone (HR = 0.51; 95% CI, 0.39–0.66; P < 0.001; Table 3). After adjustment for potential confounding factors, it was translated that the relative reduction of breast cancer-special death was 34%, and the absolute risk reduction at 15 years was 1.8%. In addition, heterogeneity tests of the interaction term were not significant among the matched population, suggesting that the protective prognostic value of adjuvant RT were consistent among different populations.
Our research has several potential strengths. To our best knowledge, this is a large cohort used to evaluate the effect on postoperative RT following lumpectomy among patients with early-stage pure mucinous breast cancer. Our study only aims to patient with tumor size less than 3 cm, which minimizes the impact of endocrine therapy on results. Propensity score matching was generated to reduce the confounding factors, leading to the baseline was comparable between treatment groups. In addition, the heterogeneity of RT effect was tested in subgroup interaction, which further verified the benefit of BCSS was attributable to radiotherapy rather than a baseline imbalance in clinicopathologic features.
Only a few studies have assessed the role of postoperative RT in this special type of breast cancer. Histological types of breast cancer, as prognostic risk factors, have rarely been evaluated in randomized trials related to radiation therapy [29]. Single-center experiences did not demonstrate that adjuvant RT improve recurrence free survival among patient with pure mucinous breast cancer [11]. In several Single-center retrospective studies, they were also failed to show that receiving adjuvant RT could improve the OS or DFS in pure mucinous breast cancer [1, 3, 6, 11, 19, 30, 31]. These negative results may be related to small sample sizes and limited follow-up periods in retrospective studies.A previous SEER analysis including 11,422 patients with pure mucinous breast cancer between 1973 and 2002, with a mean follow-up period of 84 months, showed that the addition of radiotherapy was not significantly asscosiated with prognosis using multivariable Cox regression analysis [3]. On the contrast, another SEER database study, including 8048 I-IV stage pure mucinous breast cancer from 2004 to 2014, found that radiotherapy was an independent factor for both OS and BCSS [22]. The opposing results may be related to the rapid development of radiotherapy and breast conserving surgery in the 1990s. A recent SEER research presented that radiotherapy following lumpectomy improved BCSS in pure mucious breast cancer patients aged ≥ 65 years diagnosed with T1–2N0 [23].Here, we assessed BCSS benefit of adjuvant RT following lumpectomy compared with lumpectomy alone in T1-2N0M0(tumor size ≤ 3 cm) pure mucinous breast cancer by using propensity score matching method and multivariable Cox regression analysis. Combined with the above, we believe that adjuvant RT is a value option for patients underwent lumpectomy with pure mucinous breast cancer, even in those with low-risk factors.
In the cohort, the risk prediction stratified score basing on clinical features and molecular biomarkers is low among patients with pure mucinous breast cancer, which might explain why absolute reductions in 15-year risk of breast cancer death tend to be modest. Besides, we believe patterns of intrinsic tumorigenesis of pure mucinous breast cancer may contribute to the result. This special type of breast cancer is distinct from other ER-positive/HER2-negative form of breast cance in terms of the tumorigenicity of mutated genes, suggesting that the genomic profiling of unusual variants of breast cancer should be taken into account in developing suitable personalized management for patients [5]. The PIK3CA mutation rate is 30–40% in ER-positive invasive ductal carcinoma and 7% in pure mucious breast cancer. The p53 mutation rate is 20% in ER positive invasive ductal carcinoma, but only within 5% in pure mucinous breast cancer. The probability of 1q gains and 16q losses is 10% in pure mucinous breast caner, which is 50% lower than that of ER-positive invasive ductal carcinoma [16]. In addition, pure mucious breast cancer had a relatively lower percentage of high 21-gene recurrence score patients than the infiltrating ductal carcinoma [32]]. In the future, for those with specific types of breast cancer, it is required to further study the prediction of clinical benefit from radiation therapy, and the identification of low-risk patients in whom radiation can be safely omitted.
Nevertheless, we must acknowledge several limitations of this study. There are inherent biases in retrospective study inevitably. The SEER database at present cannot provide the code on surgical margins, lymphovascular invasion, Ki-67 and hormone therapy. Data are missing in some cases for fundamental variables such as tumor size, grade, TNM stage, hormone receptor status. Fortunately, missing data in TNM stage and tumor size less than 5% of the total data. The radiotherapy treatment was not assigned at random. Although the propensity score matching method is efficient for reducing the confounding bias, a significant proportion of samples are censored in the paired matching process.
Conclusion
In patients with localized pure mucinous breast cancer receiving lumpectomy, our results indicated that the management with adjuvant RT slightly improved BCSS compared with its omission. The adjvuant radiotherapy is an appropriate therapeutic option for patients received lumpectomy with localized pure mucinous breast cancer.
Abbreviations
- RT:
-
Radiotherapy
- SEER:
-
The surveillance, epidemiology, and end results database
- PSM:
-
Propensity score matching
- BCSS:
-
Breast cancer-specific survival
- PR:
-
Progesterone receptor
- ER:
-
Estrogen receptor
- HER-2:
-
Human epidermal growth factors 2
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- AJCC:
-
American joint committee on cancer
- HR:
-
Hazard ratio
- 95%CI:
-
95% Confidence interval
References
Diab SG, Clark GM, Osborne CK, Libby A, Allred DC, Elledge RM. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol. 1999;17(5):1442–8. https://doi.org/10.1200/jco.1999.17.5.1442.
The world Health Organization Histological Typing of Breast Tumors--Second Edition. The World Organization. Am J Clin Pathol. 1982;78(6):806–16. https://doi.org/10.1093/ajcp/78.6.806.
Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008;111(3):541–7. https://doi.org/10.1007/s10549-007-9809-z.
Lei L, Yu X, Chen B, Chen Z, Wang X. Clinicopathological characteristics of mucinous breast cancer: a retrospective analysis of a 10-year study. PLoS ONE. 2016. https://doi.org/10.1371/journal.pone.0155132.
Lacroix-Triki M, Suarez PH, MacKay A, Lambros MB, Natrajan R, Savage K, et al. Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. The J pathol. 2010;222(3):282–98. https://doi.org/10.1002/path.2763.
Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011;14(4):308–13. https://doi.org/10.4048/jbc.2011.14.4.308.
Thurman SA, Schnitt SJ, Connolly JL, Gelman R, Silver B, Harris JR, et al. Outcome after breast-conserving therapy for patients with stage I or II mucinous, medullary, or tubular breast carcinoma. Int J Radiat Oncol, Biol, Phys. 2004;59(1):152–9. https://doi.org/10.1016/j.ijrobp.2003.10.029.
Anderson WF, Pfeiffer RM, Dores GM, Sherman ME. Comparison of age distribution patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol, Biomark & Prevent : A Publ Am Assocr Cancer Res, Cospons Am Soc Prevent Oncol. 2006;15(10):1899–905. https://doi.org/10.1158/1055-9965.epi-06-0191.
Skotnicki P, Sas-Korczynska B, Strzepek L, Jakubowicz J, Blecharz P, Reinfuss M, et al. Pure and mixed mucinous carcinoma of t: a comparison of clinical outcomes and treatment resultshe breast. Breast J. 2016;22(5):529–34. https://doi.org/10.1111/tbj.12621.PubMedPMID:WOS:000383520200006.
Wasif N, McCullough AE, Gray RJ, Pockaj BA. Influence of uncommon histology on breast conservation therapy for breast cancer-biology dictates technique? J Surg Oncol. 2012;105(6):586–90. https://doi.org/10.1002/jso.22132.PubMedPMID:WOS:000302550200012.
Cao AY, He M, Liu ZB, Di GH, Wu J, Lu JS, et al. Outcome of pure mucinous breast carcinoma compared to infiltrating ductal carcinoma: a population-based study from China. Ann Surg Oncol. 2012;19(9):3019–27. https://doi.org/10.1245/s10434-012-2322-6.
Louwman MW, Vriezen M, van Beek MW, Nolthenius-Puylaert MC, van der Sangen MJ, Roumen RM, et al. Uncommon breast tumors in perspective: incidence, treatment and survival in the Netherlands. Int J Cancer. 2007;121(1):127–35. https://doi.org/10.1002/ijc.22625.
Fu J, Wu L, Jiang M, Li D, Jiang T, Hong Z, et al. Clinical nomogram for predicting survival outcomes in early mucinous breast cancer. PLoS ONE. 2016. https://doi.org/10.1371/journal.pone.0164921.
Ishikawa T, Hamaguchi Y, Ichikawa Y, Shimura M, Kawano N, Nakatani Y, et al. Locally advanced mucinous carcinoma of the breast with sudden growth acceleration: a case report. Jp J Clin Oncol. 2002;32(2):64–7. https://doi.org/10.1093/jjco/hyf012.
Fujii H, Anbazhagan R, Bornman DM, Garrett ES, Perlman E, Gabrielson E. Mucinous cancers have fewer genomic alterations than more common classes of breast cancer. Breast Cancer Res Treat. 2002;76(3):255–60. https://doi.org/10.1023/a:1020808020873.PubMedPMID:WOS:000178929800008.
Pareja F, Lee JY, Brown DN, Piscuoglio S, Gularte-Merida R, Selenica P, et al. The genomic landscape of mucinous breast cancer. J Natl Cancer Inst. 2019. https://doi.org/10.1093/jnci/djy216.PubMedPMID:MEDLINE:30649385.
Rasmussen BB, Rose C, Christensen IB. Prognostic factors in primary mucinous breast carcinoma. Am J Clin Pathol. 1987;87(2):155–60. https://doi.org/10.1093/ajcp/87.2.155.
Avisar E, Khan MA, Axelrod D, Oza K. Pure mucinous carcinoma of the breast: a clinicopathologic correlation study. Ann Surg Oncol. 1998;5(5):447–51 (Epub 1998/08/26 PubMed PMID: 9718175).
Gwark SC, Lee HS, Lee Y, Lee SB, Sohn G, Kim J, et al. Clinical implication of HER2 status in hormone receptor-positive mucinous breast cancer. Ann Surg Oncol. 2019;26(7):2166–74. https://doi.org/10.1245/s10434-019-07332-9.
Ding S, Wu J, Lin C, Chen W, Li Y, Shen K, et al. Predictors for survival and distribution of 21-gene recurrence score in patients with puremucinous breast cancer: a SEER population-based retrospective analysis. Clin Breast Cancer. 2018. https://doi.org/10.1016/j.clbc.2018.10.001.PubMedPMID:MEDLINE:30396812.
NCCN Clinical Practice Guidelines in Breast Cancer (Version 3.2022). Available from: http://www.nccn.org.
Ding S, Wu J, Lin C, Chen W, Li Y, Shen K, et al. Predictors for survival and distribution of 21-gene recurrence score in patients with pure mucinous breast cancer: a SEER population-based retrospective analysis. Clin Breast Cancer. 2019;19(1):e66-73. https://doi.org/10.1016/j.clbc.2018.10.001.
Wu SG, Li FY, Wang J, Lian CL, Zhou J, He ZY. Omission of adjuvant radiotherapy following breast-conserving surgery for elderly women with early-stage pure mucinous breast carcinoma. Radiat Oncol (London, England). 2019;14(1):190. https://doi.org/10.1186/s13014-019-1394-x.
National Cancer Institute: surveillance, epidemiology, and end results. Available from: https://seer.cancer.gov/.
Iwamoto T, Booser D, Valero V, Murray JL, Koenig K, Esteva FJ, et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J Clin Oncol. 2012;30(7):729–34. https://doi.org/10.1200/jco.2011.36.2574.
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–95. https://doi.org/10.1200/jco.2009.25.6529.
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet (London, England). 2005;366(9503):2087–106. https://doi.org/10.1016/s0140-6736(05)67887-7.
Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet (London, England). 2011;378(9804):1707–16. https://doi.org/10.1016/s0140-6736(11)61629-2.
Liljegren G, Holmberg L, Bergh J, Lindgren A, Tabar L, Nordgren H, et al. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. 1999;17(8):2326–33. https://doi.org/10.1200/jco.1999.17.8.2326.
Pan B, Yao R, Shi J, Xu Q-Q, Zhou Y-D, Mao F, et al. Prognosis of subtypes of the mucinous breast carcinoma in Chinese women: a population-based study of 32-year experience (1983–2014). Oncotarget. 2016;7(25):38864–75. https://doi.org/10.18632/oncotarget.8778.PubMedPMID:WOS:000378229100116.
Vo T, Xing Y, Meric-Bernstam F, Mirza N, Vlastos G, Symmans WF, et al. Long-term outcomes in patients with mucinous, medullary, tubular, and invasive ductal carcinomas after lumpectomy. Am J Surg. 2007;194(4):527–31. https://doi.org/10.1016/j.amjsurg.2007.06.012.
Wang W, Chen X, Lin L, Fei X, Garfield DH, Hong J, et al. Distribution and clinical utility of the 21-gene recurrence score in pure mucinous breast cancer patients: a case-control study. J Cancer. 2018;9(18):3216–24. https://doi.org/10.7150/jca.27291.PubMedPMID:WOS:000444015800001.
Funding
This work was supported by the Zhejiang Provincial Natural Science Foundation of Chian (grant no.Y19H160283).
Author information
Authors and Affiliations
Contributions
MQP contributed to study conception and design, data collection and analysis, manuscript writing. WYZ wrote and edited the manuscript. SJL,WXC reviewed the manuscript.WXC acquired the funding. All authors contributed to the submitted version. MQP, WYZ and SJL have contributed equally to this work and share first authorship. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential Competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mo, Q., Wang, Y., Shan, J. et al. Effect of postoperative radiotherapy in women with localized pure mucinous breast cancer after lumpectomy: a population-based study. Radiat Oncol 17, 119 (2022). https://doi.org/10.1186/s13014-022-02082-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-022-02082-7