Abstract
Background
Prostate cancer is the second most common malignancy worldwide, and the majority of patients are diagnosed with localized disease. We examined patients’ quality of life after stereotactic body radiation therapy (SBRT) for prostate cancer.
Methods
We included patients who were treated between 2016 and 2020. Inclusion criteria were adenocarcinoma of the prostate; class risk of low, intermediate, and high; and a World Health Organization performance status of 0–2. Quality of life was measured using the Functional Assessment of Cancer Therapy-Prostate (FACT-P).
Results
A total of 439 patients were treated with SBRT, with a median age of 73 years old. The median follow-up period was 34 months. FACT-P Trial Outcome Index (p < 0.0001), FACT-General (p = 0.0003), and FACT-P-Total (p < 0.0001) scores declined at 1 month post-SBRT, then recovered and returned to the same level as before treatment at 3–4 months post-SBRT. The decrease in quality of life in the first month was particularly remarkable in patients who received long-term hormone injections (36%). One month after the end of SBRT, about 22% of patients experienced "quite a bit” or more troubling side effects.
Conclusions
This study showed longitudinal changes in quality of life by FACT-P after SBRT for prostate cancer. Overall, prostate SBRT was well tolerated.
Similar content being viewed by others
Introduction
Prostate cancer (PC) is the second most common malignancy worldwide, and the majority of PC patients are diagnosed with localized disease [1]. There are multiple efficacious guideline-recommended treatment options for localized PC, including radical prostatectomy, external beam radiation, and brachytherapy. Recent American Society for Radiation Oncology/American Society of Clinical Oncology/American Urological Association guidelines [2] included recommendations regarding the use of stereotactic body radiotherapy (SBRT) for PC. According to the National Comprehensive Cancer Network (NCCN) Guidelines Version 3.2022 [3], SBRT combined with androgen deprivation therapy can be considered if delivering longer course of external beam radiotherapy would present medical or social hardship for unfavorable intermediate, high, and very high risk. There is growing consciousness that quality of life (QoL) with objective measurement of late adverse genitourinary (GU) and gastrointestinal (GI) toxicity are indispensable for the treatment decision of patients with PC [4,5,6]. In Japan, SBRT for localized PC was covered by insurance in 2016, which is relatively recent. Health-related QoL is an increasingly important endpoint in PC care. Although the QOL fluctuations in the SRT for PC of Japanese are a very important theme, there are no reports on it yet. To the best of our knowledge, there have been no reports evaluating QoL fluctuations after radical prostate SBRT using the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire. Therefore, the present study investigated the long-term QoL of patients after SBRT using a valid and self-administered QoL questionnaire via the FACT-P.
Methods
We conducted a retrospective study of patients with PC treated with SBRT at our institution (University of Tokyo Hospital). For clinical evaluation, all patients who underwent RT for prostate cancer were asked to fill out a FACT-P QOL questionnaire immediately before SRT and at each outpatient follow-up after SRT. This time, retrospectively, only consecutive cases that satisfy the conditions described below were selected from them, and the results of the data were summarized in this study. Staging examinations included serum prostate-specific antigen (PSA) measurement, ultrasound, digital rectal exam, pelvic magnetic resonance imaging (MRI), prostate biopsy, chest/abdominal/pelvic computed tomography (CT), and technetium-99 m-methylene diphosphonate bone scan. The inclusion criteria were as follows: (1) histologically proven adenocarcinoma of the prostate; (2) NCCN class risk by NCCN Guidelines Version 1.2021 of low, intermediate, or high [3]; and (3) World Health Organization (WHO) performance status of 0–2 [7]. The exclusion criteria were as follows: (1) lymph node metastasis (n = 12), (2) bone metastasis (n = 11), (3) castration-resistant PC (n = 7), (4) after radical prostatectomy (n = 1), (5) SBRT for local recurrence after external beam radiation therapy (n = 2), (6) local recurrence after high-intensity focused ultrasound (HIFU) (n = 3), and (7) receiving more or less than five fractions of radiotherapy (n = 1). We did not limit the prostate volume or baseline International Prostate Symptom Score to maximize the number of patients included. The nodal involvement risk was not assessed in this paper, and it was not considered as exclusion criteria.
Combination hormone therapy
Hormone therapy was administered over a short term of 4–6 months in the intermediate -risk group, and over a longer term of 1.5–2 years in the high-risk group. There was no concomitant use of bicalutamide, the first Degarelix injection, and the second and subsequent leuprorelin injections. SBRT was performed when PSA dropped to near 1.0 mg/mL. However, SBRT was started by the second month in the short-term administration group and by half a year in the long-term administration group.
SBRT method
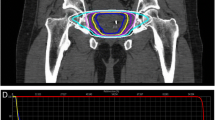
The clinical target volume (CTV) included the prostate and the proximal 2 cm of the seminal vesicle (SV) for high-risk patients, the prostate and 1 cm of the SV for intermediate-risk patients and the prostate only for low-risk patients. European Organization for Research and Treatment of Cancer (EORTC) guideline was used as a reference for our CTV delineations [8]. MRI imaging was used for delineation of the CTV. The planning target volume (PTV) consisted of a CTV with a posterior 3 mm margin and a 5 mm margin for all other margins [9].
The irradiation dose was PTV 95% prescription. 36.25 Gy in five fractions was 54%, and 40 Gy in five fractions was 43%. The former had been adopted before July 2018 and the latter after that. SBRT was performed using volumetric modulated arc therapy (VMAT) with flattening filter-free beams using a linear accelerator with image-guidance. Image guidance was performed using daily cone-beam computed tomography (CBCT). SBRT was performed five times every other day, excluding weekends. There were many cases of 177 patients in which SpaceOAR hydrogel (SpaceOAR; Augmenix Inc.) was applied before SBRT. Our previous report [10] describes the spacer insertion method, MRI planning, CT scan for SBRT, definition of the target volume, and the organs at risk. Each patient was given a glycerin enema of 30 or 40 cc 2 h before the examination or SBRT. After that, the patients were asked to drink water and hold their urine for 2 h. From 4 days before the examination to the last day of SBRT, the patients were encouraged to take elobixibat hydrate or polyethylene glycol daily. SBRT dose constraints are shown in Table 1. The Georgetown University experience was used as a reference for our dose constraints [11].
Description of the patient cohort
Baseline patient characteristics are shown in Table 2. The median follow-up period was 34 months (maximum: 54 months). Between May 2016 and December 2020, 439 patients answered the FACT-QoL questionnaire. These 439 patients were all that had been treated with SBRT for PC with curative intent at our institution during this period. The median age of the sample was 73 years and the median PSA before all treatments was 9.2 ng/mL. Clinical stage T2a was the most prevalent T-stage, with 47% of cases diagnosed as such. Regarding Gleason Score, 28% and 27% of patients were classified as Group 2 and 3, respectively. Moreover, 53% of patients were classified as intermediate-risk, while 42% were classified as high-risk. Short-term hormones were given for 4–6 months to 42% of patients, while long-term hormones were given to 36% of patients.
A total of 177 patients (40%) had a hydrogel spacer inserted between their prostate and rectum before SBRT. Regarding baseline characteristics of patients with SpaceOAR application, 36.25 Gy, 40 Gy, and 42.5 Gy were 55%, 39%, and 6%, and low, intermediate, and high-risk were 7%, 52%, and 40%, and short-term and long-term combined hormones were 40% and 31%, respectively.
Follow-up
Follow-up intervals were calculated from the date of the last SBRT dose. Outpatient follow-up was conducted at 1 month and 3–4 months after the end of SBRT, then at every 3 months until 2-year follow-up, and finally at every 6 months after the second year. We asked patients to complete a QoL questionnaire at each follow-up visit from May 2016. PSA was measured at every visit, and when it exceeded 2.0 ng/mL twice in a row, contrast-enhanced MRI and/or 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) examination was added to check for recurrence, since prostate specific membrane antigen (PSMA) was not be approved at the moment in Japan. When treatment such as chemotherapy was started in the case of double cancers, it was excluded from the survey.
Statistical analysis
Risk classification was based on the NCCN risk classification v1. 2021 for PC. The paired t-test was used for comparison of the baseline. All p-values were two-sided, and p < 0.05 was considered statistically significant. The FACT-P instrument is a multidimensional, self-administered, 39-item questionnaire. A FACT-P total score (FACT-P-Total) was obtained by adding together each patient’s emotional well-being (EWB), family well-being (FWB), physical well-being (PWB), social well-being (SWB), and prostate cancer subscale (PCS) (range 0–152) scores. The FACT-General (FACT-G) total score by determined by adding together the EWB, FWB, PWB, and SWB (range 0–104) scores. The FACT-P Trial Outcome Index (TOI) was determined by adding the FWB, PWB, and PCS subscale scores (range 0–104). Scores were obtained according to version 4 of the FACT scoring guidelines [12]. Prorating was carried out only if more than 50% of the items on each subscale were answered. For all scales, the higher the score, the better the QoL. This study was approved by the Institutional Review Board of Tokyo University (No. 3372–6). All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all participants.
Results
The baseline prostate volume was 39.3 cc (SD: 20.9) and the baseline international prostate symptom score was 9.9 (SD: 1.5).
Since we included all patients received SBRT in this survey, 28.5% of patients did not complete the questionnaire before treatment. In other words, although the questionnaire was distributed to all 439 patients, 314 patients (71.5%) answered it before treatment and 242 (55%) at one month. Even after that, the questionnaire continued to be distributed to all 439 patients every time they came to the outpatient department, everyone answered it at least once at some point. The number of the returned questionnaires at each point was shown in the bottom of Fig. 1. Across the FACT P-TOI, FACT-G, and FACT-P-Total, the QoL score at 1 month after the end of SBRT was significantly lower than that before SBRT (Fig. 1a). The average QoL score dropped from 78.84 (standard error: 0.77) before SBRT to 72.35 (1.02) at 1 month in the FACT P-TOI (p < 0.0001 by t-test), from 79.91 (0.79) to 75.38 (0.96) in the FACT-G (p = 0.0003), and from 114.22 (1.05) to 105.78 (1.31) in the FACT-P-Total (p < 0.0001). An improving trend was then seen in the second month (average score: 74.62 [SE: 2.57], 77.13 [2.09], and 107.88 [3.23], respectively). Furthermore, at 3–4 months, the scores of the FACT P-TOI (p = 0.0011, average score: 76.80 [SE: 0.88]), FACT-G (p = 0.0410, 78.15 [0.94]), and FACT-P-Total (p = 0.0029, 111.19 [1.24]) improved significantly when compared with those recorded in the first month. There was no significant decrease in the QoL score during the subsequent follow-up period, until months 49–54 (Fig. 1a). Figure 1b shows the QOL fluctuations excluding 125 cases who did not respond to the questionnaire before SBRT. The average QoL score dropped to 72.92 (SE: 1.15) at 1 month in the FACT P-TOI, 75.77 (1.11) in the FACT-G, and 106.46 (1.49) in the FACT-P-Total. The fluctuations are almost the same as those in Fig. 1a.
Mean Functional Assessment of Cancer Therapy-Prostate (FACT-P) Trial Outcome Index score (TOI, blue line), Functional Assessment of Cancer Therapy-General (FACT-G) Total score (orange line), and FACT-P-Total score (gray line). Time from last SBRT dose (months). For all patients (a) and excluding 125 cases who did not respond to the questionnaire before SBRT (b)
In the long-term hormone group, the QoL reduction which was seen in the FACT P-TOI (67.99) and FACT P-Total (102.08) at 1 month was larger than those in the short-term hormone group and the hormone-free group (Fig. 2). In the hormone-free group, QoL was lowest at two months instead of one month. Similar to the QOL fluctuations in all cases, both the mean FACT P-TOI scores and FACT P-Total scores by none, short term, and long term hormonal therapy had dropped to the bottom in the first or second month and is recovering quickly (Fig. 2). Until about the second year, the mean value was lowest in the long-term administration group than in the other two groups, and the mean value was about the same in the no- and the short-term administration group all the time both in FACT P-TOI and P-Total scores (Fig. 2).
With respect to use of a hydrogel spacer, the score of FACT P-TOI, FACT-G, and FACT P-Total at 1 month was 71.35 (SE: 1.02), 74.41 (0.96), and 104.26 (1.31) without spacer versus 74.97 (1.76) (p = 0.11 by unpaired t-test), 77.94 (1.69) (p = 0.10), and 109.74 (2.28) (p = 0.062) with spacer, respectively. With respect to the SBRT dose, the FACT P-TOI, FACT-G, and FACT-P-Total scores at 1 month were 72.58 (SE: 1.17), 75.22 (1.09), and 105.60 (1.46) after 36.25 Gy (p = 0.33 of 36.25 Gy versus 40 Gy, p = 0.68 of 36.25 Gy versus 42.5 Gy, and p = 0.99 of 40 Gy versus 42.5 Gy), and 71.77 (2.21), 76.05 (2.16), and 106.39 (3.02) (p = 0.71, 0.82, and 0.74) after 40 Gy, 71.85 (6.29), 74.15 (5.47), and 105.15 (7.88) (p = 0.79, 0.84, and 0.88) after 42.5 Gy, respectively (Fig. 3).
P7 (I have difficulty urinating) (average score: 3.25 before SBRT, 2.49 at 1 month, and 3.14 at 2 months) and BL2 (I urinate more frequently than usual) (2.58, 1.62, and 2.22, respectively) were the lowest 1 month after the end of SBRT (both p < 0.0001) (Fig. 4). Improvement was observed at 2 months and was fully recovered by months 3–4. P6 (I have trouble moving my bowels) and P8 (My problems with urinating limit my activities) continued to decline until the second month and recovered by months 3–4 (Fig. 4).
The percentage of those who answered that they were suffering from the side effects of GP5 (I am bothered by side effects of treatment) was also the highest in the first month after SBRT at 22%. While this figure had increased from the 8% recorded during the pre-SBRT period, it then dropped to 11% by months 3–4 (Fig. 5). Subsequently, this percentage gradually decreased over time. By including those who were "somewhat,” bothered by side effects, this result changed from 15% before SBRT to 45% at the 1st month, 32% at the 2nd month, and 28% at 3–4 months.
Discussion
In this retrospective study, we evaluated the QoL of 439 patients treated with SBRT for low-, intermediate-, and high-risk PC. To date, most QoL evaluations using FACT-P after SBRT for PC have only done so for metastatic cases [4]. However, since these studies did not irradiate the primary prostate lesion, their results cannot be compared with the QoL after radical SBRT. Using the FACT-P questionnaire, it is highly novel to see the time-series changes in QoL after radical PC SBRT. The FACT-P has also been used in many phase III studies on PC [5, 6, 13].
According to the previous reports [13,14,15,16,17,18,19,20], physician-reported acute GI and GU toxicity of grade 3–5 after SBRT of 2, 4, or 5 fractions were 0–2% and 0–2.5%, respectively. According to Kishan et al. [21], the crude incidence of acute grade 3–5 GU toxic events after SRT was 0.60% (13/2142 patients) and of GI toxic events was 0.09% (n = 2), and the 7-year cumulative incidence of late grade 3–5 GU toxic events was 2.4% (95% CI, 1.8%-3.2%) and of late grade 3–5 GI toxic events was 0.4% (95% CI,0.2%-0.8%). In our study, 45% of patients answered “somewhat” or more to GP5 (I am bothered by side effects of treatment) at 1 month after the last SBRT and 10–30% and 0–13% of patients answered “somewhat” or more and “quite a bit” or more in GP5 after 3–4 months, respectively. Moreover, 22% of patients responded that they were “quite a bit” or more troubled by the treatment’s side effects. In general, patient-reported side effects are more frequent than physician-reported side effects.
Previous studies of a systematic review have investigated the QoL change using FACT-P after conventional intensity-modulated radiation therapy (IMRT), but unlike our study, included an initial questionnaire 3 months after irradiation and found no decrease in QoL [22]. In contrast, we collected novel QoL data before SBRT, as well as during the first and second months after SBRT.
In a single-arm, phase II trial from multiple centers in Singapore [23], QoL of 74 men was assessed by EPIC at pretreatment, 12, and 24 months after SBRT of 36.25 Gy in 5 fractions. Overall, no significant change was observed in QoL scores over time across the three domains of urinary, bowel, and sexual scores [23]. The Dutch HYPO-RT-PC trial with the updated long-term QoL analyses at baseline, the end of radiotherapy, months 3, 6, 12, and 24 after radiotherapy, every other year thereafter up to 10 years, and at 15 years using the validated Prostate Cancer Symptom Scale (PCSS) and EORTC Quality-of-Life Questionnaire (QLQ-C30) confirmed that ultra-hypofractionated radiotherapy of 42.7 Gy in 7 fractions (n = 583) was equally well tolerated as conventionally fractionated RT of 78 Gy in 39 fractions (n = 582) up to 6 years after treatment [24]. According to Boyer et al. [22], the median AUASS (n = 60) increased from 4.5 points before SRT to 11 points while on SRT of 37 Gy in 5 fractions (p < 0.01), but was 5 points at 36 months after SRT (p = 0.65). These results were also consistent with the data after one year in the present study. According to Georgetown University’s report [11], which is the only existing study looking at QoL one month after SBRT of 35 Gy or 36.25 Gy in 5 fractions, the median baseline American Urological Association symptom score (AUASS) of 8 in 96 men significantly increased to 11 at 1 month (p = 0.001), but returned to baseline at 3 months (p = 0.60). Although a sole questionnaire after one month might have been really too short, it was conducted even in this survey by according to Georgetown University’s report [11]. According to Katz et al. [25], mean EPIC urinary (n = 262) and bowel QoL (n = 263) declined at 1 month post-SRT of 35–36.25 Gy in 5 fractions using Cyberknife technology, returned to baseline by 2 years, and remained stable thereafter and of patients potent at baseline evaluation, 67% remained potent at last follow-up with follow-up as long as 8 years. In a meta-analysis of curative-intent SBRT for localized PC [26], the EPIC-26 was used to assess 3,293 patients. Information for urinary, bowel, and sexual domain scales was readily extractable, and the data were pooled. The EPIC urinary and bowel scores returned to baseline by 2 years post-treatment (p = 0.90 and 0.09, respectively) and did not significantly differ at 5 years post-SBRT (p = 0.50 and 0.80, respectively), although these studies did not include early 1 or 2 month QoL measures. These results do not differ substantially from those of our study.
There are several points that could limit the strength of the study. First of all, there were times when questionnaire response rate was small, such as after the 38th month. Since we included all patients treated with SBRT during this period, only 71% of patients had a baseline assessment and 55% were evaluated at 1 month. Thus, our QoL assessments may follow a heterogeneous distribution. Secondly, we did not compare patient-reported outcome measures (PROMS) with physician reported outcomes nor toxicity scale of CTCAE in this study.
Conclusions
QoL declined at 1 month after the end of prostate SBRT, then recovered, and returned to the same level as before treatment 3–4 months after SBRT. The decrease in QoL in the first month was particularly remarkable in patients who received long-term hormone injections. One month after the end of SBRT, about 22% of people were "quite a bit or very much" troubled by the side effects of the treatment. Our findings support that SBRT could be considered a standard radiotherapeutic strategy for localized PC.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUASS:
-
American Urological Association symptom score
- CBCT:
-
Cone-beam computed tomography
- CT:
-
Computed tomography
- CTV:
-
Clinical target volume
- EORTC QLQ:
-
European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire
- EPIC:
-
Expanded Prostate Cancer Index Composite
- EWB:
-
Emotional well-being
- FACT-G:
-
FACT-General
- FACT-P:
-
Functional Assessment of Cancer Therapy-Prostate
- FDG:
-
Fluorodeoxyglucose
- FWB:
-
Family well-being
- GI:
-
Gastrointestinal
- GU:
-
Genitourinary
- HDR:
-
High dose rate
- HIFU:
-
High-intensity focused ultrasound
- HRQoL:
-
Health related quality of life
- IMRT:
-
Intensity-modulated radiation therapy
- LDR:
-
Low dose rate
- MRI:
-
Magnetic resonance imaging
- NCCN:
-
National Comprehensive Cancer Network
- PC:
-
Prostate cancer
- PCS:
-
Prostate cancer subscale
- PCSS:
-
Prostate Cancer Symptom Scale
- PET:
-
Positron emission tomography
- PROMS:
-
Patient-reported outcome measures
- PSA:
-
Prostate-specific antigen
- PSMA:
-
Prostate specific membrane antigen
- PTV:
-
Planning target volume
- PWB:
-
Physical well-being
- QoL:
-
Quality of life
- SBRT:
-
Stereotactic body radiotherapy
- SV:
-
Seminal vesicle
- SWB:
-
Social well-being
- TOI:
-
Trial Outcome Index
- VMAT:
-
Volumetric modulated arc therapy
- WHO:
-
World Health Organization
References
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A, ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. https://doi.org/10.1056/NEJMoa0810084.
Morgan SC, Hoffman K, Loblaw DA, Buyyounouski MK, Patton C, Barocas D, Bentzen S, Chang M, Efstathiou J, Greany P, Halvorsen P, Koontz BF, Lawton C, Leyrer CM, Lin D, Ray M, Sandler H. Hypofractionated radiation therapy for localized prostate cancer: executive summary of an ASTRO, ASCO and AUA evidence-based guideline. J Urol. 2019;201(3):528–34. https://doi.org/10.1097/JU.0000000000000071.
National Comprehensive Cancer Network®. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): Prostate Cancer. Version 3.2022. 10 Jan 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed 19 Mar 2022.
Smith MR, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, Jievaltas M, Luz M, Alekseev B, Kuss I, Le Berre MA, Mohamed AF, Odom D, Bartsch J, Snapir A, Sarapohja T, Fizazi K. Darolutamide and health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the phase III ARAMIS trial. Eur J Cancer. 2021;154:138–46. https://doi.org/10.1016/j.ejca.2021.06.010.
Yokomizo A, Koga H, Ito K, Takezawa Y, Komiyama M, Nishimura K, Yonese J, Hashine K, Masumori N, Arai G, Saito S, Shinohara M, Shimizu N, Yamauchi A, Satoh T, Tochigi T, Kobayashi M, Fujimoto H, Kakimoto KI, Fukui I, Tsukamoto T, Nozaki M, Karasawa K, Hasumi M, Ohtani M, Ishiyama H, Kuwahara M, Harada M, Ohashi Y, Kotake T, Kakizoe T, Suzuki K, Naito S, Yamanaka H, National Research Project on Endocrine-Radiation Combination Therapy for Locally Advanced Prostate Cancer investigators. Patient-reported outcomes following neoadjuvant endocrine therapy, external beam radiation, and adjuvant continuous/intermittent endocrine therapy for locally advanced prostate cancer: a randomized phase III trial. Cancer Med. 2021;10(10):3240–8. https://doi.org/10.1002/cam4.3895.
Thiery-Vuillemin A, Fizazi K, Sartor O, Oudard S, Bury D, Ozatilgan A, Poole EM, Eisenberger M, de Bono J. Post hoc health-related quality of life analysis according to response among patients with prostate cancer in the PROSELICA and FIRSTANA studies. Oncologist. 2021;26(7):e1179–88. https://doi.org/10.1002/onco.13803.
https://www.who.int/classifications/international-classification-of-functioning-disability-and-health/who-disability-assessment-schedule. WHO Disability Assessment Schedule 2.0 (WHODAS 2.0) (Report). WHO. (2014-10-29).
Boehmer D, Maingon P, Poortmans P, Baron MH, Miralbell R, Remouchamps V, Scrase C, Bossi A, Bolla M, EORTC radiation oncology group. Guidelines for primary radiotherapy of patients with prostate cancer. Radiother Oncol. 2006;79(3):259–69. https://doi.org/10.1016/j.radonc.2006.05.012.
Gurka MK, Chen LN, Bhagat A, Moures R, Kim JS, Yung T, Lei S, Collins BT, Krishnan P, Suy S, Dritschilo A, Lynch JH, Collins SP. Hematuria following stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer. Radiat Oncol. 2015;10:44. https://doi.org/10.1186/s13014-015-0351-6.
Ogita M, Yamashita H, Sawayanagi S, Takahashi W, Nakagawa K. Efficacy of a hydrogel spacer in three-dimensional conformal radiation therapy for prostate cancer. Jpn J Clin Oncol. 2020;50(3):303–9. https://doi.org/10.1093/jjco/hyz171.
Chen LN, Suy S, Uhm S, Oermann EK, Ju AW, Chen V, Hanscom HN, Laing S, Kim JS, Lei S, Batipps GP, Kowalczyk K, Bandi G, Pahira J, McGeagh KG, Collins BT, Krishnan P, Dawson NA, Taylor KL, Dritschilo A, Lynch JH, Collins SP. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol. 2013;8:58. https://doi.org/10.1186/1748-717X-8-58.
Madsen BL, Hsi RA, Pham HT, Fowler JF, Esagui L, Corman J. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: First clinical trial results. Int J Radiat Oncol Biol Phys. 2007;67(4):1099–105. https://doi.org/10.1016/j.ijrobp.2006.10.050.
Fuller DB, Falchook AD, Crabtree T, Kane BL, Medbery CA, Underhill K, Gray JR, Peddada A, Chen RC. Phase 2 multicenter trial of heterogeneous-dosing stereotactic body radiotherapy for low- and intermediate-risk prostate cancer: 5-year outcomes. Eur Urol Oncol. 2018;1(6):540–7. https://doi.org/10.1016/j.euo.2018.06.013.
Meier RM, Bloch DA, Cotrutz C, Beckman AC, Henning GT, Woodhouse SA, Williamson SK, Mohideen N, Dombrowski JJ, Hong RL, Brachman DG, Linson PW, Kaplan ID. Multicenter trial of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer: survival and toxicity endpoints. Int J Radiat Oncol Biol Phys. 2018;102(2):296–303. https://doi.org/10.1016/j.ijrobp.2018.05.040.
Zelefsky MJ, Kollmeier M, McBride S, Varghese M, Mychalczak B, Gewanter R, Garg MK, Happersett L, Goldman DA, Pei I, Lin M, Zhang Z, Cox BW. Five-year outcomes of a phase 1 dose-escalation study using stereotactic body radiosurgery for patients with low-risk and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2019;104(1):42–9. https://doi.org/10.1016/j.ijrobp.2018.12.045.
Alayed Y, Cheung P, Chu W, Chung H, Davidson M, Ravi A, Helou J, Zhang L, Mamedov A, Commisso A, Commisso K, Loblaw A. Two StereoTactic ablative radiotherapy treatments for localized prostate cancer (2STAR): Results from a prospective clinical trial. Radiother Oncol. 2019;135:86–90. https://doi.org/10.1016/j.radonc.2019.03.002.
McBride SM, Wong DS, Dombrowski JJ, Harkins B, Tapella P, Hanscom HN, Collins SP, Kaplan ID. Hypofractionated stereotactic body radiotherapy in low-risk prostate adenocarcinoma: preliminary results of a multi-institutional phase 1 feasibility trial. Cancer. 2012;118(15):3681–90. https://doi.org/10.1002/cncr.26699.
Loblaw A, Cheung P, D’Alimonte L, Deabreu A, Mamedov A, Zhang L, Tang C, Quon H, Jain S, Pang G, Nam R. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: toxicity, biochemical, and pathological outcomes. Radiother Oncol. 2013;107(2):153–8. https://doi.org/10.1016/j.radonc.2013.03.022.
Hannan R, Tumati V, Xie XJ, Cho LC, Kavanagh BD, Brindle J, Raben D, Nanda A, Cooley S, Kim DWN, Pistenmaa D, Lotan Y, Timmerman R. Stereotactic body radiation therapy for low and intermediate risk prostate cancer-results from a multi-institutional clinical trial. Eur J Cancer. 2016;59:142–51. https://doi.org/10.1016/j.ejca.2016.02.014.
Kishan AU, Dang A, Katz AJ, Mantz CA, Collins SP, Aghdam N, Chu FI, Kaplan ID, Appelbaum L, Fuller DB, Meier RM, Loblaw DA, Cheung P, Pham HT, Shaverdian N, Jiang N, Yuan Y, Bagshaw H, Prionas N, Buyyounouski MK, Spratt DE, Linson PW, Hong RL, Nickols NG, Steinberg ML, Kupelian PA, King CR. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw Open. 2019;2(2):e188006. https://doi.org/10.1001/jamanetworkopen.2018.8006.
Boyer MJ, Papagikos MA, Kiteley R, Vujaskovic Z, Wu J, Lee WR. Toxicity and quality of life report of a phase II study of stereotactic body radiotherapy (SBRT) for low and intermediate risk prostate cancer. Radiat Oncol. 2017;12(1):14. https://doi.org/10.1186/s13014-016-0758-8.
Tan JSH, Teh JYH, Tan LLY, Tan SXF, Li YQ, Tan TWK, Wang MLC, Kanesvaran R, Ong EHW, Tay KJ, Lee LS, Tuan JKL, Tan DYH, Chua MLK. Efficacy, toxicity, and quality-of-life outcomes of ultrahypofractionated radiotherapy in patients with localized prostate cancer: a single-arm phase 2 trial from Asia. Asia Pac J Clin Oncol. 2021. https://doi.org/10.1111/ajco.13742.
Fransson P, Nilsson P, Gunnlaugsson A, Beckman L, Tavelin B, Norman D, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, Kindblom J, Ginman C, Johansson B, Björnlinger K, Seke M, Agrup M, Zackrisson B, Kjellén E, Franzén L, Widmark A. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer (HYPO-RT-PC): patient-reported quality-of-life outcomes of a randomised, controlled, non-inferiority, phase 3 trial. Lancet Oncol. 2021;22(2):235–45. https://doi.org/10.1016/S1470-2045(20)30581-7.
Katz AJ, Kang J. Quality of life and toxicity after SBRT for organ-confined prostate cancer, a 7-year study. Front Oncol. 2014;4:301. https://doi.org/10.3389/fonc.2014.00301.
Jackson WC, Silva J, Hartman HE, Dess RT, Kishan AU, Beeler WH, Gharzai LA, Jaworski EM, Mehra R, Hearn JWD, Morgan TM, Salami SS, Cooperberg MR, Mahal BA, Soni PD, Kaffenberger S, Nguyen PL, Desai N, Feng FY, Zumsteg ZS, Spratt DE. Stereotactic body radiation therapy for localized prostate cancer: a systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biol Phys. 2019;104(4):778–89. https://doi.org/10.1016/j.ijrobp.2019.03.051.
Acknowledgements
Not applicable.
Funding
No applicable.
Author information
Authors and Affiliations
Contributions
HY wrote the main manuscript text. MO, SS, YN, and OA helped to collect the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This was a retrospective study conducted with the approval of the ethics committee of the University of Tokyo (No. 3372-6). Informed consent was obtained by receiving patients’ answer to the questionnaire.
Consent for publication
Patients gave written consent for data collection and analysis.
Competing interests
The authors declare that they have no competing interests in association with this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yamashita, H., Ogita, M., Sawayanagi, S. et al. Quality of life after definitive linear accelerator-based stereotactic radiotherapy for prostate cancer: a longitudinal study. Radiat Oncol 17, 90 (2022). https://doi.org/10.1186/s13014-022-02061-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-022-02061-y