Abstract
Background
The neutrophil-lymphocyte ratio (NLR) has been proposed as an indicator of systemic inflammatory response and may predict the clinical outcome in some cancers, such as head and neck cancer and gastric cancer. However, the value of this ratio is variable in different cancers. Studies of the relationship between NLR and both survival and response to chemoradiation have been limited with respect to locally advanced rectal cancer.
Methods and materials
From 2006 to 2011, 199 consecutive locally advanced rectal cancer patients who were treated with neoadjuvant chemoradiation in the Shanghai Cancer Center were enrolled and analysed retrospectively. Tumor response was evaluated by pathological findings. The baseline total white blood cell count (WBC) and the neutrophil, lymphocyte, platelet counts were recorded. The neutrophil-lymphocyte ratio (NLR) and the relationship with clinical outcomes such as overall survival (OS) and disease-free survival (DFS) was analyzed.
Results
With ROC analysis, the baseline NLR value was found to significantly predict prognosis in terms of OS well in locally advanced rectal cancer patients. A multivariate analysis identified that a cut-off value of NLR ≥ 2.8 could be used as an independent factor to indicate decreased OS (HR, 2.123; 95% CI, 1.140-3.954; P = 0.018). NLR ≥ 2.8 was also associated with worse DFS in univariate analysis (HR, 1.662; 95% CI, 1.037-2.664; P = 0.035), though it was not significant in the multivariate analysis (HR, 1.363; 95% CI, 0.840-2.214; P = 0.210). There was no observed significant correlation of mean value of NLR to the response to neoadjuvant chemoradiation. The mean NLR in the ypT0-2 N0 group was 2.68 ± 1.38, and it was 2.77 ± 1.38 in the ypT3-4/N+ group, with no statistical significance (P = 0.703). The mean NLR in the TRG 0–1 group was 2.68 ± 1.42, and it was 2.82 ± 1.33 in the TRG 2–3 group with no statistical significance (P = 0.873).
Conclusions
An elevated baseline NLR is a valuable and easily available prognostic factor for OS in addition to tumor response after neoadjuvant therapy. Baseline NLR could be a useful candidate factor for stratifying patients and making treatment decisions in locally advanced rectal cancer.
Similar content being viewed by others
Background
Neoadjuvant chemoradiation therapy (NACRT) has been established as a standard treatment for locally advanced rectal cancer (LARC). Patients who achieve complete pathological response after chemoradiation show a better survival [1],[2]. Although pathological examination and conventional clinicopathological prognostic variables remain the primary assessment, researchers have attempted to quantify tumor response to neoadjuvant chemoradiation and predict prognosis using other factors. Many studies have focused on the prognostic significance of genes, proteins and inflammatory factors, but no consensus has been reached. Identifying patients with differential therapeutic responses and prognosis according to affordable and reliable markers is significant for following risk-stratified therapy in locally advanced rectal cancer.
Recently, the local and systemic inflammatory response has been reported as an important determinant of disease progression and survival in colorectal cancer [3]. The Glasgow prognostic score, which is based on elevated circulating concentrations of C-reactive protein and hypoalbuminaemia, is independently associated with poor survival [4]. Other inflammatory parameters such as interleukins, TGFβ and VEGF have also been found to be associated with outcome [5]. However, these parameters are not routinely measured in daily clinical practice. The systemic inflammatory response measured using the surrogate neutrophil-lymphocyte ratio (NLR) has been proposed as an inexpensive and widely available marker to predict cancer patient survival [6]. Several studies have demonstrated that elevated NLR was associated with inferior survival in several common cancers, including colorectal cancer [7]-[9], gastric cancer [10], renal cancer [11], breast cancer [12] and pancreatic cancer [13]. The causes of this inferior survival have not yet been identified, but an elevated NLR is thought to correlate with the decline of nutrition and immune function. More specifically in locally advanced rectal cancer (LARC) patients, some studies have demonstrated that an elevated lymphocyte count is associated with increased downstaging following neoadjuvant chemoradiation [14], while elevated NLR is associated with short time to local recurrence (TTLR) and worse overall survival (OS) and disease-free survival (DFS) [15]. However, with regard to the response of neoadjuvant chemoradiation and prognosis in locally advanced rectal cancer patients, there are still relatively few studies also with small number of patients have focused on this issue. In addition, it’s very important to discriminate different risk groups up front, because this may influence the treatment modality choosing for these patients. If the patients’ baseline characteristics can be stratified into different risk groups, we may avoid over-treatment for patients with good prognosis while intensify treatment for patients with poor prognosis.
Therefore, the aim of our study is to further clarify the prognostic significance of the baseline NLR in locally advanced rectal cancer with neoadjuvant chemoradiation and its relationship with radiation response.
Methods
Patients and evaluation
A consecutive cohort of 224 patients with locally advanced (cT3-4 and/or cN1-2) rectal cancer treated with neoadjuvant chemoradiotherapy followed by surgery at the Fudan University Shanghai Cancer Center between January 2006 and December 2011 was identified from the colorectal cancer database. All medical records were retrospectively reviewed.
Primary tumors were staged by MRI. All patients underwent conventional radiation with concurrent 5-fluorouracil-based chemotherapy. The mean radiation dose was 50 Gy (range 45–55 Gy) with daily fraction of 1.8-2.0 Gy. Radiation treatments were performed according to the institutional protocols. For the analysis in this study, the exclusion criteria included patients who did not have rectal adenocarcinoma, were diagnosed with a non–skin cancer within 5 years of the diagnosis of rectal cancer, did not complete neoadjuvant chemoradiation, were found to have metastatic disease before or at the time of surgery or had an interval from the completion of radiation to surgery greater than 16 weeks.
In this data set, 208 patients met the criteria, and 199 patients had retrievable baseline blood sampling reports within 7 days before the start of chemoradiation. The blood routine examination was detected by fluorescence flow cytometry in our center. The total white blood cell count (WBC) and the neutrophil, lymphocyte and platelet counts were recorded. The neutrophil-lymphocyte ratio (NLR) was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. The pre-treatment carcinoembryonic antigen (CEA) levels were categorised as normal (<5 ng/ml) or elevated (≥5 ng/ml). Pathologic tumor staging of the surgical specimen was performed in accordance with the 7th guidelines of the American Joint Committee on Cancer [16]. Written informed consent was obtained from the patient for the publication of this report and any accompanying images and this study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center.
The primary endpoint of the study was overall survival (OS) and disease-free survival (DFS). The secondary endpoint was neoadjuvant chemoradiation response. OS was defined as the time from the date of surgery to death from any cause. DFS was defined as the time from the date of surgery to the date of tumor relapse (local recurrence and/or distant metastases) or death. We evaluated tumor response with both ypTNM staging and TRG score. In the ypTNM staging system, good response was defined as ypT0 to 2 without lymph node metastases, and poor response was defined as ypT3 to 4 or with identified lymph node metastases. The four-point TRG system graded on a scale of 0(complete response; no viable cancer cells) to 3 (poor response; minimal or no regression, extensive residual cancer) [16]. Patients with TRG0-1 had good response while TRG2-3 had poor response.
Statistical analysis
Receiver operating characteristic (ROC) analysis and relative area under the curve (AUC) statistics were used, and the ratio closest to the point with the maximum sensitivity and specificity was selected as the optimal cut-off value. The NLR was correlated with the clinicopathological variables using the chi-square test or Fisher’s exact test. Continuous data were expressed as the median (range) or mean ± standard deviation (SD) and compared using an independent two-sample t-test. Survival curves were made using the Kaplan-Meier method, and groups were compared using the log-rank test. Univariate and multivariate analyses to identify prognostic predictors were performed using Cox proportional hazard regression models. Variables with P < 0.10 on univariate analysis were entered into multivariate analyses.
Statistical analysis was performed using the SPSS statistical software package, version 18.0 (SPSS Inc., Chicago, IL, USA). A two-sided P < 0.05 was considered statistically significant.
Results
The baseline patient characteristics and tumor pathological factors are shown in Table 1. Seventy-five percent of the patients were male, with a mean age of 55 years (range 22–76 years). Of these patients, 185 (93%) patients had curative resection, and 14 (7%) patients had palliative surgery. Clear surgical margins were achieved in 195 (98%) patients. There were 88 (44.2%) patients who were at ypT0-2 N0 after neoadjuvant chemoradiation and 38 (19.1%) achieved pathological complete response (pCR). One hundred seventeen (58.8%) patients achieved TRG0-1. For all patients, the baseline median lymphocyte count was 1.6 × 109/l (range 0.6-3.8), the median neutrophil count was 3.9 × 109/l (range 1.3-15.7), and the median NLR was 2.4 (range 1.0-8.9).
Of the 199 rectal cancer patients with baseline blood sample reports, 19 (9.5%) patients developed local recurrence, 49 (24.6%) patients developed distant metastasis and 43 (21.6%) patients had died by the time of last follow-up. The median follow-up for survivors was 31 months (range 1–84 months). In the ROC analysis, the AUC (area under the curve) for NLR was 0.635 (P = 0.007) for OS and 0.569 (P = 0.107) for DFS. The optimal cut-off value for NLR was 2.8 and 3.6 for OS and DFS, respectively. Because the NLR could not statistically discriminate DFS, the cut-off value of 2.8 was chosen for further analysis. An NLR ≥2.8 was defined as high NLR, and an NLR < 2.8 was defined as low NLR in our study.
Table 1 shows the difference in clinicopathological characteristics between the NLR groups. Overall, 133 (66.8%) patients had a low NLR, and 66 patients (33.2%) had a high NLR. A high NLR was only significantly associated with a higher clinical stage before treatment. None of the other clinicopathological characteristics were found to associate with the NLR.
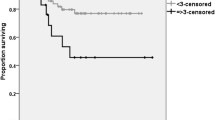
An elevated NLR was a significantly poor prognostic factor. The 5-year overall survival (OS) in patients with NLR <2.8 was 0.717 and 0.437 in patients with NLR ≥2.8 (P = 0.002) (Figure 1). The 5-year disease-free survival (DFS) in patients with NLR <2.8 was 0.625, and it was 0.334 in patients with NLR ≥2.8 (P = 0.032) (Figure 2).
The results of the univariate analysis are shown in Table 2. An elevated NLR before chemoradiation was significantly associated with decreased OS (HR, 2.526; 95% CI, 1.384-4.610; P = 0.003) and decreased DFS (HR, 1.662; 95% CI, 1.037-2.664; P = 0.035). A multivariate analysis was performed for the characteristics with P < 0.1 by univariate analysis (Table 3). This analysis indicated that NLR (HR, 2.123; 95% CI, 1.140-3.954; P = 0.018), ypTNM staging (HR, 3.928; 95% CI, 1.651-9.345; P = 0.002) and adjuvant chemotherapy completion (HR, 3.506; 95% CI, 1.251-9.827; P = 0.017) were associated with OS. NLR showed no association with DFS (HR, 1.363; 95% CI, 0.840-2.214; P = 0.210), and ypTNM staging (HR, 3.477; 95% CI, 1.857-6.509; P < 0.001) was the only factor associated with DFS in multivariate analysis.
We also validated the cut-off values from a previous study in our patient cohort. Carruthers et al. [15] found that an NLR ≥5 could predict outcome in patients undergoing neoadjuvant chemoradiation for locally advanced rectal cancer. In our study, a NLR ≥5 was significantly associated with worse OS (HR, 3.286; 95% CI, 1.362-7.932; P = 0.008) in the univariate analysis and remained significant in the multivariate analysis (HR, 2.849; 95% CI, 1.097-7.398; P = 0.032). For DFS, NLR ≥5 did not show statistical significance in either the univariate or multivariate analysis. However, there were only 15 (7.5%) patients with NLR ≥5 in our cohort.
The relationship of the tumor response to the mean NLR and the mean lymphocyte count was analysed. When we evaluated with ypTNM staging system, the mean NLR in the ypT0-2 N0 group was 2.68 ± 1.38, and it was 2.77 ± 1.38 in the ypT3-4/N+ group with no statistical significance (P = 0.703). The mean lymphocyte count was 1.70 ± 0.57 and 1.70 ± 0.60 for the good and poor response groups (P = 0.884), respectively. When we evaluated with TRG system, the mean NLR in the TRG 0–1 group was 2.68 ± 1.42, and it was 2.82 ± 1.33 in the TRG 2–3 group with no statistical significance (P = 0.873). The mean lymphocyte count was 1.66 ± 0.54 and 1.76 ± 0.64 for the good and poor response groups (P = 0.260), respectively. ROC analysis showed a minimal sensitivity of NLR to predict ypTNM staging (AUC = 0.526; P = 0.534) or TRG score (AUC = 0.546; P = 0.268). The chi-square test in Table 1 also showed no significant association between an NLR of 2.8 with ypTNM staging or TRG score. Therefore, NLR and lymphocyte count were unable to predict neoadjuvant chemoradiation response in these LARC patients.
Discussion
Our study has highlighted the potential ability of NLR to serve as a prognostic marker for overall survival in locally advanced rectal cancer (LARC) patients. NLR ≥2.8 is associated with worse survival in these patients undergoing neoadjuvant chemoradiation, but no correlation was found between NLR and radiation response. Several translational studies have demonstrated that NLR can serve as a marker of systemic inflammatory and immune response and can predict clinically useful outcomes for multiple cancers, including LARC. Taken together, these results indicate that the NLR is a simple and robust laboratory variable for risk stratification in LARC patients.
The mechanisms of inflammation that promote tumor progression and immune response suppression remain under investigation. A high NLR reflects an enhanced neutrophil response and/or relative lymphopenia. Neutrophils are a type of inflammatory cell that could secrete circulating vascular endothelial growth factor (VEGF), chemokines and proteases that stimulate angiogenesis, and these inflammatory cytokines may establish a tumor microenvironment and promote tumor development and progression [17],[18]. Moreover, an elevated neutrophil count may activate immunosuppression through the inhibition of natural killer cells and activated T cells [19],[20]. Furthermore, lymphocytes are thought to be responsible for the adaptive immune response and participate in cancer immunosurveillance and immunoediting [21]. Tumor-infiltrating lymphocytes appear to play an anti-tumorigenic role in colorectal cancer [22]. The proportion of different subsets of T cells can also influence the clinical outcome. High densities of CD3+ T cells, CD8+ cytotoxic T cells and CD45RO+ memory T cells are typically associated with a longer disease-free survival (after surgical resection of the primary tumor) and/or overall survival [23]. Therefore, the NLR may influence the survival outcome by influencing the tumor microenvironment and immune system in several types of cancer. More specific studies are needed to combine such immunohistochemical and bio-molecular analyses.
Although there is a body of evidence suggesting that an elevated NLR indicates worse survival, the threshold of NLR ranges between 2 and 5 in different studies of colorectal cancer [24], and the relationship between NLR and tumor response to neoadjuvant chemoradiotherapy remains controversial. Carruthers et al. found that an elevated NLR was associated with worse survival, but they found no correlation between NLR and tumor downstaging [15], which is in agreement with the findings of our study. A validation cut-off value of 5, as used in their study [15], could discriminate OS in our cohort as well, but with less sensitivity. Therefore, a much larger population is needed for identifying the optimal cut-off value. Kitayama et al. analyzed 73 locally advanced rectal cancer patients retrospectively and showed that the lymphocyte ratio in WBC was significantly higher (P = 0.020) and the neutrophil ratio tended to be lower (P = 0.099) in a pathological complete response (pCR) group [14]. Krauthamer et al. reported a significantly higher probability of pCR to NACRT in patients with clinical stage III LARC patients who had an NLR value <5 (OR = 2.54; P = 0.04) by multivariate analysis, but it should be noted that only 10 patients achieved pCR [25]. Additional parameters in routine blood measurements such as platelets may have implications for the metastasis and prognosis of colorectal cancer [26]. However, we found that neither NLR nor lymphocyte counts was associated with tumor response evaluated with ypTNM staging or TRG score, and no relationship was found between platelet count and tumor response or survival. A further validation of these differences is necessary.
Morever, Kitayama J, et al. found circulating lymphocyte count was most markedly decreased in the CRT period and gradually recovered by the time of surgery, while the numbers of neutrophils were comparatively stable [14]. We have observed that the NLR was mainly influenced by concomittant chemotherapy and granulocyte colony-stimulating factors during the therapeutic process of chemoradiation. That is, the NLR would like to decrease after concomittant chemotherapy while increase after treating with granulocyte colony-stimulating factors. Therefore, the baseline NLR which is not interfered by any therapy is more important as a prognostic factor for patients.
It should be noted that although the NLR is easy to measure, its utility as a marker of systemic inflammation may be affected by many conditions, including coronary disease [27], metabolic syndrome [28], inflammatory diseases and any medication related to inflammatory conditions in the patients. These limitations must be considered because this is a retrospective study, and no specific data were available about co-morbidities and the cause of death. These variables will influence the specificity of NLR as a prognostic factor in our rectal cancer patient cohort. Another limitation is that 86.9% of the patients in our cohort with stage III have a worse 5-year OS (62%) compared with well-organised randomized clinical trial patients (76%) [29]. This result is likely because our cohort is a routine locally advanced rectal cancer population without strict inclusion criteria for neoadjuvant chemoradiation. Although there was no significant difference in the DFS between the NLR groups according to the multivariate analysis, the difference may be significant with a longer follow-up time and a larger patient population.
Even considering these limitations, our data indicate that an elevated pre-treatment NLR may serve as a prognostic marker for risk stratification in locally advanced rectal cancer patients who received neoadjuvant chemoradiation. In addition, tumor response, which is the tumor stage after treatment, and the ability to receive adjuvant chemotherapy remain the most significant prognostic factors for overall survival in the multivariate analysis. In this study, the NLR did not represent a alternate factor for either of them. Although systemic adjuvant chemotherapy is an independent prognostic factor for OS in our study, the benefit of adjuvant chemotherapy remains controversial. The EORTC 22921 study showed that the addition of adjuvant chemotherapy benefited only the group of patients who had a reduction in tumor stage to ypT0-2, while there was no benefit in the group who had no tumor reduction (ypT3-4) [30]. The ongoing NCT01941979 study is the first phase III randomized trial comparing adjuvant chemotherapy and observation for rectal adenocarcinoma patients with ypT3-4, N0-1 after neoadjuvant chemoradiotherapy. The investigator believes that this subgroup of patients will benefit from adjuvant therapy [31]. Therefore, it may make sense for patients with elevated NLR to receive intensive preoperative treatment because the postoperative chemotherapy remains controversial.
Conclusion
In conclusion, our study demonstrated that the pre-treatment NLR may be an independent, significant predictor of long-term mortality in locally advanced rectal cancer patients undergoing neoadjuvant chemoradiation. Large-scale prospective studies and a validation study are warranted to confirm the optimal NLR cut-off value and its prognostic significance.
References
Zorcolo L, Rosman AS, Restivo A, Pisano M, Nigri GR, Fancellu A, Melis M: Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol 2012, 19: 2822-2832. 10.1245/s10434-011-2209-y
de Campos-Lobato LF, Stocchi L, da Luz MA, Geisler D, Dietz DW, Lavery IC, Fazio VW, Kalady MF: Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol 2011, 18: 1590-1598. 10.1245/s10434-010-1506-1
Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC: The relationship between the local and systemic inflammatory responses and survival in patients undergoing curative surgery for colon and rectal cancers. J Gastrointest Surg 2009, 13: 2011-2018. discussion 2018–2019 10.1007/s11605-009-1034-0
Miki C, Tanaka K, Toiyama Y, Inoue Y, Uchida K, Mohri Y, Kusunoki M: Comparison of the prognostic value of inflammation-based pathologic and biochemical criteria in patients undergoing potentially curative resection for colorectal cancer. Ann Surg 2010, 251: 389-390. author reply 390–381 10.1097/SLA.0b013e3181cb8b31
Lippitz BE: Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 2013, 14: e218-228. 10.1016/S1470-2045(12)70582-X
Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ: The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013, 88: 218-230. 10.1016/j.critrevonc.2013.03.010
Malietzis G, Giacometti M, Askari A, Nachiappan S, Kennedy RH, Faiz OD, Aziz O, Jenkins JT: A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg 2014, 260: 287-292. 10.1097/SLA.0000000000000216
Absenger G, Szkandera J, Pichler M, Stotz M, Arminger F, Weissmueller M, Schaberl-Moser R, Samonigg H, Stojakovic T, Gerger A: A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer 2013, 109: 395-400. 10.1038/bjc.2013.346
Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K: Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer 2013, 109: 401-407. 10.1038/bjc.2013.350
Lee DY, Hong SW, Chang YG, Lee WY, Lee B: Clinical significance of preoperative inflammatory parameters in gastric cancer patients. J Gastric Cancer 2013, 13: 111-116. 10.5230/jgc.2013.13.2.111
Pichler M, Hutterer GC, Stoeckigt C, Chromecki TF, Stojakovic T, Golbeck S, Eberhard K, Gerger A, Mannweiler S, Pummer K, Zigeuner R: Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer 2013, 108: 901-907. 10.1038/bjc.2013.28
Azab B, Shah N, Radbel J, Tan P, Bhatt V, Vonfrolio S, Habeshy A, Picon A, Bloom S: Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol 2013, 30: 432. 10.1007/s12032-012-0432-4
Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, Stojakovic T, Samonigg H, Hoefler G, Pichler M: Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 2013, 109: 416-421. 10.1038/bjc.2013.332
Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H: Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol 2010, 5: 47. 10.1186/1748-717X-5-47
Carruthers R, Tho LM, Brown J, Kakumanu S, McCartney E, McDonald AC: Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Colorectal Dis 2012, 14: e701-707. 10.1111/j.1463-1318.2012.03147.x
Edge S, Byrd D, Carducci M, Compton C. AJCC Cancer Staging Manual. 7. 2009.
Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, Mignatti P: Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol 2001, 189: 197-206. 10.1002/jcp.10014
Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH: Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 2003, 6: 283-287. 10.1023/B:AGEN.0000029415.62384.ba
Shau HY, Golub SH: Inhibition of lymphokine-activated killer- and natural killer-mediated cytotoxicities by neutrophils. J Immunol 1989, 143: 1066-1072.
Di Carlo E, Forni G, Musiani P: Neutrophils in the antitumoral immune response. Chem Immunol Allerg 2003, 83: 182-203. 10.1159/000071561
Dunn GP, Old LJ, Schreiber RD: The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21: 137-148. 10.1016/j.immuni.2004.07.017
Ohtani H: Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun 2007, 7: 4.
Fridman WH, Pages F, Sautes-Fridman C, Galon J: The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012, 12: 298-306. 10.1038/nrc3245
Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, Lv Y: Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer 2014, 134: 2403-2413. 10.1002/ijc.28536
Krauthamer M, Rouvinov K, Ariad S, Man S, Walfish S, Pinsk I, Sztarker I, Charkovsky T, Lavrenkov K: A study of inflammation-based predictors of tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Oncology 2013, 85: 27-32. 10.1159/000348385
Wan S, Lai Y, Myers RE, Li B, Hyslop T, London J, Chatterjee D, Palazzo JP, Burkart AL, Zhang K, Xing J, Yang H: Preoperative platelet count associates with survival and distant metastasis in surgically resected colorectal cancer patients. J Gastrointest Cancer 2013, 44: 293-304. 10.1007/s12029-013-9491-9
Adamsson Eryd S, Smith JG, Melander O, Hedblad B, Engstrom G: Incidence of coronary events and case fatality rate in relation to blood lymphocyte and neutrophil counts. Arterioscler Thromb Vasc Biol 2012, 32: 533-539. 10.1161/ATVBAHA.111.240416
Balta S, Cakar M, Demirkol S, Arslan Z, Akhan M: Higher neutrophil to lymhocyte ratio in patients with metabolic syndrome. Clin Appl Thromb Hemost 2013, 19: 579. 10.1177/1076029612475023
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R: German Rectal Cancer Study G: Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004, 351: 1731-1740. 10.1056/NEJMoa040694
Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, Radosevic-Jelic L, Pierart M, Calais G: European Organisation for R, Treatment of Cancer Radiation Oncology G: Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 2007, 25: 4379-4386. 10.1200/JCO.2007.11.9685
A Study Comparing Adjuvant Chemotherapy Versus Observation for Patients With Rectal Adenocarcinoma After Neoadjuvant Chemo-Radiotherapy Treatment. Available at: http://clinicaltrials.gov/ct2/show/NCT01941979
Acknowledgements
This work was supported by the grants from Natural Science Foundation of China (81372432).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LS, HZ and LL participated in manuscript drafting, table creation, and manuscript revision. GL and MF participated the study design and revised the manuscript. JZ revised the manuscript and provided important intellectual content. YW performed statistical analyses of data. ZZ participated in the study design and was responsible for final approval of the manuscript. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Shen, L., Zhang, H., Liang, L. et al. Baseline neutrophil-lymphocyte ratio (≥2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Radiat Oncol 9, 295 (2014). https://doi.org/10.1186/s13014-014-0295-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-014-0295-2