Abstract
Background
The purpose of the study was to evaluate the impact of multiple prognostic factors on the acute skin reaction in adjuvant breast cancer radiotherapy, in particular the impact of hypofractionation (HF) compared to conventional fractionation (CF) and tangential beam (TB) IMRT compared to three-dimensional conformal radiotherapy (3DCRT).
Methods
Two-hundred and sixty-six breast cancer patients with postoperative radiotherapy after breast conserving surgery or mastectomy were retrospectively evaluated. Patients were treated with HF (15 fractions of 2.67 Gy; n = 121) or CF (28 fractions of 1.8 Gy or 25 fractions of 2.0 Gy; n = 145) and TB-IMRT (n = 151) or 3DCRT (n = 115). The acute skin reactions were prospectively assessed using the CTCAE v4 grading scale. Ordinal regression analysis was used to assess the impact of possible prognostic factors on the maximal acute skin reaction.
Results
Grade 2 skin reactions were observed in 19 % of the patients treated with CF compared to 2 % treated with HF. On univariate analysis, the fractionation regimen, the PTV (breast versus chest wall), the volume of the PTV and the body mass index were significant prognostic factors for the maximum acute skin reaction. On multivariate analysis, the fractionation regimen (p < 0.00001) and the volume of the PTV (p = 0.0002) remained as independent significant factors.
Conclusions
Our data suggest that HF is associated with a significantly reduced maximal acute skin reaction compared to CF.
Similar content being viewed by others
Background
Breast cancer is the most common cancer in women worldwide, and after lung cancer the second most common cancer overall. In 2012, nearly 1.7 million new breast cancer cases were diagnosed representing about 12 % of all new cancer cases and 25 % of all cancers in women [1].
Adjuvant radiotherapy is an important part of breast cancer management. Conventional fractionation regimens (CF) consisting of 25 daily fractions of 2.0 Gy or 28 daily fractions of 1.8 Gy have generally been considered the “standard” adjuvant radiotherapy prescription. Several large, well-conducted randomized trials have established that hypofractionated regimens (HF) such as 15 daily fractions of 2.67 Gy can be equally effective in terms of long-term disease control and late radiation effects compared to the excellent outcomes of more protracted conventional fractionation schedules [2–5]. The HF evaluated in the recent trials were characterized by an increase of the daily fraction dose and a decrease of the total dose at the same time. The reduced number of daily fractions compared to CF results in a benefit to patients and health services in terms of convenience and cost. Most human cancer types respond to the total dose rather than to the size of the daily fractions [6]. In contrast, the late reacting normal tissues respond to the daily fraction size (higher fraction doses increase the risk of late toxicity) and the total dose [7]. The late adverse effects are dose limiting. CF typically use “small” daily fraction doses of 2.0 Gy or 1.8 Gy to deliver the highest possible tolerated total dose, thereby ensuring the highest rate of tumor control. The finding of comparable late toxicity and long-term tumor control with HF (using “higher” fraction doses) compared to CF (using “small” fraction doses) of the recent studies suggests that breast cancer is an exception in showing comparable sensitivity to fraction size as the normal tissues of the breast and ribcage [8].
Like most human cancer types, early reacting normal tissues respond to the total dose rather than to the size of the daily fractions. Due to the lower total dose used with HF compared to CF it can be expected that the acute radiation reactions are lower in patients treated with HF. However, the reports of randomized trials to date have provided little information comparing acute toxic effects with HF as compared with CF [2, 3, 9–11].
Several randomized [12, 13] and retrospective studies [14–16] have suggested that the radiation technique may influence the acute toxic effects in adjuvant breast cancer radiotherapy. Patients treated with intensity modulated radiotherapy (IMRT) showed less acute skin reactions compared to patients treated with standard tangential beam technique using wedge compensation. This observation has been explained with the improvement in the dose distribution homogeneity using breast IMRT compared to standard radiotherapy using wedges [12]. Due to the mathematical form of the linear-quadratic dose effect relationship, hot spots are penalized more severely in a hypofractionated treatment, so-called ‘triple trouble’ [8, 17]. It can therefore be expected that IMRT is in particular beneficial in patients treated with HF. Commonly used beam configurations for breast IMRT are tangential beams (TB-IMRT) or multiple beams from four to seven directions.
Although not a dose limiting factor in breast cancer radiotherapy, acute skin reactions are of clinical importance. Acute skin toxicity affects multiple dimensions of quality of life. They cause physical discomfort, body image disturbance, emotional distress, and impair both day-to-day functioning and satisfaction with radiation treatment [12, 18]. Goal of this study was to evaluate the impact of multiple prognostic factors on the acute skin reaction in breast cancer radiotherapy, in particular the impact of HF compared to CF and TB-IMRT compared to 3DCRT.
Patients and methods
Patient population
Two-hundred and sixty-six breast cancer patients with postoperative radiotherapy after breast conserving surgery or mastectomy between March 2014 and April 2016 were evaluated in this study. The acute skin radiation reaction of all patients was prospectively assessed once weekly during and 6 weeks after radiotherapy by two observers using the “dermatitis radiation” grade of the Common Terminology Criteria for Adverse Events (CTCAE v4.03) (Table 1). The dermatitis radiation grade was documented immediately after assessment in the Local Area Network Therapy Information System “Lantis” (Siemens Healthcare, Germany), and a table with all weekly assessments was included in the “End of Treatment Report” of all patients. The two observers were not involved in the statistical analysis of the study. Patient and treatment related data were transferred into a database, anonymized and retrospectively analyzed using specific statistical software. The study was approved by the local institutional ethical committee and conducted in accordance with the Helsinki Declaration in its current version.
Patients with the histologically proven diagnosis of breast cancer or breast cancer in situ receiving postoperative radiotherapy of the whole breast after breast conserving surgery or of the chest wall after mastectomy were eligible for the study. Patients with bilateral breast cancer or a history of previous radiotherapy of the chest were excluded from the analysis. Patients were offered a hypofractionated or conventionally fractionated radiotherapy using inverse planned tangential beam intensity modulated radiotherapy (TB-IMRT) or three-dimensional planned conformal radiotherapy using wedge compensation (3DCRT). The decision about the fractionation regimen and radiation technique was based on patient preference. Main factors considered for the choice of the fractionation regimen by the patients were distance to the radiotherapy department, personal commitments limiting the overall treatment time and recommendation of the treating physicians. Patients not or not fully covered by medical insurance tended to opt for 3DCRT for financial reasons. A few patients with unfavourable thoracic geometry and left-sided breast cancer were treated with seven-field IMRT. These patients were not considered in the analysis.
Fractionation regimen and assessment of the acute skin reaction
The conventional fractionation regimen (CF) for the postoperative radiotherapy of the breast consisted of 28 fractions (fraction dose 1.8 Gy; total dose 50.4 Gy) and for the chest wall of 25 fractions (fraction dose 2.0 Gy; total dose 50.0 Gy). The hypofractionated regimen (HF) for the breast or chest wall consisted of 15 fractions (fraction dose 2.67 Gy; total dose 40.05 Gy). Where indicated, the supraclavicular lymph nodes were treated with the same fractionation regimen used for the breast or the chest wall. Patients were treated once per day and five times per week. If radiation fractions were missed, patients were treated on weekends in order not to exceed the prescribed overall treatment time. Where indicated, an electron boost was applied with five or eight additional fractions with a fraction dose of 2.0 Gy.
For the analysis of the radiation reaction of the skin, the maximal “dermatitis radiation” grade according to CTCAE v4.03 was used observed during the CF or HF of the whole breast or chest wall (before a possible boost to the PTV; for CF at the planned dose of 50.4 Gy or 50.0 Gy, for HF at the planned dose of 40.05 Gy). Likewise, the time to the dermatitis radiation grade 1 or grade 2 was defined as the time in days from the first radiation fraction to the corresponding dermatitis radiation grade observed during the course of the CF of HF of the whole breast or chest wall (before a possible boost to the PTV).
Treatment planning
A non-contrast CT-simulation was performed in the supine position on a carbon breast board with the ipsilateral arm up and head turned to the contralateral side. Radio-opaque wires were used to mark the clinical boundaries. A CT scan was performed using 5 mm slice thickness. The CT scanning reference point was defined using the CT simulation software Coherence Dosimetrist (Siemens Medical, Germany), and target volumes (PTV and OARs) using the software Coherence Oncologist (Siemens Medical, Germany). The 3DCRT and IMRT plans were generated using the treatment planning system XIO 4.4 (CMS, Inc. of St. Louis, Mo, USA). Two Siemens Oncor Anvantgarde linear accelerators with a 160 MLC Multileaf Collimator were used for the treatment. The leaf width was 0.5 cm at the isocenter. The dose calculation was determined using the “Superposition” algorithm. Dose volume histograms (DVH) of the PTV and OARs of the 3D-CRT and IMRT plans were generated. The target volumes were defined and the dose prescribed according to the International Commission on Radiation Units and Measurement (ICRU) Reports 50 and 62 recommendations. Accordingly, the target volume should be surrounded by the 95 % isodose line of the prescribed dose. The planning target volume (PTV) definition for the whole breast or chest wall was done according to the recommendations of the breast cancer atlas for radiation therapy planning consensus definitions of the Radiation Therapy Oncology Group (RTOG) (http://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx). The PTV of the breast included the apparent computed tomography (CT) glandular breast tissue and the PTV of the chest wall the pectoralis muscle, chest wall muscles, and ribs. For the statistical analysis the volume of the PTV (breast or chest wall) was obtained from the dose volume histograms of the 3DCRT or TB-IMRT plans.
Daily online verification and correction of the patient positioning error prior to radiotherapy was performed in all patients using orthogonal megavoltage electronic portal images [19]. No respiratory gating techniques were applied in this study.
3DCRT plans
The dose was prescribed to the ICRU reference point which was usually the isocenter located in the PTV volume centroid. Two tangential semi-opposed beams (to avoid divergence), physical wedges (usually 15° or 30°), a 160 MLC Multileaf Collimator and 6 MV photons were used for 3DCRT. A few patients received a mixed beam technique (6 MV and 15 MV photons). The beam angles, wedge angles, and beam weighting (usually minimal) were chosen to optimize coverage of the PTV, while minimizing exposure to the ipsilateral lung, heart and contralateral breast. Gantry angles ranged from 42° to 55° for the medial fields and from 224° to 232° for the lateral fields for patients treated on the right side, and from 305° to 322° for the medial fields and from 133° to 147° for the lateral fields for patients treated on the left side.
TB-IMRT plans
The same PTV and tangential beam orientation of the 3DCRT plans were used for the TB-IMRT plans. An extension into the air anteriorly of the chest of 1.5 cm was added to the PTV to ensure appropriate opening of the 160 MLC Multileaf Collimator. Inverse treatment planning and 6MV photons were used for all IMRT plans. The dose was prescribed to the PTV, and as initial dose volume constraints the IMRT prescription table provided by the XIO treatment planning system was used (Table 2). The IMRT plans were optimized to cover the PTV and spare the surrounding tissues as much as possible. A step-and-shoot technique was applied. An optimization with 100 iterations was then applied, and followed by a semiautomatic segmentation (minimum 3 cm step size). Segments equal or less than 2 MU were expelled from the plan. Tissue inhomogeneities were considered in the treatment planning optimization process, and the dose calculation algorithm used was “Superposition”. The plans were developed to deliver 95 % of the prescribed dose to the full PTV, and to minimize dose to the OARs lung and heart.
Statistical analysis
Differences between patient groups treated with CF or HF were assessed using the Chi-square test for categorical variables, the t-test for normally distributed and the U-test for non-normally distributed continuous variables. All tests were two-sided, and a p-value of ≤0.05 was considered significant. To assess the impact of possible prognostic factors on the maximal dermatitis radiation grade an ordinal logistic regression analysis was performed. The model selection of the multivariate analysis was performed by a backward stepwise strategy. The possible prognostic factors included in the analysis are listed in Table 4.
Results
The patient (Table 2) and treatment characteristics (Table 3) were for the most part well balanced between the patients treated with CF and HF. The volumes of the PTV as well as the body mass index (BMI) were slightly higher in the HF group. Furthermore, 97 % of the patients of the HF group completed their radiotherapy within the prescribed overall treatment time compared to 71 % of the CF group.
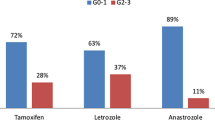
On univariate analysis, the fractionation regimen (Fig. 1), the PTV (breast versus chest wall), the volume of the PTV, and the BMI were significantly associated with the maximal acute skin reaction. On multivariate analysis, only the factors volume of the PTV (p = 0.0002) and fractionation regimen (p < 0.00001) had a significant independent impact on the maximal acute skin reaction (Table 4). Furthermore, patients treated with HF developed the same acute skin reaction grade significantly earlier compared to patients treated with CF (Table 3, Fig. 2).
Discussion
Our data clearly show that a moderately hypofractionated fractionation regimen (15 daily fractions of 2.67 Gy) results in a significantly less acute skin reaction rate compared to CF in breast cancer radiotherapy.
Two recent randomized [20] and non-randomized studies [21] comparing HF with CF have reported similar results. The randomized study allocated breast cancer patients stage Tis-T2, N0-N1a, M0 (n = 287) to adjuvant radiotherapy of the whole breast with either HF (16 daily fractions of 2.67 Gy) or CF (25 daily fractions of 2.0 Gy. Both study arms were followed by a tumor bed boost. Patients were treated with megavoltage tangential portals and forward- or inverse-planned segmental fields in supine or prone position. Acute skin reactions were significantly lower with HF compared to CF (CTCAE v4.0 dermatitis grade 2: 36 % versus 69 %; Ptrend < 0.001) [20]. The retrospective study compared the acute toxic effects of 2309 patients receiving HF (daily fraction dose >2 Gy (95 % were between 2.6 Gy and 2.7 Gy); mean total dose (SD) 45.3 Gy (2.5 Gy); n = 578) versus CF (daily fraction dose ≤2 Gy (62.9 % were 1.8 Gy and 37.1 % were 2.0 Gy); mean total dose (SD) 52.1Gy (2.8 Gy); n = 1732) to the whole breast after breast conserving surgery. Sixty percent of the patients treated with HF and 92.9 % of the patients treated with CF received a tumor bed boost. The radiation techniques used were not described in detail in this report. Acute skin reactions were significantly lower with HF compared to CF (CTCAE v4.0 dermatitis grade 2: 28 % versus 61 %; P < 0.001) [21]. Our study showed a reduction of the CTCAE 4.0 dermatitis radiation grade 2 from 19 % with CF to 2 % with HF. The lower incidence of grade 2 dermatitis observed in our study compared to the two above studies may be related to differences in the distribution of multiple prognostic factors, for example the total dose. In the above two studies the acute skin reactions were assessed during the radiotherapy of the whole breast and the tumor bed boost whereas in our study only during the radiotherapy to the whole breast or chest wall (before the possible application of a boost). Interestingly, the reported incidence of CTCAE v.2–4 dermatitis radiation grade 2 varies considerably between the studies with CF (9 % [22], 14 % [23], 19 % [this study], 34 % [24], 37 % [12], 61 % [21], 68 % [20] and 68 % [25], 72 % [14]), suggesting that prognostic factors other than the daily fraction dose and total dose significantly influence the acute skin reaction. In our study, the fractionation regimen and the volume of the PTV were identified as the only significant independent prognostic factors for the maximal acute skin reaction grade. The breast volume as prognostic factor for the acute skin reaction has been reported by multiple studies [12, 14, 16, 22, 26–31]. This observation has been explained with the association of large breasts with increased dose inhomogeneity and hot spots [31].
Several studies have reported an impact of the radiation technique on the acute skin reaction [12–16]. IMRT can produce more homogenous dose distributions compared to conventional tangential beam breast cancer radiotherapy using wedge compensation, in particular if compared to two-dimensionally planned breast cancer radiotherapy [12, 32]. However, no significant difference of the superficial dose in breast cancer radiotherapy has been found between tangential beam IMRT (TB-IMRT) and three-dimensional conformal radiotherapy using tangential beams with wedge compensation (3DCRT) in a phantom study [18] and a clinical study using GafChromic film in vivo dosimetry [33]. The superficial dose can be considered as a good surrogate parameter of the skin dose. In agreement with the two in vitro and in vivo dosimetry studies, no significant difference of the acute skin reaction using TB-IMRT compared to 3DCRT was found in our study on univariate and multivariate analysis. An interesting observation in our study is that patients treated with HF developed the same dermatitis radiation grade significantly earlier than the patients treated with CF. The data suggest that the daily fraction size has a significant impact on the time to develop the acute skin reaction, whereas the total dose is more relevant for the severity of the acute skin reaction.
The strength of our study is the uniform prospective assessment of the skin reaction and application of the TB-IMRT and 3DCRT plans by the same team. Limitations of our study are related to the non-randomized study design where a selection bias cannot be excluded with certainty. Furthermore, to obtain the maximal acute skin reaction grade at comparable total doses, the skin reaction during the radiotherapy of the whole breast or chest wall (before the possible application of a boost) was used in the analysis of our study. Some patients may develop their maximal skin reaction after the completion of the radiotherapy of the whole breast or chest wall, and in these patients the maximal skin reaction would have been underestimated. Some possible prognostic factors for the acute skin reaction have not been included in our multivariate analysis, for example genetic markers or smoking during radiotherapy [22, 26, 34].
Conclusion
Large randomized trials have established that hypofractionated regimens can be equally effective in terms of long-term disease control and late radiation effects compared to conventional fractionation schedules. Our study shows that a moderately hypofractionated regimen is also significantly associated with a reduced maximal acute skin reaction.
Abbreviations
3DCRT, three-dimensional conformal radiotherapy; BMI, Body mass index; CF, conventional fractionation; CT, computed tomography; CTCAE v4.03, common terminology criteria for adverse events version 4.03; HF, hypofractionationation; PTV, planning target volume; TB-IMRT, inverse planned tangential beam intensity modulated radiotherapy
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386.
Yarnold J, Ashton A, Bliss J, Homewood J, Harper C, Hanson J, Haviland J, Bentzen S, Owen R. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol. 2005;75(1):9–17.
Owen JR, Ashton A, Bliss JM, Homewood J, Harper C, Hanson J, Haviland J, Bentzen SM, Yarnold JR. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7(6):467–71.
Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362(6):513–20.
Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, Dobbs HJ, Hopwood P, Lawton PA, Magee BJ, et al. The UK standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14(11):1086–94.
Thames HD, Bentzen SM, Turesson I, Overgaard M, van den Bogaert W. Fractionation parameters for human tissues and tumors. Int J Radiat Biol. 1989;56(5):701–10.
Thames Jr HD, Withers HR, Peters LJ, Fletcher GH. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8(2):219–26.
Yarnold J, Somaiah N, Bliss JM. Hypofractionated radiotherapy in early breast cancer: clinical, dosimetric and radio-genomic issues. Breast. 2015;24(2):S108–113.
Whelan T, MacKenzie R, Julian J, Levine M, Shelley W, Grimard L, Lada B, Lukka H, Perera F, Fyles A, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94(15):1143–50.
Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, Brown J, Dewar JA, Dobbs HJ, Haviland JS, et al. The UK standardisation of breast radiotherapy (START) trial a of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9(4):331–41.
Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bentzen SM, Bliss JM, Brown J, Dewar JA, Dobbs HJ, et al. The UK standardisation of breast radiotherapy (START) trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371(9618):1098–107.
Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, Vu TT, Truong P, Ackerman I, Paszat L. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13):2085–92.
Donovan E, Bleakley N, Denholm E, Evans P, Gothard L, Hanson J, Peckitt C, Reise S, Ross G, Sharp G, et al. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiol Oncol. 2007;82(3):254–64.
Freedman GM, Anderson PR, Li J, Eisenberg DF, Hanlon AL, Wang L, Nicolaou N. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am J Clin Oncol. 2006;29(1):66–70.
Freedman GM, Li T, Nicolaou N, Chen Y, Ma CC, Anderson PR. Breast intensity-modulated radiation therapy reduces time spent with acute dermatitis for women of all breast sizes during radiation. Int J Radiat Oncol Biol Phys. 2009;74(3):689–94.
Harsolia A, Kestin L, Grills I, Wallace M, Jolly S, Jones C, Lala M, Martinez A, Schell S, Vicini FA. Intensity-modulated radiotherapy results in significant decrease in clinical toxicities compared with conventional wedge-based breast radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68(5):1375–80.
Jones B, Dale RG, Deehan C, Hopkins KI, Morgan DA. The role of biologically effective dose (BED) in clinical oncology. Clin Oncol. 2001;13(2):71–81.
Schnur JB, Ouellette SC, Dilorenzo TA, Green S, Montgomery GH. A qualitative analysis of acute skin toxicity among breast cancer radiotherapy patients. Psycho-Oncology. 2011;20(3):260–8.
Rudat V, Hammoud M, Pillay Y, Alaradi AA, Mohamed A, Altuwaijri S. Impact of the frequency of online verifications on the patient set-up accuracy and set-up margins. Radiat Oncol. 2011;6:101.
Shaitelman SF, Schlembach PJ, Arzu I, Ballo M, Bloom ES, Buchholz D, Chronowski GM, Dvorak T, Grade E, Hoffman KE, et al. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: a randomized clinical trial. JAMA oncol. 2015;1(7):931–41.
Jagsi R, Griffith KA, Boike TP, Walker E, Nurushev T, Grills IS, Moran JM, Feng M, Hayman J, Pierce LJ. Differences in the acute toxic effects of breast radiotherapy by fractionation schedule: comparative analysis of physician-assessed and patient-reported outcomes in a large multicenter cohort. JAMA oncol. 2015;1(7):918–30.
Kraus-Tiefenbacher U, Sfintizky A, Welzel G, Simeonova A, Sperk E, Siebenlist K, Mai S, Wenz F. Factors of influence on acute skin toxicity of breast cancer patients treated with standard three-dimensional conformal radiotherapy (3D-CRT) after breast conserving surgery (BCS). Radiat Oncol. 2012;7:217.
Karasawa K, Kunogi H, Hirai T, Hojo H, Hirowatari H, Izawa H, Ito K, Sasai K, Kawashima M, Furuya T, et al. Comparison of hypofractionated and conventionally fractionated whole-breast irradiation for early breast cancer patients: a single-institute study of 1,098 patients. Breast cancer. 2014;21(4):402–8.
Morganti AG, Cilla S, Valentini V, Digesu C, Macchia G, Deodato F, Ferrandina G, Cece MG, Cirocco M, Garganese G, et al. Phase I-II studies on accelerated IMRT in breast carcinoma: technical comparison and acute toxicity in 332 patients. Radiol Oncol. 2009;90(1):86–92.
Hardee ME, Raza S, Becker SJ, Jozsef G, Lymberis SC, Hochman T, Goldberg JD, DeWyngaert KJ, Formenti SC. Prone hypofractionated whole-breast radiotherapy without a boost to the tumor bed: comparable toxicity of IMRT versus a 3D conformal technique. Int J Radiat Oncol Biol Phys. 2012;82(3):e415–423.
De Langhe S, Mulliez T, Veldeman L, Remouchamps V, van Greveling A, Gilsoul M, De Schepper E, De Ruyck K, De Neve W, Thierens H. Factors modifying the risk for developing acute skin toxicity after whole-breast intensity modulated radiotherapy. BMC Cancer. 2014;14:711.
Dorn PL, Corbin KS, Al-Hallaq H, Hasan Y, Chmura SJ. Feasibility and acute toxicity of hypofractionated radiation in large-breasted patients. Int J Radiat Oncol Biol Phys. 2012;83(1):79–83.
Goldsmith C, Haviland J, Tsang Y, Sydenham M, Yarnold J. Large breast size as a risk factor for late adverse effects of breast radiotherapy: is residual dose inhomogeneity, despite 3D treatment planning and delivery, the main explanation? Radiother Oncol. 2011;100(2):236–40.
Barnett GC, Wilkinson JS, Moody AM, Wilson CB, Twyman N, Wishart GC, Burnet NG, Coles CE. The Cambridge breast intensity-modulated radiotherapy trial: patient- and treatment-related factors that influence late toxicity. Clin Oncol. 2011;23(10):662–73.
Vicini FA, Sharpe M, Kestin L, Martinez A, Mitchell CK, Wallace MF, Matter R, Wong J. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2002;54(5):1336–44.
Moody AM, Mayles WP, Bliss JM, A’Hern RP, Owen JR, Regan J, Broad B, Yarnold JR. The influence of breast size on late radiation effects and association with radiotherapy dose inhomogeneity. Radiol Oncol. 1994;33(2):106–12.
Rudat V, Alaradi AA, Mohamed A, Ai-Yahya K, Altuwaijri S. Tangential beam IMRT versus tangential beam 3D-CRT of the chest wall in postmastectomy breast cancer patients: a dosimetric comparison. Radiol Oncol. 2011;6:26.
Rudat V, Nour A, Alaradi AA, Mohamed A, Altuwaijri S. In vivo surface dose measurement using GafChromic film dosimetry in breast cancer radiotherapy: comparison of 7-field IMRT, tangential IMRT and tangential 3D-CRT. Radiol Oncol. 2014;9:156.
Mangoni M, Bisanzi S, Carozzi F, Sani C, Biti G, Livi L, Barletta E, Costantini AS, Gorini G. Association between genetic polymorphisms in the XRCC1, XRCC3, XPD, GSTM1, GSTT1, MSH2, MLH1, MSH3, and MGMT genes and radiosensitivity in breast cancer patients. Int J Radiat Oncol Biol Phys. 2011;81(1):52–8.
Acknowledgments
Not applicable.
Funding
Not applicable.
Availability of data and materials
The presented data is summarized in this paper. The complete datasets can be retrieved from the authors upon formal request from interested readers.
Authors’ contributions
All authors participated in the collection and interpretation of the data, and drafting of the manuscript. VR designed and supervised the study, and performed the statistical analysis. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study was approved by the institutional ethical committee of Saad Specialist Hospital and was conducted in accordance with the Helsinki Declaration in its current version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Rudat, V., Nour, A., Ghaida, S.A. et al. Impact of hypofractionation and tangential beam IMRT on the acute skin reaction in adjuvant breast cancer radiotherapy. Radiat Oncol 11, 100 (2016). https://doi.org/10.1186/s13014-016-0674-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-016-0674-y