Abstract
Background
The use of low-value care (LVC) is a persistent problem that calls for knowledge about strategies for de-implementation. However, studies are dispersed across many clinical fields, and there is no overview of strategies that can be used to support the de-implementation of LVC. The extent to which strategies used for implementation are also used in de-implementing LVC is unknown. The aim of this scoping review is to (1) identify strategies for the de-implementation of LVC described in the scientific literature and (2) compare de-implementation strategies to implementation strategies as specified in the Expert Recommendation for Implementing Change (ERIC) and strategies added by Perry et al.
Method
A scoping review was conducted according to recommendations outlined by Arksey and O’Malley. Four scientific databases were searched, relevant articles were snowball searched, and the journal Implementation Science was searched manually for peer-reviewed journal articles in English. Articles were included if they were empirical studies of strategies designed to reduce the use of LVC. Two reviewers conducted all abstract and full-text reviews, and conflicting decisions were discussed until consensus was reached. Data were charted using a piloted data-charting form. The strategies were first coded inductively and then mapped onto the ERIC compilation of implementation strategies.
Results
The scoping review identified a total of 71 unique de-implementation strategies described in the literature. Of these, 62 strategies could be mapped onto ERIC strategies, and four strategies onto one added category. Half (50%) of the 73 ERIC implementation strategies were used for de-implementation purposes. Five identified de-implementation strategies could not be mapped onto any of the existing strategies in ERIC.
Conclusions
Similar strategies are used for de-implementation and implementation. However, only a half of the implementation strategies included in the ERIC compilation were represented in the de-implementation studies, which may imply that some strategies are being underused or that they are not applicable for de-implementation purposes. The strategies assess and redesign workflow (a strategy previously suggested to be added to ERIC), accountability tool, and communication tool (unique new strategies for de-implementation) could complement the existing ERIC compilation when used for de-implementation purposes.
Similar content being viewed by others
Background
Recognition is growing with regard to the importance of reducing low-value care (LVC), i.e., “care that is unlikely to benefit the patient given the harms, cost, available alternatives, or preferences of the patient” [1]. Common examples of LVC are non-indicated antibiotics, unnecessary imaging, potentially inappropriate medications for the elderly and unnecessary lab tests [2]. LVC has become a pervasive problem in health care in high-income countries [1, 3,4,5], with around 30% of care estimated to be of low value [6]. Furthermore, estimations show that about 7% of the care considered to be best practice 1 year becomes LVC the next [7]. Thus, the rapid development of new practices (e.g., diagnostics and treatments) not only calls for continuous implementation of new evidence but also requires the de-implementation of LVC. De-implementation entails a structured process with the purpose of reducing or ceasing the use of LVC [8].
Similar to implementing evidence-based interventions, de-implementing LVC is a complex process influenced by multilevel factors [9]. Determinants of LVC use and de-implementation include various patient characteristics such as age, gender, ethnicity, and socio-economic factors, although there are no consistent patterns as to their positive or negative influence on LVC [2]. For instance, older age is usually associated with use of LVC [10,11,12], but some studies have linked younger age with higher LVC use [13, 14]. Patients’ health conditions—e.g., the severity of illness and characteristics of the disease [15, 16]—often contribute to use of LVC. Patient expectations, e.g., patients who request non-indicated prescriptions, also tend to increase the occurrence of LVC [17, 18]. Health professionals’ characteristics are also associated with LVC use. As with patient characteristics, the results are inconsistent regarding professionals’ age, gender, and length of experience [2]. However, a lack of or inadequate training has consistently been linked to use of LVC [17, 19]. Professionals’ knowledge of LVC contributes to and protects against the use of LVC [2]. For example, a lack of knowledge about cost-effectiveness [20] and poor cost-awareness [21] are associated with use of LVC. Professionals’ expectations and attitudes also influence LVC use, e.g., their fear of malpractice and desire to meet patient requests [2]. Interaction between patients and professionals can also impact the use of LVC, e.g., communication about unnecessary antibiotics or tests [22].
Determinants of LVC also exist at the contextual level [2]. Inner context, including setting characteristics, care processes (e.g., lack of care continuity), perceived lack of time and time pressure when performing work tasks, accessibility of decision support, staffing levels, and composition and organizational incentives for LVC use have been identified [2]. Outer context determinants have included location of the health care organization (e.g., metropolitan, urban, suburban, or rural), financing and financial incentives (e.g., fee-for-service funding), policy and political support, and marketing initiatives such as promotion of screening directed to the population and direct-to-consumer advertising about drugs or treatments.
Yet, knowing the potential determinants of LVC is not sufficient for changing them. Strategies to address determinants constitute the “how-to” component of changing practice. Strategies are methods and techniques to facilitate implementation of evidence-based practices and/or de-implementation of LVC [23]. Some implementation strategies are likely to be applicable for de-implementation, while other strategies may be unique or more applicable for de-implementation [24]. However, the evidence for de-implementation strategies is dispersed across multiple clinical fields, which makes it difficult to document and survey findings [2]. Studies investigating strategies to reduce LVC have been published in a broad range of journals, typically within specific clinical and medical care areas, from microbiological research on antimicrobial resistance to potentially inappropriate medication for the elderly [2]. Studies on strategies for de-implementation have also focused on specific LVC within fields such as nursing [25], low-value blood management techniques in primary hip and knee arthroplasty [26], pharmacological prescriptions [27], and cancer [28]. An exception is a systematic review of de-implementation strategies covering a wide range of clinical areas [29] that found promising results for clinical decision support and performance feedback, concluding that multicomponent strategies addressing both clinicians and patients had the greatest potential for reducing LVC. Another review [28] focused specifically on cancer care similarly found that most de-implementation strategies were multifaceted. The most widely used strategies were audit and feedback, use of clinical champions, educating clinicians through developing and disseminating guidelines, and decision-support tools. Integrating a clinical decision-support tool in the electronic health record system for real-time alerts was the most effective strategy.
The de-implementation field suffers from a lack of established nomenclature for how strategies to de-implement LVC are named, defined, and organized. This makes it difficult to compare strategies across studies, hindering an accumulation of a generalizable body of knowledge related to effectively de-implementing LVC. A notable exception is a recent study in which researchers used the behavior-change techniques taxonomy to categorize de-implementation categories based on data from three systematic reviews [30]. They also compared de-implementation strategies to implementation strategies and showed that behavior substitution, monitoring of behavior by others without feedback, and restructuring social environment were more frequently used in de-implementation efforts than in implementation. However, this study was limited to strategies aiming to change individual clinicians’ behavior and did not cover strategies on a system or policy level.
In contrast, researchers studying implementation have developed taxonomies to guide the identification, selection, and reporting of strategies, thus making it easier to compare strategies across studies [31,32,33,34]. One such taxonomy is the ERIC compilation [31], which consists of 73 discrete implementation strategies belonging to nine categories [35]. ERIC has been widely used in implementation science and is useful in evaluations of implementation strategies [36,37,38,39]. However, it is unknown whether the same types of strategies are also used in de-implementation. It is likely that some of the 73 strategies and nine categories of the ERIC compilation are also relevant for de-implementation purposes. Still, findings from a recent study [30] using behavior change taxonomy suggest that this might not be the case, since de-implementation differs in the primary behavior-change techniques utilized. Thus, it is necessary to investigate to what extent de-implementation and implementation strategies are the same.
This study addresses two important knowledge gaps in the literature. First, due to the dispersion of studies on strategies for de-implementation across many clinical fields, no overview of the strategies that can be used to support LVC de-implementation exists. This fragmentation inhibits the systematic development of knowledge about effective strategies for the de-implementation of LVC. Second, it is unknown whether and to what extent implementation strategies are also applicable for de-implementing LVC. Addressing these key knowledge gaps, the aim of this review is to evaluate the scope of the literature to (1) identify strategies for the de-implementation of LVC described in the scientific literature and (2) compare de-implementation strategies to implementation strategies as specified in ERIC and strategies added by Perry et al. [36].
Methods
Design
We conducted a scoping review based on the steps outlined by Arksey and O´Malley [40] and reported the review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) checklist [41] (Additional file 1).
Protocol and registration
We described the method in a previously published study protocol [42].
Eligibility criteria
We included English-language articles describing empirical studies published in peer-reviewed journals. Using the PCC mnemonic (Population, Concept, Context) recommended for scoping reviews [43], we specified that the content of the articles should focus on interventions or strategies aiming to reduce LVC within health care (context). Health care was defined as all types of primary, hospital (secondary), community, and mental health care. We did not specify a population since we were interested in strategies for reducing LVC in health care in general. All study designs were considered. We included LVC practices as defined based on a published guideline or recommendation, as stated in the article. All eligibility criteria are reported in Table 1.
Information sources
We searched four electronic databases: MEDLINE, Embase, CINAHL, and Web of Science. We also searched the journal Implementation Science manually and searched reference lists in relevant articles for additional papers.
Search
We identified keywords for the search by means of an extensive discussion among ourselves, a review outlining potential terminology for de-implementation [44], an inspection of key articles, and discussions with representatives from the Swedish Agency for Health Technology Assessment and Assessment of Social Services. We identified 18 key articles used to inform the search strategy. In collaboration with the Karolinska Institutet library, we defined, tested, and refined a search strategy three times to ensure that it was broad enough to capture the 18 key articles and sufficiently discriminant to generate a reasonable number of articles. We conducted the first search in June 2018, and the search strategy included articles published from 2013 to June 2018. We chose to limit our time frame to make the number of papers feasible and chose 2013 as a starting point due to comparatively higher number of articles published during these years. We conducted a second search in September 2021 because a considerable number of studies had been published during the finalization of the review. The second search included articles published between June 2018 and September 2021. Table 2 shows the search strategy for Web of Science for the first search. All other strategies, including the second search, can be found as an attachment (Additional file 2). The only difference between the first and second search was the focus solely on strategies in the second one.
Selection of sources
We imported all articles to Rayyan [45] to screen. In total, eight people (authors 1, 2, 6, and 7 and four research assistants) were involved in the process. In the first step, authors 1 and 7, along with two research assistants, reviewed a sample of 40 abstracts independently to test the inclusion and exclusion criteria. They discussed differences in judgements and adjusted the criteria based on the discussion to ensure a consistent assessment among screeners. Authors 1, 2, and 7 and two research assistants then screened the abstracts independently. All abstracts were screened by two screeners, who discussed any inconsistencies. If these were not resolved, all authors discussed the inconsistencies. In the next step, two reviewers (authors 1, 6, and 7 and two other research assistants) assessed all full-text articles and applied the same process for resolving inconsistencies. Corresponding authors were contacted for clarification if the screeners were uncertain whether an article fulfilled the criteria for a formal guideline used to define LVC.
Data charting
Authors 1 and 6 developed a data-charting table in Excel and tested it on five articles. The authors did not find any inconsistencies between them. One author [6] continued with the data extraction of all articles from the first search and consulted the rest of the authors when needed. Two research assistants who had been trained by the first author conducted extraction from the second search. The first author reviewed all the extracted data.
Data items
The data that were charted included aim and research question; setting; study design; data collection method; type of evaluation (e.g., effectiveness/efficacy, process evaluation, or cost effectiveness); type of LVC; guideline used to define LVC; single or multicomponent strategy; and activities included in the strategy (as described in the original article).
Synthesis of results
The data were first coded inductively regarding the type of strategy described in the study. Inductive coding made it possible to capture a detailed description of the strategies and was chosen since no existing taxonomy for de-implementation strategies exists. The coding was performed in pairs. Strategies were excluded if they could not be understood as a result of limited reporting. All inductive codes were then compared and mapped onto ERIC strategies, including three new strategies suggested by Perry et al. [36]. Authors 1 and 5 performed the mapping onto ERIC, and authors 1 and 7 solved inconsistencies. Author 2 checked the samples of these codes to make sure the transfer from inductive codes to ERIC categories did not change the intent of the strategy described in the studies. All inductive codes and their ERIC mapping can be found in Additional file 3. The results of the mapping were then summarized based on the nine categories of ERIC strategies outlined by Waltz et al. [35].
Results
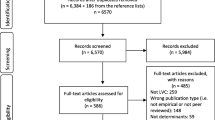
The first search resulted in 9642 citations and the second search in an additional 5816 (Fig. 1). An additional 186 citations in the first search and 70 in the second search were identified through searches in reference lists. Removing duplicates resulted in 6570 unique citations from the first search and 1,550 from the second search for abstract screening. After abstract screening, 605 citations from the first search and 370 from the second search were included for full-text review. Following full-text screening, 310 citations remained and were included in the study.
Study characteristics
The 310 studies originated from 38 countries, with almost half conducted in the USA (n = 136). The most frequent type of LVC was non-indicated antibiotics (n = 84), followed by potentially inappropriate medication for the elderly (n = 73), imaging (n = 41), and lab tests (n = 31). The most common study setting was hospitals (n = 153), followed by primary care (n = 87). Only three studies focused on influencing on a systems level whereas the rest of the studies targeted individual health care professionals.
Of the 310 studies, 279 were based on quantitative methods, 25 were mixed methods, and 10 were qualitative. The most common study design was a pre–post study design (n = 147), followed by quasi-experimental study design (n = 66) and randomized controlled trial (n = 39). The most frequent type of evaluation was efficacy/effectiveness (n = 260), followed by process evaluation (n = 21). Of the 310 studies, 217 used multicomponent strategies to reduce LVC, and 93 used single-component strategies.
Identified de-implementation strategies
The inductive coding yielded 71 unique strategies, of which 62 could be mapped onto ERIC strategies. A total of 36 of the 73 ERIC strategies were covered. Four of the identified strategies could be mapped onto one of the additional strategies suggested by Perry et al.: assess and redesign workflow [36]. Five of the identified strategies could not be mapped onto the ERIC compilation or the suggested additions from Perry et al. [36].
The de-implementation strategies used most commonly were related to the ERIC categories training and education of stakeholders, use of evaluative and iterative strategies, and support of clinicians (Table 3).
Starting with the most common category, Table 3 presents the identified de-implementation strategies for each of the nine categories in ERIC [35], the added strategies by Perry et al. [36], and strategies not reflected in any of the previous strategies. A table with all identified strategies in each study is provided in Additional file 4.
Train and educate stakeholders
A majority of the identified de-implementation strategies were related to the category train and educate stakeholders. Develop educational materials (n = 106) was the most frequently used strategy in that category. This strategy was comprised of information tailored to various target audiences such as patients (e.g., [46, 47]) and practitioners (e.g., [48, 49]). Make training dynamic (n = 85) was the second most common strategy. This strategy included various types of staff trainings with active participation from the participants, including case studies, handouts, and a pre- and post-education knowledge test (e.g., [50, 51]). Another frequently used strategy included distribute educational materials (n = 59), which consisted of a more passive distribution of guidelines to practitioners (e.g., [52, 53]).
Use evaluative and iterative strategies
Use of evaluative and iterative strategies was the second most common category of ERIC strategies. Within this category, audit and provide feedback (n = 89) was the most frequently used de-implementation strategy. It included various types of targets for the feedback such as individuals (e.g., [49, 54] and teams (e.g., [55, 56]), as well as feedback targeted to high prescribers only (e.g., [57]) and combined with social comparisons (e.g., [58]) or benchmark data (e.g., [59]). Another common strategy within this group was develop and organize a quality monitoring system (n=86). This strategy consisted of monitoring systems electronically (e.g., [60, 61]) or via a pharmacist (e.g., [62, 63]) or peer (e.g., [64]). It also included feedback on the clinical outcomes of the reduced use of LVC (e.g., [65]).
Support clinicians
This category of ERIC strategies was the third most common. The majority of the identified de-implementation strategies in the category used the ERIC strategy remind clinicians (n = 92). This entailed digital (e.g., [66, 67]) or analog (e.g., [68, 69]) clinical decision support or other types of reminders such as stickers (e.g., [70]).
Develop stakeholder interrelationships
In this category of ERIC strategies, use advisory boards and workgroups (n = 28) was the most common de-implementation strategy. This category consisted of studies that had involved staff in planning the strategy (e.g., [71, 72]). Sixteen studies also used the strategy identify and prepare champions (e.g., [73, 74]), and 10 studies used obtain formal commitments (e.g., [75, 76]).
Change infrastructure
Within this category of ERIC strategies, the most frequently used strategy for de-implementation was change physical structure and equipment (n = 38). This encompassed changes in the ordering system for lab tests (e.g., [53, 77], changes in prescription process concerning medications (e.g., [78, 79]), facilitation of testing to only prescribe to patients with a certain test result (e.g., [80, 81]), facilitation of alternative practice (e.g., [82, 83]), and restricted availability of LVC practices (e.g., [84]).
Utilize financial strategies
In this category, alter incentive/allowance structure (n = 6) was the most common ERIC strategy. This strategy involved changing the level of reimbursement for LVC practices or the addition of criteria in the incentive system related to LVC use (e.g., [85]. The category alter patient/consumer fees (n = 3) included both increased patient costs for LVC (e.g., [86]) and reduced patient costs for diagnostic tests that may hinder non-indicated antibiotic prescriptions (e.g., [65]).
Adapt and tailor to context
In this category, tailor strategies (n = 6) was the most common ERIC strategy. The researchers used various methods to tailor the strategies to specific contexts, such as an educational strategy based on previously assessed knowledge among staff (e.g., [87]).
Provide interactive assistance
The only strategy that had been used for de-implementation in this category was provide clinical supervision (n = 5), which involved studies in which clinicians received supervision regarding when and how to use LVC practices and when other practices would be more beneficial for the patients. The supervision was either tailored to high prescribers (e.g., [88] or on demand for clinicians who requested it (e.g., [89]).
Engage consumers
The only strategy identified within this category was use mass media (n = 2). This strategy was represented by two studies in which an education campaign targeting the general population was conducted [90, 91].
De-implementation strategies mapped to the strategies suggested by Perry et al. [36]
Twenty-one studies were based on strategies that matched the suggested added category: assess and redesign workflow. In the identified studies, the de-implementation strategies used included changes in coordinating patient follow-up within primary care (e.g., [62, 92]), the clinical pathway (e.g., [58, 93], and more general changes in work process (e.g., [87, 94]). The other two suggested strategies were not found in the literature.
Strategies not found in ERIC
Four inductively coded strategies were not found in ERIC or the additional strategies suggested by Perry et al. [36]. These were accountability tool (n = 22), black box warning (n = 1), policy and regulations (n = 5), and communication tool (n = 9). Accountability tools included various tools that provided a gatekeeping function through which the clinicians were held accountable for their decision to use a low-value practice. They were requested to provide an argument as to why they were planning to use a low-value practice either by means of a written answer in the electronic health record or a verbal response to a colleague, specialist, or pharmacist (e.g., [95, 96]). Black box warnings were warning text on drug packages about the risk of the LVC drug so as to make both the clinician and patient aware of its risks (e.g., [97]). Policy and regulations had to do with directives instructing people to avoid using LVC (e.g., [47, 90]). Communication tools comprised a written script describing a process for communicating with patients about why they are not receiving a low-value practice, including processes for shared decision-making (e.g., [98, 99]).
Discussion
This scoping review identified a total of 71 unique de-implementation strategies described in the literature. Of these, 62 strategies could be mapped onto ERIC compilation strategies, whereas four strategies could be mapped onto one of the added strategies [36]. Thus, 87% of de-implementation strategies reported across various fields overlapped with strategies used in implementation, while four identified strategies could not be mapped onto any existing implementation strategy. Two of these strategies (i.e., policy and regulations and international collaboration) are likely to be useful for both de-implementation and implementation, whereas accountability tool, communication tool and black box warning may be unique to de-implementation.
The most commonly used category of strategies was train and educate stakeholders, ranging from distribute educational materials to make training dynamic. These types of strategies are also prevalent strategies for implementation [100]. However, previous studies have suggested that education alone is insufficient for successful de-implementation [29] and implementation [101]. Colla et al. [29] found that educational strategies, combined with patient information and/or audit and feedback (i.e., multicomponent strategies), were more effective at reducing LVC. Regardless, as many as 24 of the studies in our review were based on the train and educate stakeholders category as the sole strategy. This suggests that de-implementation strategies may be chosen pragmatically, without much regard for research findings as to what is most effective.
Four de-implementation strategies were not possible to map onto ERIC or the additional strategies suggested by Perry et al. [36]. Three of these strategies may be unique to de-implementation and thus differ from the implementation process of introducing a new practice. Of these, the strategy accountability tool (n = 22) was most common and had the purpose of holding clinicians accountable when prescribing an LVC practice. It serves to disrupt the habitual use of a practice and forces clinicians to stop and reflect on whether they should prescribe the practice. This could be considered a more important strategy for de-implementation than implementation. The other strategies that may be unique for de-implementation were communication tool and black box warning. Communication tools consisted of a structured method for communicating with patients or next of kin about why a patient did not receive a practice, and black box warning consisted of a clear written warning on the packaging for certain medications. The other two strategies identified in this scoping review and not captured by ERIC or the additions by Perry et al. were policy and regulation and international collaboration. These two strategies might be relevant for both implementation and de-implementation, which could suggest that the ERIC compilation should be extended. Thus, as suggested in the study by Perry et al. [36], a potential limitation of the ERIC compilation is that all possible implementation strategies may not be covered. The original authors of ERIC similarly stated that the compilation should not be seen as the final word and welcomed comments and critique [31].
We found that only half (50%) of the 73 ERIC strategies had been used in the included studies. However, it is unclear whether the remaining strategies lack relevance or applicability for de-implementation or whether they have not been used for other reasons. Strategies from the category labeled adapt and tailor to context may be less applicable for de-implementation, where drift from protocols and guidelines may be the very reason for a practice becoming LVC. Examples include indication creep (when a practice is used for purposes for which it has not been proven efficient) and prevention creep (when a practice developed for symptomatic disease is used for asymptomatic individuals [102]. Other exemplary strategies that were rare in this review included the category develop stakeholder relationships. This might have considerable potential as a de-implementation strategy, since one determinant for the use of LVC is professionals’ expectations, attitudes, and behaviors [2]. Strategies such as informing local opinion leaders, identifying early adopters, or conducting local consensus discussions can influence professionals’ expectations, attitudes, and behaviors to support de-implementation.
Several of the de-implementation strategies that matched ERIC strategies involved more than one inductive code. For instance, the ERIC strategy develop educational materials involved development of information materials that comprised several codes in the inductive coding based on the material’s target: staff, providers, or patients. In fact, patient expectations have been found to be an important determinant for the use of LVC [2, 103], which suggests that strategies involving information for patients may be more important for de-implementation. The de-implementation strategies within the ERIC strategy audit and provide feedback entailed many types of audit and feedback. Some of these researchers used individual feedback, and others delivered group-level feedback. More innovative examples included quality-improvement contests and setting a goal for prescription and delivering rewards when reaching the goal. Finally, one frequent example was delivering feedback only to high prescribers, which seems to be a more specific de-implementation strategy because a small number of clinicians can have a large impact on the total amount of prescriptions [104]. Both of these examples indicate that some strategies are more multifaceted and heterogeneous than others, making it challenging to compare the effectiveness of strategies across studies. For future research on the de-implementation of LVC, various components of the strategies must be reported transparently and in detail, preferably using guidelines for specifying and reporting strategies [105].
Very few of the identified studies used any of the ERIC strategies that could be classified as pre-analysis approaches to assist in choosing the most suitable de-implementation strategies. The pre-analysis strategies found were assess readiness and identify barriers and facilitators (5% of the studies), stage implementation scale-up (1% of the studies), conduct a local needs assessment (3% of the studies), and tailor strategies (3% of the studies). This finding implies that the choice of de-implementation strategies is rarely tailored to the determinants of LVC use. In contrast, the importance of a comprehensive analysis of the current practice is considered crucial for successful implementation [101].
It is noteworthy that we could not find any studies within the behavioral health field (i.e., all studies were related to medicine). This could be due to the fact that it may be easier to determine that a medical practice is of low value because the efficacy or effectiveness of such practices can often be tested in trials that produce more unequivocal evidence. For instance, the problem of overprescribing antibiotics was defined in parallel with the development of the medication [106], whereas the side effects within psychotherapy research have only been investigated in recent years [107].
Knowledge gaps and implications
The findings provide an overview of the most-used strategies within de-implementation. Most strategies could be found within ERIC, suggesting that the same type of strategies used for implementation purposes are also relevant for de-implementation. However, some new strategies were found that could be interpreted as more relevant to de-implementation than to implementation, including accountability and communication tool. The accountability tool provides a hurdle for routine use of LVC, and the communication tool helps the professional communicate their decision not to use LVC to patients or their families. Only half of the strategies described in ERIC was found in our review. This could be because some implementation strategies are irrelevant or under-utilized for de-implementation. Future studies could determine if some unused implementation strategies are also beneficial for de-implementation purposes.
Almost one-third of the studies in this review were focused on non-indicated antibiotics, implying that the most common strategies found in this review are a reflection of the most common strategies within the de-implementation of this type of LVC. One question that remains unanswered is whether different strategies could be beneficial for different types of LVC. For instance, patient centered care was suggested as an alternative to potentially inappropriate medications where individual strategies were described based on what caused anxiety to patients with dementia and how to best calm them without the unnecessary use of medications (e.g., [108]). This strategy is perhaps most suitable for patients within nursing homes or similar facilities and perhaps not for other patient populations. However, the lack of studies within behavioral health makes it difficult to assess the generalizability of the findings to this field.
Methodological considerations
A considerable strength of this research is the number of studies included and the breadth of clinical fields covered. The study was also rigorous in its processes. We designed and performed the literature search in collaboration with the university library. Two reviewers screened all references independently. Two of the authors completed all coding and mapping and solved issues through discussions within the entire author group. However, there were also some limitations. The inconsistent terminology for LVC and de-implementation makes it plausible that studies may have been missed. Moreover, the review only covered literature written in English and published in peer-reviewed sources. We did not report the efficacy or effectiveness of various strategies because this was not the aim of the study. This allowed us to include a wider range of study designs including qualitative, process evaluations and cost-effectiveness studies and as an effect identify a wider range of strategies. Furthermore, the amount of information concerning the described strategies in the included studies varied, which may affect the trustworthiness of the inductive coding and the mapping onto the ERIC compilation. Data were charted individually, which may have influenced the information extracted from the articles. To ensure that relevant data were charted in a consistent way, an additional author piloted the data charting form, all individuals conducting the data charting were trained, and the charted data for a subset of articles were compared across individuals before starting the data charting. Finally, it is unknown if we would have received other results if we had coded the data based on another taxonomy. Future studies could investigate how de-implementation strategies differ depending on which taxonomy is used to code the strategies (e.g., [109]).
Conclusions
The de-implementation strategies described in the literature overlap with implementation strategies to a large extent. However, only a limited number of the implementation strategies included in ERIC were represented as de-implementation strategies. This could imply that some strategies are underused or not applicable for de-implementation purposes. Nevertheless, the findings show that ERIC can be used to classify de-implementation strategies. We suggest some adaptions when using the ERIC compilation for de-implementation. The strategy assess and redesign workflow [36] and two new strategies, accountability tool and communication tool should complement the existing compilation when used for de-implementation purposes.
Availability of data and materials
The datasets used will be available from the corresponding author on reasonable request.
Abbreviations
- LVC:
-
Low-value care
- ERIC:
-
Expert Recommendation for Implementing Change
References
Verkerk EW, Tanke MAC, Kool RB, van Dulmen SA, Westert GP. Limit, lean or listen? A typology of low-value care that gives direction in de-implementation. Int J Qual Health Care. 2018;30(9):736–9.
Augustsson H, Ingvarsson S, Nilsen P, von Thiele Schwarz U, Muli I, Dervish J, et al. Determinants for the use and de-implementation of low-value care in health care: a scoping review. Implement Sci Commun. 2021;2(1):13.
Willson A. The problem with eliminating ‘low-value care’. BMJ Qual Saf. 2015;24(10):611–4.
Davidson KW, Ye S, Mensah GA. Commentary: de-implementation science: a virtuous cycle of ceasing and desisting low-value care before implementing new high value care. Ethn Dis. 2017;27(4):463–8.
Brownlee S, Chalkidou K, Doust J, Elshaug AG, Glasziou P, Heath I, et al. Evidence for overuse of medical services around the world. Lancet. 2017;390(10090):156–68.
Braithwaite J, Glasziou P, Westbrook J. The three numbers you need to know about healthcare: the 60-30-10 Challenge. BMC Med. 2020;18(1):102.
Shojania KG, Sampson M, Ansari MT, Ji J, Doucette S, Moher D. How quickly do systematic reviews go out of date? A survival analysis. Ann Intern Med. 2007;147(4):224–33.
van Bodegom-Vos L, Davidoff F, de Mheen PJ M-v. Implementation and de-implementation: two sides of the same coin? BMJ Qual Saf. 2017;26(6):495–501.
Norton WE, Chambers DA. Unpacking the complexities of de-implementing inappropriate health interventions. Implement Sci. 2020;15(1):2.
Dallas A, Magin P, Morgan S, Tapley A, Henderson K, Ball J, et al. Antibiotic prescribing for respiratory infections: a cross-sectional analysis of the ReCEnT study exploring the habits of early-career doctors in primary care. Fam Pract. 2014;32(1):49–55.
Bhatia RS, Bouck Z, Ivers NM, Mecredy G, Singh J, Pendrith C, et al. Electrocardiograms in low-risk patients undergoing an annual health examination. JAMA Intern Med. 2017;177(9):1326–33.
Faustino CG, Passarelli MC, Jacob-Filho W. Potentially inappropriate medications among elderly Brazilian outpatients. Sao Paulo Med J. 2013;131(1):19–26.
Simos D, Hutton B, Graham ID, Arnaout A, Caudrelier JM, Clemons M. Imaging for metastatic disease in patients with newly diagnosed breast cancer: are doctor’s perceptions in keeping with the guidelines? J Eval Clin Pract. 2015;21(1):67–73.
Ramsey SD, Henry NL, Gralow JR, Mirick DK, Barlow W, Etzioni R, et al. Tumor marker usage and medical care costs among older early-stage breast cancer survivors. J Clin Oncol. 2015;33(2):149–55.
Dekker AR, Verheij TJ, van der Velden AW. Inappropriate antibiotic prescription for respiratory tract indications: most prominent in adult patients. Fam Pract. 2015;32(4):401–7.
Irfan N, Brooks A, Mithoowani S, Celetti SJ, Main C, Mertz D. A controlled quasi-experimental study of an educational intervention to reduce the unnecessary use of antimicrobials for asymptomatic bacteriuria. PLoS One. 2015;10(7):e0132071.
Sawan M, Jeon YH, Fois RA, Chen TF. Exploring the link between organizational climate and the use of psychotropic medicines in nursing homes: a qualitative study. Res Soc Adm Pharm. 2016;13(3):513–23.
Selby K, Cornuz J, Cohidon C, Gaspoz J-M, Senn N. How do Swiss general practitioners agree with and report adhering to a top-five list of unnecessary tests and treatments? Results of a cross-sectional survey. Eur J Gen Pract. 2018;24(1):32–8.
Kerns JW, Winter JD, Winter KM, Boyd T, Etz RS. Primary care physician perspectives about antipsychotics and other medications for symptoms of dementia. J Am Board Fam Med. 2018;31(1):9–21.
Hines JZ, Sewell JL, Sehgal NL, Moriates C, Horton CK, Chen AH. “Choosing wisely” in an academic department of medicine. Am J Med Qual. 2014;30(6):566–70.
Sedrak MS, Patel MS, Ziemba JB, Murray D, Kim EJ, Dine CJ, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med. 2016;11(12):869–72.
Munoz-Plaza CE, Parry C, Hahn EE, Tang T, Nguyen HQ, Gould MK, et al. Integrating qualitative research methods into care improvement efforts within a learning health system: addressing antibiotic overuse. Heal Res Policy Syst. 2016 Aug;14(1):63.
Leeman J, Birken SA, Powell BJ, Rohweder C, Shea CM. Beyond “implementation strategies”: classifying the full range of strategies used in implementation science and practice. Implement Sci. 2017;12(1):125.
Patey AM, Hurt CS, Grimshaw JM, Francis JJ. Changing behaviour `more or less’---do theories of behaviour inform strategies for implementation and de-implementation? A critical interpretive synthesis. Implement Sci. 2018;13(1):134.
Rietbergen T, Spoon D, Brunsveld-Reinders AH, Schoones JW, Huis A, Heinen M, et al. Effects of de-implementation strategies aimed at reducing low-value nursing procedures: a systematic review and meta-analysis. Implement Sci. 2020;15(1):38.
Voorn VMA, de Mheen PJ, van der Hout A, Hofstede SN, So-Osman C, van den Akker-van Marle ME, et al. The effectiveness of a de-implementation strategy to reduce low-value blood management techniques in primary hip and kneearthroplasty: a pragmatic cluster-randomized controlled trial. Implement Sci. 2017;12(1):72.
Sanchez A, Pijoan JI, Pablo S, Mediavilla M, de Rozas RS, Lekue I, et al. Addressing low-value pharmacological prescribing in primary prevention of CVD through a structured evidence-based and theory-informed process for the design and testing of de-implementation strategies: the DE-imFAR study. Implement Sci. 2020;15(1):8.
Alishahi Tabriz A, Turner K, Clary A, Hong Y-R, Nguyen OT, Wei G, et al. De-implementing low-value care in cancer care delivery: a systematic review. Implement Sci. 2022;17(1):24.
Colla CH, Mainor AJ, Hargreaves C, Sequist T, Morden N. Interventions aimed at reducing use of low-value health services: a systematic review. Med Care Res Rev. 2017;74(5):507–50.
Patey AM, Grimshaw JM, Francis JJ. Changing behaviour, “more or less”: do implementation and de-implementation interventions include different behaviour change techniques? Implement Sci. 2021;16(1):20.
Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10(1):21.
Effective Practice and Organisation of Care (EPOC). EPOC Taxonomy; 2015. https://epoc.cochrane.org/epoc-taxonomy. Accessed 20 Oct 2022.
Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The Behavior Change Technique Taxonomy (v1) of 93 Hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95.
Kok G, Gottlieb NH, Peters G-JY, Mullen PD, Parcel GS, Ruiter RAC, et al. A taxonomy of behaviour change methods: an Intervention Mapping approach. Health. Psychol Rev. 2016;10(3):297–312.
Waltz TJ, Powell BJ, Matthieu MM, Damschroder LJ, Chinman MJ, Smith JL, et al. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: results from the Expert Recommendations for Implementing Change (ERIC) study. Implement Sci. 2015;10(1):109.
Perry CK, Damschroder LJ, Hemler JR, Woodson TT, Ono SS, Cohen DJ. Specifying and comparing implementation strategies across seven large implementation interventions: a practical application of theory. Implement Sci. 2019;14(1):32–32.
Rogal SS, Yakovchenko V, Waltz TJ, Powell BJ, Kirchner JE, Proctor EK, et al. The association between implementation strategy use and the uptake of hepatitis C treatment in a national sample. Implement Sci. 2017;12(1):60.
Rogal SS, Yakovchenko V, Waltz TJ, Powell BJ, Gonzalez R, Park A, et al. Longitudinal assessment of the association between implementation strategy use and the uptake of hepatitis C treatment: Year 2. Implement Sci. 2019;14(1):36.
Yakovchenko V, Miech EJ, Chinman MJ, Chartier M, Gonzalez R, Kirchner JE, et al. Strategy configurations directly linked to higher hepatitis c virus treatment starts: an applied use of configurational comparative methods. Med Care. 2020;58(5):e31–8.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Hasson H, Nilsen P, Augustsson H, von Thiele Schwarz U. Empirical and conceptual investigation of de-implementation of low-value care from professional and health care system perspectives: a study protocol. Implement Sci. 2018;13(1):67.
The Joanna Briggs Institute. The Joanna Briggs Institute Manual 2015: Methodology for JBI scoping reviews. 2015.
Niven DJ, Mrklas KJ, Holodinsky JK, Straus SE, Hemmelgarn BR, Jeffs LP, et al. Towards understanding the de-adoption of low-value clinical practices: a scoping review. BMC Med. 2015;13(1):255.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Clyne B, Cooper JA, Hughes CM, Fahey T, Smith SM, Team O-S study. A process evaluation of a cluster randomised trial to reduce potentially inappropriate prescribing in older people in primary care (OPTI-SCRIPT study). Trials. 2016;17(1):386.
Adeola M, Azad R, Kassie GM, Shirkey B, Taffet G, Liebl M, et al. Multicomponent Interventions Reduce High-Risk Medications for Delirium in Hospitalized Older Adults. J Am Geriatr Soc. 2018;66(8):1638–45.
Angelidou A, Bell K, Gupta M, Tropea Leeman K, Hansen A. Implementation of a Guideline to Decrease Use of Acid-Suppressing Medications in the NICU. Pediatrics. 2017;140(6):e20171715.
Bruno C, Pearson SA, Daniels B, Buckley NA, Schaffer A, Zoega H. Passing the acid test? Evaluating the impact of national education initiatives to reduce proton pump inhibitor use in Australia. BMJ Qual Saf. 2020;29(5):365–73.
Cooper DL, Titler M, Struble L, Redman R. A multifaceted, evidence-based program to reduce inappropriate antibiotic treatment of suspected urinary tract infections. Ann Long-Term Care. 2017;25(2):36–43.
Couturier Y, Lanneville D, Lane J, Bruneau MA, Morin M, Gilbert S, et al. Implementation conditions leading to the scale-up of an innovation involving the optimal use of antipsychotics in long-term care centers: The Optimizing Practices, Use, Care and Services-Antipsychotics (OPUS-AP) program. Res Soc Adm Pharm. 2022;18(3):2484–8.
Sigmund AE, Stevens ER, Blitz JD, Ladapo JA. Use of Preoperative Testing and Physicians’ Response to Professional Society Guidance. JAMA Intern Med. 2015;175(8):1352–9.
Berg K, Nedved A, Richardson T, Montalbano A, Michael J, Johnson M. Actively doing less: deimplementation of unnecessary interventions in bronchiolitis care across urgent care, emergency department, and inpatient settings. Hosp Pediatr. 2020;10(5):385–91.
Lin IB, Coffin J, O’Sullivan PB. Using theory to improve low back pain care in Australian Aboriginal primary care: a mixed method single cohort pilot study. BMC Fam Pract. 2016;17:44.
Bhatia RS, Milford CE, Picard MH, Weiner RB. An educational intervention reduces the rate of inappropriate echocardiograms on an inpatient medical service. JACC Cardiovasc Imaging. 2013;6(5):545–55.
Alcorn S, van der Hoek J, Shaban RZ. Reducing inappropriate third-generation cephalosporin use for community-acquired pneumonia in a small Australian emergency department. Infect Dis Heal. 2018;23(3):163–9.
Elnenaei MO, Campbell SG, Thoni AJ, Lou A, Crocker BD, Nassar BA. An effective utilization management strategy by dual approach of influencing physician ordering and gate keeping. Clin Biochem. 2016;49(3):208–12.
Drees M, Fischer K, Consiglio-Ward L, Caruano J, BCIDP, Chan S, et al. Statewide Antibiotic Stewardship: : An eBrightHealth Choosing Wisely Initiative. Dela J Public Heal. 2019;5(2):50–8.
Del Giorno R, Ceschi A, Pironi M, Zasa A, Greco A, Gabutti L. Multifaceted intervention to curb in-hospital over-prescription of proton pump inhibitors: a longitudinal multicenter quasi-experimental before-and-after study. Eur J Intern Med. 2018;50:52–9.
Kelley D, Aaronson P, Poon E, McCarter YS, Bato B, Jankowski CA. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35(2):193–5.
Bejjanki H, Mramba LK, Beal SG, Radhakrishnan N, Bishnoi R, Shah C, et al. The role of a best practice alert in the electronic medical record in reducing repetitive lab tests. Clin Outcomes Res. 2018;10:611–8.
Gillespie U, Alassaad A, Hammarlund-Udenaes M, Morlin C, Henrohn D, Bertilsson M, et al. Effects of pharmacists’ interventions on appropriateness of prescribing and evaluation of the instruments’ (MAI, STOPP and STARTs’) ability to predict hospitalization--analyses from a randomized controlled trial. PLoS One. 2013;8(5):e62401.
Masood U, Sharma A, Bhatti Z, Carroll J, Bhardwaj A, Sivalingam D, et al. A Successful Pharmacist-Based Quality Initiative to Reduce Inappropriate Stress Ulcer Prophylaxis Use in an Academic Medical Intensive Care Unit. Inq. 2018;55:1–5.
Monette J, Monette M, Sourial N, Vandal AC, Wolfson C, et al. Effect of an interdisciplinary educational program on antipsychotic prescribing among residents with dementia in two long-term care centers. J Appl Gerontol. 2013;32(7):833–54.
Boonyasiri A, Thamlikitkul V. Effectiveness of multifaceted interventions on rational use of antibiotics for patients with upper respiratory tract infections and acute diarrhea. J Med Assoc Thail. 2014;97:S13–9.
Depinet H, von Allmen D, Towbin A, Hornung R, Ho M, Alessandrini E, et al. Risk stratification to decrease unnecessary diagnostic imaging for acute appendicitis. Pediatrics. 2016;138(3):e20154031.
May A, Hester A, Quairoli K, Wong JR, Kandiah S. Impact of clinical decision support on azithromycin prescribing in primary care clinics. J Gen Intern Med. 2021;36(8):2267–73.
Fleet E, Gopal Rao G, Patel B, Cookson B, Charlett A, Bowman C, et al. Impact of implementation of a novel antimicrobial stewardship tool on antibiotic use in nursing homes: a prospective cluster randomized control pilot study. J Antimicrob Chemother. 2014;69(8):2265–73.
Campins L, Serra-Prat M, Palomera E, Bolibar I, Martínez MÀ, Gallo P. Reduction of pharmaceutical expenditure by a drug appropriateness intervention in polymedicated elderly subjects in Catalonia (Spain). Gac Sanit. 2019;33(2):106–11.
Giles M, Watts W, O’Brien A, Berenger S, Paul M, McNeil K, et al. Does our bundle stack up! Innovative nurse-led changes for preventing catheter-associated urinary tract infection (CAUTI). Healthc Infect. 2015;20(2):62–71.
Boggan JC, Schulteis RD, Donahue M, Simel DL. Guideline-based decision support has a small, non-sustained effect on transthoracic echocardiography ordering frequency. BMJ Qual Saf. 2015;25(1):57–62.
Cateau D, Ballabeni P, Niquille A. Effects of an interprofessional Quality Circle-Deprescribing Module (QC-DeMo) in Swiss nursing homes: a randomised controlled trial. BMC Geriatr. 2021;21(1):289.
Stinnett-Donnelly JM, Stevens PG, Hood VL. Developing a high value care programme from the bottom up: a programme of faculty-resident improvement projects targeting harmful or unnecessary care. BMJ Qual Saf. 2016;25(11):901–8.
Naimer MS, Munro J, Singh S, Permaul JA. Improving family medicine residents’ opioid prescribing: a nurse practitioner-led model. J Nurse Pract. 2019;15(9):661–5.
Meeker D, Knight TK, Friedberg MW, Linder JA, Goldstein NJ, Fox CR, et al. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med. 2014;174(3):425–31.
Kullgren JT, Krupka E, Schachter A, Linden A, Miller J, Acharya Y, et al. Precommitting to choose wisely about low-value services: a stepped wedge cluster randomised trial. BMJ Qual Saf. 2018;27(5):355–64.
Martins CM, da Costa Teixeira AS, de Azevedo LF, Sa LM, Santos PA, do Couto ML, et al. The effect of a test ordering software intervention on the prescription of unnecessary laboratory tests - a randomized controlled trial. BMC Med Informatics Decis Mak. 2017;17(1):20.
Fagan M, Lindbaek M, Reiso H, Berild D. A simple intervention to reduce inappropriate ciprofloxacin prescribing in the emergency department. Scand J Infect Dis. 2014;46(7):481–5.
Abdul-Moheeth M, Valencia V, Schaefer S, Brode WM, Nieto K, Moriates C. Improving Healthcare Value: Effectiveness of a Program to Reduce Laboratory Testing for Non-Critically-Ill Patients With COVID-19. J Hosp Med. 2021;16(8):495–8.
Njuguna J, Menge D, Nzou J, Chege C. Impact of an intervention to minimize overdiagnosis of malaria cases in a low risk kenyan sub-county. J Heal Care Poor Underserved. 2015;26(3):802–10.
Rautemaa-Richardson R, Rautemaa V, Al-Wathiqi F, Moore CB, Craig L, Felton TW, et al. Impact of a diagnostics-driven antifungal stewardship programme in a UK tertiary referral teaching hospital. J Antimicrob Chemother. 2018;73(12):3488–95.
Linder JA, Meeker D, Fox CR, Friedberg MW, Persell SD, Goldstein NJ, et al. Effects of behavioral interventions on inappropriate antibiotic prescribing in primary care 12 months after stopping interventions. JAMA. 2017;318(14):1391–2.
Morgan T, Wu J, Ovchinikova L, Lindner R, Blogg S, Moorin R. A national intervention to reduce imaging for low back pain by general practitioners: a retrospective economic program evaluation using Medicare Benefits Schedule data. BMC Health Serv Res. 2019;19(1):983.
Petrou P. Failed attempts to reduce inappropriate laboratory utilization in an emergency department setting in Cyprus: lessons learned. J Emerg Med. 2016;50(3):510–7.
Bou-Antoun S, Costelloe C, Honeyford K, Mazidi M, Hayhoe BWJ, Holmes A, et al. Age-related decline in antibiotic prescribing for uncomplicated respiratory tract infections in primary care in England following the introduction of a national financial incentive (the Quality Premium) for health commissioners to reduce use of antibiotic. J Antimicrob Chemother. 2018;73(10):2883–92.
Schaffer AL, Buckley NA, Cairns R, Pearson S. Comparison of Prescribing Patterns Before and After Implementation of a National Policy to Reduce Inappropriate Alprazolam Prescribing in Australia. JAMA Netw Open. 2019;2(9):e1911590.
Schondelmeyer AC, Simmons JM, Statile AM, Hofacer KE, Smith R, Prine L, et al. Using quality improvement to reduce continuous pulse oximetry use in children with wheezing. Pediatrics. 2015;135(4):e1044–51.
Strother MK, Robert EC, Cobb JG, Pruthi S, Feurer ID. Reduction in the number and associated costs of unindicated dual-phase head CT examinations after a quality improvement initiative. AJR Am J Roentgenol. 2013;201(5):1049–56.
Garcia-Gollarte F, Baleriola-Julvez J, Ferrero-Lopez I, Cuenllas-Diaz A, Cruz-Jentoft AJ. An educational intervention on drug use in nursing homes improves health outcomes resource utilization and reduces inappropriate drug prescription. J Am Med Dir Assoc. 2014;15(12):885–91.
Holloway KA, Rosella L, Henry D. The Impact of WHO Essential Medicines Policies on Inappropriate Use of Antibiotics. PLoS One. 2016;11(3):e0152020.
Walker AJ, Curtis HJ, Goldacre B. Impact of Chief Medical Officer activity on prescribing of antibiotics in England: an interrupted time series analysis. J Antimicrob Chemother. 2019;74(4):1133–6.
Sara GG, Antoni LA, Ana Cris CL, Oreto RM, Pilar CA, Juan Pablo OB, et al. Deprescribing program in pluripathological elderly patients at a general hospital. Eur J Clin Pharmacol. 2020;22(3):132–41.
Jenkins TC, Irwin A, Coombs L, Dealleaume L, Ross SE, Rozwadowski J, et al. Effects of clinical pathways for common outpatient infections on antibiotic prescribing. Am J Med. 2013;126(4):327–335.e12.
Jefferson BK, King JE. Impact of the acute care nurse practitioner in reducing the number of unwarranted daily laboratory tests in the intensive care unit. J Am Assoc Nurse Pract. 2018;30(5):285–92.
Ip IK, Gershanik EF, Schneider LI, Raja AS, Mar W, Seltzer S, et al. Impact of IT-enabled intervention on MRI use for back pain. Am J Med. 2014;127(6):512–518.e1.
Buckley MS, Agarwal SK, Lansburg JM, Kopp BJ, Erstad BL. Clinical pharmacist–led impact on inappropriate albumin utilization and associated costs in general ward patients. Ann Pharmacother. 2021;55(1):44–51.
Seetasith A, Holdford D, Shah A, Patterson J. On-label and off-label prescribing patterns of erythropoiesis-stimulating agents in inpatient hospital settings in the US during the period of major regulatory changes. Res Soc Adm Pharm. 2017;13(4):778–88.
Jennings RM, Burtner JJ, Pellicer JF, Nair DK, Bradford MC, Shaffer M, et al. Reducing head CT use for children with head injuries in a community emergency department. Pediatrics. 2017;139(4):e20161349.
Coronado-Vázquez V, Gómez-Salgado J, de los Monteros JCE, Ayuso-Murillo D, Ruiz-Frutos C. Shared decision-making in chronic patients with polypharmacy: an interventional study for assessing medication appropriateness. J Clin Med. 2019;8(6):904.
Godin G, Bélanger-Gravel A, Eccles M, Grimshaw J. Healthcare professionals’ intentions and behaviours: a systematic review of studies based on social cognitive theories. Implement Sci. 2008;3(1):36.
Grimshaw JM, Eccles MP, Lavis JN, Hill SJ, Squires JE. Knowledge translation of research findings. Implement Sci. 2012;7(1):50.
Djulbegovic B, Paul A. From efficacy to effectiveness in the face of uncertainty: indication creep and prevention creep. JAMA. 2011;305(19):2005–6.
Ingvarsson S, Augustsson H, Hasson H, Nilsen P, von Thiele Schwarz U, von Knorring M. Why do they do it? A grounded theory study of the use of low-value care among primary health care physicians. Implement Sci. 2020;15(1):1–93.
Schwartz AL, Jena AB, Zaslavsky AM, McWilliams JM. Analysis of physician variation in provision of low-value services. JAMA Intern Med. 2018;179(1):16–25.
Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8(1):139.
New York times (June 26, 1945). Penicillin’s finder assays its future: Sir Alexander Fleming says improved dosage method is needed to extend use other scientists praised self-medication decried: The New York Times; 1945.
Rozental A, Kottorp A, Boettcher J, Andersson G, Carlbring P. Negative effects of psychological treatments: an exploratory factor analysis of the negative effects questionnaire for monitoring and reporting adverse and unwanted events. PLoS One. 2016;11(6):e0157503.
Westaway K, Frank O, Shute R, Pall D, Moffat A, LeBlanc V, et al. Gathering tips from carers to support people with dementia: an adaptation of the TOP 5 program for community use. Int J Evid Based Healthc. 2018;16(2):128–35.
McHugh S, Presseau J, Luecking CT, Powell BJ. Examining the complementarity between the ERIC compilation of implementation strategies and the behaviour change technique taxonomy: a qualitative analysis. Implement Sci. 2022;17(1):56.
Acknowledgements
The authors would like to thank Irene Muli, Jessica Dervish, Lana al Soufi, and My Sjunnestrand for assistance with screening articles and extracting data. We are also grateful to Carl Gornitzki, Sabina Gillsund, and GunBrit Knutssön at the Karolinska Institutet university library, who assisted with the development of the search strategy and the data base searches.
Funding
Open access funding provided by Karolinska Institute. This study is funded by the Swedish Research Council for Health, Working Life and Welfare (FORTE) (project beslutsbryderi 2020-01197) (project no. 2017-00449). Open-access funding provided by Karolinska Institutet. BJP was supported by the U.S. National Institute of Mental Health through K01MH113806.
Author information
Authors and Affiliations
Contributions
HA, HH, UvTS, PN, and SI designed the study. HA, SI, HH, and four research assistants conducted the abstract screening, SI, CL, HA and two research assistants conducted the full-text screening, and CL and two research assistants the data charting. HA, SI, HH, UvTS, and PN coded and categorized the extracted data. SI and BP mapped the coded strategies onto ERIC. SI drafted the first version of the article with assistance from HH, PN, UvTS, and HA. All authors discussed the draft, revised it, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist.
Additional file 2.
Documentation of search strategies.
Additional file 3.
All inductive codes and their ERIC mapping.
Additional file 4.
All identified strategies in each study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ingvarsson, S., Hasson, H., von Thiele Schwarz, U. et al. Strategies for de-implementation of low-value care—a scoping review. Implementation Sci 17, 73 (2022). https://doi.org/10.1186/s13012-022-01247-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13012-022-01247-y