Abstract
Water dropwort (Oenanthe javanica (Blume) DC), an aquatic perennial plant from the Apiaceae family, rich in dietary fibert, vitamins, and minerals. It usually grows in wet soils and water. Despite accumulating the transcriptomic data, gene function research on water dropwort is still far behind than that of the other crops. The cucumber mosaic virus (CMV) induced gene silencing was established to study the functions of gene and microRNA (miRNA) in the water dropwort. CMV Fast New York strain (CMV-Fny) genomic RNAs 1, 2, and 3 were individually cloned into pCB301 vectors. We deleted part of the ORF 2b region and introduced recognition sites. A CMV-induced gene silencing vector was employed to suppress the expression of endogenous genes, including phytoene desaturase (PDS). In order to assess the efficacy of gene silencing, we also cloned conserved sequence of gibberellin insensitive dwarf (GID1) cDNA sequences into the vector and inoculated the water dropwort. The height of CMV-GID1-infected plants was marginally reduced as a result of GID1 gene silencing, and their leaves were noticeably longer and thinner. Additionally, we also used a CMV-induced silencing vector to analyze the roles of endogenous miRNAs. We used a short tandem target mimic approach to clone miR319 and miR396 from water dropwort into the CMV vector. Plants with CMV-miRNA infection were driven to exhibit the distinctive phenotypes. We anticipate that functional genomic research on water dropwort will be facilitated by the CMV-induced gene silencing technique.
Similar content being viewed by others
Introduction
Water dropwort (Oenanthe javanica (Blume) DC) is a perennial aquatic herb belonging to the genus Oenanthe of the Apiaceae family [1]. It is commonly grown in East Asian nations such South Korea, China, and Japan [2]. Having a large customer base due to its high vitamin, protein, dietary fiber, and mineral nutrient content is water dropwort, a well-liked vegetable [3, 4]. It also has various medicinal benefits such as, antithrombotic [5], hepatoprotective [6], neuroprotective [7], anti-inflammatory [8], antioxidant [9], antiviral [10], and antisenescence effects [11]. Moreover, recent years have witnessed increasing research on water dropwort. Despite the fact that Feng created a complete water dropwort transcriptome using PacBio SMRT and Illumina RNA sequencing, research on its gene function is fairly limited compared to other crops [12]. However, the genetic transformation, gene silencing, and gene knockout systems of water dropwort have not been established, along with a lack of corresponding technical support for gene function research. It is necessary that the genetic tools will be developed to address these gaps in functional genomic studies and water dropwort’s breeding.
The usability of transcriptomic data promotes the progress and development of reverse genetics research [2]. Virus-induced gene silencing (VIGS) has been extensively used to characterize gene functions in various plant species [13,14,15]. Viral infections activate a mechanism that defends against the viral RNA in host plants. This system is exploited by VIGS, which utilises posttranscriptional gene silencing (PTGS) that occurs when a virus infects host cells [16]. By inserting a part of the gene sequence into the virus, VIGS is a mechanism that can specifically target the mRNA of interest to initiate viral defence. When the recombinant virus replicates, double-stranded RNA (dsRNA) intermediates are generated, which activate RNA-mediated antiviral defence mechanisms that result in the formation of short interfering RNAs (siRNAs). These siRNAs then target the RNase complex to the corresponding RNA, ultimately leading to the disruption of the protein encoded by that RNA. [17]. The versatility of VIGS technology has made it an essential tool in simplifying the application of plant virus vectors in various plant species. Recently, some plant RNA or DNA viruses including, tobacco mosaic virus (TMV) [18], potato virus X (PVX) [19], tobacco rattle virus (TRV) [13, 20], barley stripe mosaic virus (BSMV) [21], tobacco mosaic virus satellite (TMVS) [22], pea early browning virus (PEBV) [23], tomato mosaic virus (ToMV) [24], bean pod mottle virus (BPMV) [25], cucumber mosaic virus (CMV) [26], brome mosaic virus (BMV) [27, 28], citrus tatter leaf virus (CTLV), apple latent spherical virus (ALSV) [29], tobacco ringspot virus (TRSV) [30], and rice tungro bacilliform virus (RTBV) [31], have been identified.
CMV is a typical member of the genus Cucumovirus of the family Bromoviridae. It is known to infect more than 1200 host species in over 100 plant families, including water dropwort [32]. CMV consists of three positively sensed single-stranded genomic RNAs (RNA1, 2, and 3) that express five proteins [33]. CMV express a total of five proteins, with RNA1 encoding the 1a protein and RNA2 encoding the 2a protein, both of which are responsible for viral genome replication [34]. RNA2 also includes the 2b gene, which encodes for the suppressor of RNA silencing [33, 35], a protein ultimately impairing protein activities associated with Argonaute 1 and Argonaute 4 by direct interaction [12, 25, 36,37,38]. Previous research studies suggest that the absence of a 2b gene results in CMV infecting plants systemically with only mild symptoms [39]. Furthermore, the 3a movement and coat proteins were encoded by RNA3, both of which play crucial roles in viral packaging and systemic movement. CMV is a prominent plant virus that is responsible for numerous plant diseases worldwide, while also serving as a pivotal model in studying plant-virus interactions. CMV has been successfully deployed as a VIGS agent in dicotyledonous plants such as Nicotiana benthamiana [26], Glycine max [40], Antirrhinum majus [41], Solanum lycopersicum, and Capsicum annuum [42], as well as monocotyledonous maize; whether CMV can induce silencing in water dropwort remains unknown.
We modified CMV-Fny into pCB301 vector. It was used to reduce the expression of the PDS and GID1 genes in water dropwort and N. benthamiana. Following this, we proceeded to introduce CMV-Fny into the VbMS vector in order to explore the functional significance of two distinct miRNAs in both water dropwort and N. benthamiana. Through our experimentation, we demonstrated that the CMV-VIGS vector serves as a highly effective and efficient solution for conducting functional genomic research pertaining to water dropwort.
Results
Construction of CMV VIGS vector
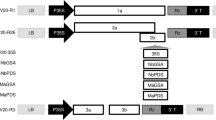
To analyse the suitability of the cucumber mosaic virus Fast New York strain (CMV-Fny) to induce gene silencing, the three chains of CMV-Fny from Prof. Xianbing Wang's laboratory were initially transferred into the pCB301 vector to build pCB301CMV-Fny1, pCB301CMV-Fny2, and pCB301CMV-Fny3 (Fig. 1A). The three Agrobacterium tumefaciens strains, individually harbouring the three different pCB301CMV-Fny constructs, were used together to infect four varieties of water dropwort, including ‘Fq1’, ‘Yzcbq’, ‘sq02’ and ‘sq09’. Mild shrinking appeared in the non-inoculated upper (that is, systemic) leaves at four weeks post agroinfiltration (wpi) (Fig. 1B). In addition to symptom observation, we extracted RNA and total protein from its systemic leaves to prepare samples; the utilization of both RT-PCR and western blotting techniques identified the presence of CMV in plant specimens that had been deliberately inoculated with CMV-Fny, thereby establishing the occurrence of CMV infection. (Fig. 1C).
Construction of the CMV VIGS vector. A, Construct map of CMV-Fny. B, Symptoms of CMV-Fny in O. javanica (4 wpi). Scale bars are 2 cm. C, Detection of CMV-Fny-infected O. javanica by agro-infection (4 wpi). The vector plasmid with the CMV RNA3 insert was amplified as the positive (lane P) control. D, Construct map of the CMV-Fny 2b deletion mutant. E, Symptoms of the CMV-Fny 2b deletion mutant in O. javanica. Scale bars are 2 cm. F, Detection of CMV-Fny 2b deletion mutant-infected O. javanica by agro-infection (4 wpi). The vector plasmid with the CMV RNA3 insert was amplified as the positive (lane P) control
In order to create a gene silencing vector for CMV, a modified form of pCB301CMV-Fny2 was utilized. The vector was customized by the addition of restriction sites that facilitated the cloning of a sequence. The RNA 2 consists of two ORFs, namely ORF 2a and 2b. It is noteworthy that the 2b ORF is integral for CMV symptom development, yet it is dispensable for viral replication and systemic infection. Fny 2b del BamSmaSpe-F and Fny 2b del Bam-R were used as primers for reverse PCR amplification to delete the nonoverlapping region of ORF 2b [43]. We introduced new BamH I, Sma I, and Spe I recognition sites (Fig. 1D, Additional file 1: Fig. S1). The 2b deletion mutant pCB301-Fny2-2b del was used to complete the transformation of the CMV infection clone. In addition, A. tumefaciens cells harbouring pCB301CMV-Fny1, pCB301CMV-Fny3 and pCB301-Fny2-2b del were infiltrated into leaves of N. benthamiana and O. javanica. Since infected plants showed no obvious symptoms, the treated plant leaves were used to prepare samples, and the target bands’ CMV-CP were detected by RT-PCR and western blotting (Fig. 1E and F; Additional file 1: Fig. S2). The results indicated that the modified CMV VIGS vector could be used in subsequent studies.
Development of CMV-Fny as a virus-induced gene silencing (VIGS) vector
To build upon the accomplishments of the current inoculation method, we sought to investigate the CMV VIGS vector in promoting gene silencing within both N. benthamiana and water dropwort. A 332 bp fragment from the C-terminus of the PDS gene, necessary for carotenoid pigment production, was inserted into the CMV-Fny 2b deletion mutant (Additional file 1: S3A and B). CMV-Fny1 agro-infection, CMV-Fny3 agro-infection, and CMV-Fny2-2b-NbPDS agro-infection were infiltrated into N. benthamiana leaves and assayed for phenotypes and silencing efficacy. N. benthamiana that were subjected to recombinant CMV-NbPDS experienced the phenotypes of photobleaching on their leaves, with visible effects observed after 14 days of inoculation (Additional file 1: Fig. S3C). Total RNA and protein extracts extracted from Mock, CMVΔ2b, CMV-NbPDS, and CMV agroinfiltrated plants at 14 dpi were used for the detection of CMV by RT-PCR and western blotting (Additional file 1: Fig. S3D). CMV infection was confirmed in all CMVΔ2b, CMV-NbPDS, and CMV agroinfiltrated plants. In addition, the expression of PDS in CMV-NbPDS-infected plants decreased significantly compared to CMVΔ2b and CMV agroinfiltrated plants by semiquantitative RT-PCR (Additional file 1: Fig. S3D). The silencing efficiency was 70%–90% in N. benthamiana plants (Additional file 2: Table S1). Overall, these results demonstrate that the CMV VIGS vector can be used to silence the NbPDS gene.
Next, we tested whether the CMV VIGS vector is suitable for gene silencing in water dropwort. The PDS gene of water dropwort was identified through the transcriptome data of water dropwort, while phylogenetic analysis revealed that it had the closest genetic relationship with the Daucus carota PDS gene (Additional file 1: Fig. S4A). In this regard, the conservative sequences of the N-terminus and C-terminus of the OjPDS gene were amplified to obtain 213 bp and 249 bp fragments that were inserted into the CMV-Fny 2b deletion mutant (Fig. 2A; Additional file 1: Fig. S4). Convert the recombinant virus into Agrobacterium competent cell and infiltrated water dropwort leaves via Agrobacterium infiltration. The newly born leaves of ‘Yzcbq’, inoculated with recombinant pCB301CMV-OjPDSN and pCB301CMV-OjPDSC, showed obvious symptoms of photobleaching after 35 days of inoculation (Fig. 2C). The systematically infected leaves in 'Fq1' also became white after six weeks (Fig. 2C). The positive bands detected in agroinfiltrated plants were consistent with the size of the target band by RT-PCR and western blotting (Fig. 2D and E). Semiquantitative PCR and RT-qPCR showed that the expression levels of OjPDSN and OjPDSC, after silencing, were significantly lowered than those of the control (Fig. 2D and E). The silencing efficiency reached 37.5%–75% in ‘Fq1’ and ‘Yzcbq’ plants (Additional file 2: Table S2). The expression levels of both, PDSN and PDSC, were detected in 'Fq1' and 'Yzcbq' silencing PDS genes (Additional file 2: Tables S3 and S4). The successful silencing of PDS genes by the CMV VIGS vector thus indicated that the CMV VIGS vector of water dropwort can study the gene function of water dropwort.
Silencing of the OjPDS gene in O. javanica using the CMV VIGS vector. A, Construct diagram of infectious clones of pCB301CMV-OjPDS. B, RT‒PCR detection of the PDSN and PDSC of O. javanica. C, Phenotypes of pCB301CMV-OjPDS in O. javanica (4 wpi). Scale bars are 2 cm. D, Detection of pCB301CMV-OjPDS-infected ‘Fq1’ by agro-infection (4 wpi). The vector plasmid with the CMV RNA3 insert was amplified as the positive (lane P) control. E, Detection of pCB301CMV-OjPDS-infected ‘Yzcbq’ by agro-infection (4 wpi). The vector plasmid with the CMV RNA3 insert was amplified as the positive (lane P) control
Silencing of GID1 genes affected the growth and development of water dropwort
To further assess the ability of the CMV VIGS vector to silence endogenous genes in water dropwort, we investigated the roles of GID1 in the development of water dropwort. According to the transcriptome data of water dropwort, a total of seven OjGID1 gene sequences were found through phylogenetic analysis, closely related to the GID1 gene of D. carota and Coriandrum sativum (Additional file 1: Fig. S5A). The conserved region found by sequence alignment was amplified a fragment of size 414 bp (Fig. 3B; Additional file 1: Fig. S5B and S5C). The obtained fragment was inserted into the CMV-Fny 2b deletion mutant to construct the pCB301CMV-OjGID1 vector (Fig. 3A). Similarly, it was transformed into the Agrobacterium competent cell and injected into the water dropwort plant via Agrobacterium infiltration. After being infected with CMV-GID1, compared to the plants agroinfiltrated with CMV-Δ2b, 'Yzcbq' exhibited considerable phenotypic alterations by the 30th day post-infection. The CMV-GID1-inoculated plants were notably shorter in height and had longer and thinner leaves. (Fig. 3C). Statistical analysis of systematic leaf morphology showed that after silencing the OjGID1 gene, leaf length changed insignificantly, but the leaf width and the number of nicks were significantly lowered than those of the control (Fig. 3D). CMV infection was confirmed in agroinfiltrated plants by RT-PCR (Fig. 3E). RT-qPCR analyses confirmed that the expression of OjGID1 was significantly downregulated in water dropwort (Fig. 3F; Additional file 1: Fig. S6D and S7). The expression of GA20ox1, GA3ox1, and GAI as signaling pathway-related genes were upregulated by RT-qPCR (Fig. 3G). In addition, CMV-GID1-infected ‘Fq1’ plants showed the same silencing phenotypes (Additional file 1: Fig. S6A). The silencing efficiency was 37.5%–62.5% in ‘Fq1' and 'Yzcbq' plants (Additional file 2: Table S5). A similar phenotype was observed in N. benthamiana, where the silencing efficiency reached 50–75% (Additional file 1: Fig. S8; Additional file 2: Table S5). Overall, silencing OjGID1 had dramatic effects on the growth and development of water dropwort and N. benthamiana plants.
Silencing of the OjGID1 gene in O. javanica using the CMV VIGS vector. A, Construct diagram of infectious clones of pCB301CMV-OjGID1; B, RT‒PCR detection of GID1 of ‘Yzcbq’. C, Phenotypes of pCB301CMV-OjGID1 in ‘Yzcbq’ (30 dpi). Scale bars are 2 cm. D, Systematic leaf morphology analysis of pCB301CMV-OjGID1 in ‘Yzcbq’ (30 dpi). E Detection of pCB301CMV-OjGID1-infected O. javanica by agro-infection (30 dpi). F RT-qPCR analysis of OjGID1 expression levels in CMV△2b- or CMV-OjGID1-infected plants. G RT-qPCR analysis of GA-related genes expression levels in CMV△2b- or CMV-OjGID1-infected plants
Silencing of water dropwort miRNA by the CMV VIGS vector
We studied the silencing of water dropwort miRNA by the CMV VIGS vector to further explore the latter’s application in water dropwort. Previous studies on miRNA of Apiaceae plants and celery have shown that miR319 regulated four TCP family genes in celery, four in coriander, and three in carrot [44, 45]. The target genes’ TCP2 and TCP4 sequences for water dropwort were obtained by blasting TCP2 and TCP4 sequences in carrot and celery to the transcriptomic data of water dropwort. The well-characterized miR319 was cloned into the pCB301CMV-Fny2-2b-del vector using a short tandem target mimic (STTM) strategy (Fig. 4A). By 40 dpi, CMV-STTM319-infected ‘Fq1’ plants showed phenotypes in which the plants exhibited a dwarf phenotype, and the leaves were significantly smaller than the control (Fig. 4B). Statistical analysis of systematic leaf morphology showed that after silencing STTM319, the leaf length, width, and height were significantly lowered than those of the control (Fig. 4C). The STTM319 expression in the CMV-STTM319-infected plants was detected by RT-PCR (Fig. 4D). Additionally, the findings of RT-qPCR detection revealed that in the plants infected with CMV-STTM319, the miR319 level was dramatically decreased while the target miRNA level increased (Fig. 4E and F). CMV-STTM319-infected ‘Yzcbq’ plants showed the same silencing phenotypes (Additional file 1: Fig. S9). The silencing efficiency was 37.5%–75% in ‘Fq1' and 'Yzcbq' plants (Additional file 2: Table S6). A similar phenotype was observed in N. benthamiana, in which the silencing efficiency reached 50–62.5% (Additional file 1: Fig. S10; Additional file 2: Table S6).
Silencing of miR319 in O. javanica using the CMV-based VbMS vector. A, Schematic representation of VbMS of miR319. B, Phenotypes of plants inoculated with CMV-STTM319 at 40 dpi. C, Systematic leaf morphology analysis of pCB301CMV-STTM319 in ‘Fq1’ (40 dpi). Scale bars are 2 cm. D, RT-PCR detection confirmed the expression of STTM319 in CMV-STTM319-infected plants. E, Stem-loop RT-qPCR analysis of miR319 expression levels in CMV△2b- or CMV-STTM319-infected plants. F, Detection of the relative expression levels of the miR319 target genes TCP2 and TCP4
The miR396 sequence and target gene Growth-Regulating Factor 9-like sequences of water dropwort were obtained by blasting Growth-Regulating Factor 9-like sequences in celery to the transcriptomic data of water dropwort [45]. The well-characterized miR396 was cloned into the pCB301CMV-Fny2-2b-del vector using an STTM strategy (Fig. 5A). By 40 dpi, CMV-STTM396-infected ‘Fq1’ plants showed a silencing phenotype in which the plants exhibited a dwarf phenotype (Fig. 5B). Statistical analysis of systematic leaf morphology showed that after silencing STTM396, the leaf length, width, and height were significantly lowered than those of the control (Fig. 5C). RT-PCR results showed that STTM396 was normally expressed in CMV-STTM396-infected water dropwort plants (Fig. 5D). In addition, results of fluorescence quantitative detection showed that the expression of miR396 was increased (Fig. 5E), while that of Growth-Regulating Factor 9-like, which is a target gene of miR396, was significantly lowered than that of the control (Fig. 5F). CMV-STTM396-infected ‘Yzcbq’ plants showed the same silencing phenotypes (Additional file 1: Fig. S11). The silencing efficiency was 37.5%–62.5% in ‘Fq1' and 'Yzcbq' plants (Additional file 2: Table S7). A similar phenotype was observed in N. benthamiana, in which the silencing efficiency reached 50–75% (Additional file 1: Fig. S12; Additional file 2: Table S7). Based on the findings, it appears that the CMV VbMS technique has the ability to suppress miRNAs and offer valuable insights into the roles of miRNA and target mRNA interactions.
Silencing of miR396 in O. javanica using the CMV-based VbMS vector. A, Schematic representation of VbMS of miR396. B, Phenotypes of plants inoculated with CMV-STTM396 at 40 dpi. C, Systematic leaf morphology analysis of pCB301CMV-STTM396 in ‘Fq1’ (40 dpi). Scale bars are 2 cm. D, RT‒PCR detection confirmed the expression of STTM396 in CMV-STTM396-infected plants. E, Stem‒loop RT‒qPCR analysis of miR396 expression levels in CMV△2b- or CMV-STTM396-infected plants. F, Detection of the relative expression level of the miR396 target gene Growth-Regulating Factor 9-like
Discussion
At present, the genetic transformation, gene silencing, and gene knockout systems for water dropwort have not been well established, with no corresponding technical support for gene function research. VIGS offers an alternate method for examining how plant genes work. We created a CMV-based gene silencing vector to address this problem. Based on the CMV-Fny infectious clone, the 2b deletion mutant vector, pCB301-Fny2-2b del, was constructed to transform the CMV-Fny infectious clone of water dropwort (Fig. 1D). Otagaki et al. (2016), for the first time, carried out the deletion mutation of CMV 2b protein, successfully constructed the CMV2-A1 vector, and proved that it was suitable for the rapid induction of transcription and posttranscriptional gene silencing [26]. Hong et al. successfully silenced the PDS gene in tomatoes and peppers. In this research, the test method was used to perform deletion mutations of amino acids 81 ~ 111 of the CMV 2b protein through reverse PCR amplification, to obtain its deletion mutant [42]. Based on pCMV201, four VIGS vectors expressing 2b proteins of different lengths namely, ZMBJ-CMV-2b, ZMBJ-CMV-2bN94, ZMBJ-CMV-2bN81 and ZMBJ-CMV-2bN61, were constructed [46]. Tzean et al., in their study, inserted the restriction site at the end of the CMV 2a protein and constructed the CMV-VIGS vector in banana by deletion of the CMV 2b protein [47]. By two distinct strains, CMV-ZMBJ and CMV-Fny, Li et al. (2021) generated a pseudo recombinant-chimeric (Pr) CMV [48]. By employing a ligation-independent cloning (LIC) technique, they devised an altered Pr CMV-LIC VIGS vector, which facilitates straightforward gene cloning for carrying out extensive silencing in maize. In addition, when the modified CMV VIGS vector was inoculated into N. benthamiana, the upper leaves showed mild symptoms of shrinkage in the early stages and disappeared in the middle and late stages, which was consistent with the test results of Yao Min [43] (Additional file 1: Fig. S2). Although 2b deletion did not fully manifest disease in the early stages, it significantly affected the symptoms brought on by the virus in N. benthamiana, suggesting that the 2b protein is crucial for N. benthamiana's CMV symptoms. The specific mechanism causing this phenomenon needs to be further explored.
The PDS gene is utilized to produce visual phenotypes in plant VIGS experiments for various species, which are involved in carotenoid biosynthesis. In water dropwort, silencing OjPDSN and OjPDSC with the CMV-based VIGS system led to photobleaching that affected both newly developed leaves and stems (Fig. 2; Additional file 1: Fig. S3). This demonstrates the highly efficient nature of the CMV-based VIGS system in quickly silencing genes in water dropwort. Herefore, gene silencing approach has the potential to be extremely beneficial in carrying out functional investigations related to the developmental and biosynthetic pathways of water dropwort genes.
The gibberellin signaling pathway heavily relies on the Gibberellin receptor GID1. GA is a crucial hormone responsible for plant growth and frequently resulted in dwarfing. To analyze the GID1 gene that encodes GA receptors, specifically OjGID1, we extracted it from the water dropwort genome. VIGS was performed to silence the OjGID1 gene, which led to a dwarfish appearance (Fig. 3; Additional file 1: Fig. S6). Additionally, the OjGID1 gene silencing had a significant impact on papaya leaf morphology [49]. Our experiment revealed that CMV-OjGID1 infection triggered GID1 silencing phenotypes comparable to those in tobacco rattle virus-induced gene silencing of GID1 in petunia and Arabidopsis thaliana and tomato GID1 mutants [50,51,52]. Hence, our study provides a sound foundation for future plant breeding and production.
As well as engineering CMV for utilization as a VIGS vector, we also its miRNA silencing function. It represents the initial VbMS vector designed for the functional assessment of miRNAs relating to water dropwort. Amongst the miRNA families, miR319 is highly conserved and integral in leaf development, it regulates the expression of a multitude of genes, including teosinte branched1, cycloidea, and proliferating cell nuclear antigen binding factor (TCP) genes [53]. In this study, ‘Yzcbq’ and ‘Fq1’ were selected as the experimental materials. This variety showed obvious stem elongation characteristics during the growth of water dropwort, which were conducive to the observation of phenotype. The results show that CMV VIGS system expressed the STTM319. After 30 days of inoculation, it was observed that the leaf morphology was significantly smaller than that of the control, and the entire plant showed an obvious dwarf phenotype (Figs. 4B and 5B; Additional file 1: Fig. S9 and S10). Fang expressed STTM319 through the TRSV silencing system; after 30 days of watermelon inoculation, it was discovered that both the plant and leaf size were noticeably shortened. These results were in agreement with the phenotypic observations from the study conducted, as previously established in [54]. Additionally, the experimental findings indicated a crucial outcome, whereby the expression level of the target gene TCP4, demonstrated a substantial increase on miR319 silencing. Significantly, this finding was found to be congruent with the results obtained from the watermelon STTM319 analysis (Fig. 4F; Additional file 1: Fig. S8E and S9E). The ectopic expression of Arabidopsis miR319-resistant TCP4 (mTCP4) has been found to lead to the production of micro leaves [55]. Moreover, Jones-Rhoades and Bartel discovered miR396 in Arabidopsis and rice in 2004 and predicted that it can regulate the Growth-Regulating Factor (GRF) gene [56]. In this study, STTM396 was silenced by CMV VIGS. miR396-silenced water dropwort plants were found to be shorter than control plants 30 days after inoculation (Fig. 5B; Additional file 1: Fig. S11A and S12A). Liu et al. found that the transgenic A. thaliana overexpressing miR396 was significantly shorter than the control, which was consistent with the results of this experiment [57]. Therefore, the CMV silencing system established can be used for miRNA silencing tests. Arabidopsis miR396 has been reported to be able to target seven members of the GRF family and regulate leaf growth and shape [58, 59]. Jia measured the expression level of miR396 in water dropwort leaves at three different developmental stages and found that it showed a gradual downwards trend, indicating that miR396 was involved in the development of celery leaves [45]. Similarly, the leaf size of miR396 silenced water dropwort was smaller than that of the control, which was consistent with the experimental results by Jia [45] (Fig. 5B; Additional file 1: Fig. S11A and S12A).
According to the findings of the research, CMV-based vectors demonstrate a remarkable capacity for exploring the biological roles of genes and miRNAs in water dropwort. Considering the versatility of vectors, it is evident that functional genomic studies in water dropwort plants are likely to be significantly facilitated and wildly used. The VIGS systerm plays a good role in silencing the expression of PDS, G1D1 and miRNAs in water dropwort for subsequent studies. In our experiments, water dropwort subjected to VIGS still exhibited silencing after 60–90 dpi (data not shown). The miRNA319 silencing efficiency was also superior to previous study [30]. The tissue cultured seedlings can effectively improve the consistency and efficiency of the results. Temperature and the length of insertion fragments are essential to large-scale and efficient application of VIGS in the gene function-related validation experiments. Therefore, there is great potential for these vectors to reveal more about the molecular mechanisms of water dropwort plants and establish a theoretical foundation for future studies of this species.
Materials and methods
Plant materials
The study utilized various types of water dropwort buds. The plants subjected to VIGS assays were cultivated in a greenhouse, with a temperature of 25 °C, and exposed to a light/dark cycle of 10/14 h. Moreover, the plants were kept in an environment with relative humidity at 85%.
Vector construction
Agrobacterium-mediated infectious cDNA clones of CMV-Fny were obtained from Prof. Dawei Li's laboratory. The three chains of CMV-Fny were transferred into the mini binary vector, pCB301, to build pCB301CMV-Fny1, pCB301CMV-Fny2, and pCB301CMV-Fny3, which were then introduced into Agrobacterium and inoculated into N. benthamiana and water dropwort by agroinfiltration. pCB301CMV-Fny2 was used as the DNA template; Fny 2b del BamSmaSpe-F and Fny 2b del Bam-R were used as primers for reverse PCR amplification, and a fragment of approximately 7 566 bp size was obtained [43] (Additional file 1: Fig. S1). The PCR product was recovered, the 2b deletion mutant vector pCB301-Fny3-2b del was constructed by BamH I enzyme digestion and self-linking, and the modification of the CMV-infected clone of water dropwort was completed. It indicated that the modified CMV VIGS vector could be used in subsequent studies.
Agroinfiltration of N. benthamiana and water dropwort plants
The plasmids were transformed into individual A. tumefaciens GV3101 cells [60]. The cells were cultured at 28 °C with shaking at 220 rpm for 10–16 h. After centrifuging the cells at 3000 × g for 10 min, they were suspended in an infiltration buffer [61]. The cultures containing the construct of CMV infectious clone were adjusted to an optical density of OD600 = 0.6, and then incubated at room temperature for 2–3 h. N. benthamiana and water dropwort plants were injected with the Agrobacterium cultures using a sterile 1.0 mL syringe.
RNA extraction and RT‒PCR
The MiniBEST Plant RNA Extraction Kit (polysaccharide polyphenol Plant tissue lysis) manufactured by TAKARA in Dalian was utilized to extract total RNA from leaf tissues of N. benthamiana and water dropwort. The manufacturer’s standardized protocol was followed during the RNA extraction procedure. To determine RNA quality, a 1.0% agarose gel was utilized. Single-stranded cDNA was synthesized by the PrimeScript™ II 1st Strand cDNA Synthesis Kit, following the instructions provided by TAKARA, Dalian. The resulting cDNA solution was preserved at a temperature of − 20 °C.
The RT-PCR assay utilized specific primers that were listed in the Additional file 2: Table S10. The RT-PCR mixture had a volume of 25 μL and contained 0.2 μL of Ex Taq HS (5 U/μL) from TAKARA in Dalian, 2.0 μL of cDNA, 2.0 μL of dNTP (10 mM each), 2.5 μL of primers, 2.5 μL of 10 × PCR Buffer II, and the appropriate amount of ddH2O. Following its creation, a 5 μL sample was tested on a 1.0% agarose gel. All of the samples were then subjected to RT-PCR testing, and any positive samples. were stored at − 80 ℃.
Protein extractions and western blotting
To extract total protein, the leaf tissue infected with CMV was frozen in liquid nitrogen and then ground to a fine powder. Next, the powder was suspended in a protein extraction buffer composed of 1 M Tris–HCl (pH 6.8), 200 mL of glycerin, 2 g of bromophenol blue, and 50 mL of β-mercaptoethanol. The resultant samples were boiled for 10 min, after which they were centrifuged at 12,000 rpm for 10 min. Supernatants were selected and subjected to SDS-PAGE on a vertical electrophoresis apparatus. Next, the membrane was blocked with gentle agitation in 10 mL of non-fat milk in TBST (20 mM Tris base, 150 mM NaCl, 0.05% tween-20, pH 7.5) added with the CP-specific antibody at 4 °C overnight. Following this, the membranes were washed thrice with a 1 × solution of TBST every 10 min. A coupled anti-rabbit immunoglobulin was then incubated at an appropriate working dilution for 45 min. The NC membranes were subsequently washed with TBST for 10 min each. Finally, the membrane was photographed.
Availability of data and materials
Source data can be found in Supplementary Datasets. The data that support the finding of this Conflict of interests study are available from the corresponding authors upon request.
References
Lu CL, Li XF. A review of Oenanthe javanica (Blume) DC. as traditional medicinal plant and its therapeutic potential. Evid Based Complement Alternat Med. 2019;2019:6495819.
Feng K, Kan XY, Li R, Yan YJ, Zhao SP, Wu P, Li LJ. Integrative analysis of long- and short-read transcriptomes identify the regulation of terpenoids biosynthesis under shading cultivation in Oenanthe javanica. Front Genet. 2022;13: 813216.
Park JH, Cho JH, Kim IH, Ahn JH, Lee JC, Chen BH, Shin BN, Tae HJ, Yoo KY, Hong S, Kang IJ, Won MH, Kim JD. Oenanthe Javanica extract protects against experimentally induced ischemic neuronal damage via its antioxidant effects. Chin Med J. 2015;128:2932–7.
Feng K, Xu ZS, Que F, Liu JX, Wang F, Xiong AS. An R2R3-MYB transcription factor, OjMYB1, functions in anthocyanin biosynthesis in Oenanthe javanica. Planta. 2018;247:301–15.
Ku SK, Kim TH, Lee S, Kim SM, Bae JS. Antithrombotic and profibrinolytic activities of isorhamnetin-3-O-galactoside and hyperoside. Food Chem Toxicol. 2013;53:197–204.
Yang SA, Jung YS, Lee SJ, Park SC, Kim MJ, Lee EJ, Byun HJ, Jhee KH, Lee SP. Hepatoprotective effects of fermented field water-dropwort (Oenanthe javanica) extract and its major constituents. Food Chem Toxicol. 2014;67:154–60.
Ma CJ, Lee KY, Jeong EJ, Kim SH, Park J, Choi YH, Kim YC, Sung SH. Persicarin from water dropwort (Oenanthe javanica) protects primary cultured rat cortical cells from glutamate-induced neurotoxicity. Phytother Res. 2010;24:913–8.
Ahn H, Lee GS. Isorhamnetin and hyperoside derived from water dropwort inhibits inflammasome activation. Phytomedicine. 2017;24:77–86.
Her Y, Shin BN, Lee YL, Park JH, Kim DW, Kim KS, Kim H, Song M, Kim JD, Won MH, Ahn JH. Oenanthe Javanica Extract Protects Mouse Skin from UVB Radiation via Attenuating Collagen Disruption and Inflammation. Int J Mol Sci. 2019;20:1435.
Han YQ, Huang ZM, Yang XB, Liu HZ, Wu GX. In vivo and in vitro anti-hepatitis B virus activity of total phenolics from Oenanthe javanica. J Ethnopharmacol. 2008;118:148–53.
Moon SC, Park SC, Yeo EJ, Kwak CS. Water Dropwort (Ostericum sieboldii) and Sedum (Sedum sarmentosum) Delay H2O2-Induced Senescence in Human Diploid Fibroblasts. J Med Food. 2009;12:485–92.
Feng L, Duan CG, Guo HS. Inhibition of in vivo Slicer activity of Argonaute protein 1 by the viral 2b protein independent of its dsRNA-binding function. Mol Plant Pathol. 2013;14:617–22.
Liu Y, Schiff M, Dinesh-Kumar SP. Virus-induced gene silencing in tomato. Plant J. 2002;31:777–86.
Padmanabhan M, Dinesh-Kumar SP. Virus-induced gene silencing as a tool for delivery of dsRNA into plants. Cold Spring Harb Protoc. 2009;2009:5139.
Senthil-Kumar M, Mysore KS. Tobacco rattle virus-based virus-induced gene silencing in Nicotiana benthamiana. Nat Protoc. 2014;9:1549–62.
Robertson D. VIGS vectors for gene silencing: many targets, many tools. Annu Rev Plant Biol. 2004;55:495–519.
Lu R, Martin-Hernandez AM, Peart JR, Malcuit I, Baulcombe DC. Virus-induced gene silencing in plants. Methods. 2003;30:296–303.
Kumagai MH, Donson J, Della-Cioppa G, Harvey D, Hanley K, Grill LK. Cytoplasmic inhibition of carotenoid biosynthesis with virus-derived RNA. Proc Nat Acad Sci United States Am. 1995;92:1679–83.
Ruiz MT, Voinnet O, Baulcombe DC. Initiation and maintenance of virus-induced gene silencing. Plant Cell. 1998;10:937–46.
Ratcliff FG, MacFarlane SA, Baulcombe DC. Gene silencing without DNA: RNA-medicated cross-protection between viruses. Plant Cell. 1999;11:1207–16.
Holzberg S, Brosio P, Gross C, Pogue GP. Barley stripe mosaic virus-induced gene silencing in a monocot plant. Plant J. 2002;30:315–27.
Gosselé V, Faché I, Meulewaeter F, Cornelissen M, Metzlaff M. SVISS—a novel transient gene silencing system for gene function discovery and validation in tobacco plants. Plant J. 2002;32:859–66.
Constantin GD, Krath BN, MacFarlane SA, Nicolaisen M, Johansen IE, Lund OS. Virus-induced gene silencing as a tool for functional genomics in a legume species. Plant J. 2004;40:622–31.
Hori K, Takizawa M, Watanabe Y. Use of an attenuated strain of Tobamovirus for early detection of virus-induced gene silencing. Plant Biotechnol. 2004;21:135–42.
Zhang C, Ghabrial SA. Development of Bean pod mottle virus-based vectors for stable protein expression and sequence-specific virus-induced gene silencing in soybean. Virology. 2006;344:401–11.
Otagaki S, Arai M, Takahashi A, Goto K, Hong JS, Masuta C, Kanazawa A. Rapid induction of transcriptional and post-transcriptional gene silencing using a novel Cucumber mosaic virus vector. Plant Biotechnol. 2006;23:259–65.
Ding XS, Schneider WL, Chaluvadi SR, Mian MA, Nelson RS. Characterization of a Brome mosaic virus strain and its use as a vector for gene silencing in monocotyledonous hosts. Mol Plant Microbe Interact. 2006;19:1229–39.
Ding XS, Mannas SW, Bishop BA, Rao X, Lecoultre M, Kwon S, Nelson RS. An improved Brome mosaic virus silencing vector: greater insert stability and more extensive VIGS. Plant Physiol. 2018;176:496–510.
Kon T, Yoshikawa N. Induction and maintenance of DNA methylation in plant promoter sequences by apple latent spherical virus-induced transcriptional gene silencing. Front Microbiol. 2014;5:595.
Fang L, Wei XY, Liu LZ, Zhou LX, Tian YP, Geng C, Li XD. A tobacco ringspot virus-based vector system for gene and microRNA function studies in cucurbits. Plant Physiol. 2021;186:853–64.
Purkayastha A, Mathur S, Verma V, Sharma S, Dasgupta I. Virus-induced gene silencing in rice using a vector derived from a DNA virus. Planta. 2010;232:1531–40.
Wang LQ, Dong TT, Kan XY, Chen W, Liu X, Zhang Y, He Z, Li LJ. First report of cucumber mosaic virus infecting water dropwort (Oenanthe javanica (Blume). DC) in Jiangsu province in China. J Plant Pathol. 2023;105:621.
Ding SW, Anderson BJ, Haase HR, Symons RH. New overlapping gene encoded by the Cucumber Mosaic Virus genome. Virology. 1994;198:593–601.
Hayes RJ, Buck KW. Complete replication of a eukaryotic virus RNA in vitro by a purified RNA-dependent RNA polymerase. Cell. 1990;63:363–8.
Guo HS, Ding SW. A viral protein inhibits the long range signaling activity of the gene silencing signal. EMBO J. 2002;21:398–407.
Duan CG, Fang YY, Zhou BJ, Zhao JH, Hou WN, Zhu H, Ding SW, Guo HS. Suppression of Arabidopsis ARGONAUTE1-mediated slicing, transgene-induced RNA silencing, and DNA methylation by distinct domains of the Cucumber mosaic virus 2b protein. Plant Cell. 2012;24:259–74.
González I, Martínez L, Rakitina DV, Lewsey MG, Atencio FA, Llave C, Kalinina NO, Carr JP, Palukaitis P, Canto T. Cucumber mosaic virus 2b protein subcellular targets and interactions: their significance to rna silencing suppressor activity. Mol Plant Microbe Interact. 2010;23:294–303.
Fang YY, Zhao JH, Liu SW, Wang S, Duan CG, Guo HS. CMV2b-AGO interaction is required for the suppression of RDR-dependent antiviral silencing in Arabidopsis. Front Microbiol. 2016;7:1329.
Lewsey M, Surette M, Robertson FC, Ziebell H, Choi SH, Ryu KH, Canto T, Palukaitis P, Payne T, Walsh JA, Carr JP. The role of the Cucumber mosaic virus 2b protein in viral movement and symptom induction. Mol Plant Microbe Interact. 2009;22:642–54.
Nagamatsu A, Masuta C, Senda M, Matsuura H, Kasai A, Hong JS, Kitamura K, Abe J, Kanazawa A. Functional analysis of soybean genes involved in flavonoid biosynthesis by virus-induced gene silencing. Plant Biotechnol J. 2007;5:778–90.
Kim BM, Ji I, Masuta C. Virus induced gene silencing in Antirrhinum majus using the Cucumber Mosaic Virus vector: functional analysis of the AINTEGUMENTA (Am-ANT) gene of A. majus. Hortic Environ Biotechnol. 2011;52:176–82.
Hong JS, Rhee S, Kim E, Kim TS, Ryu KH, Masuta C, Lee GP. Application of a reassortant cucumber mosaic virus vector for gene silencing in tomato and chili pepper plants. Plant Pathol J. 2012;28:81–6.
Yao M, Zhang TQ, Tian ZC, Wang YC, Tao XR. Construction of Agrobacterium-mediated Cucumber mosaic virus Infectious cDNA Clones and 2b Deletion Viral Vector. Scientia Agricultura Sinica. 2011;44:3060–8.
Pei QY, Li N, Bai Y, Wu T, Yang Q, Yu T, Wang ZY, Liu Z, Li Q, Lin H, Song XM. Comparative analysis of the TCP gene family in celery, coriander and carrot (family Apiaceae). Veget Res. 2021;1:5.
Jia XL, Li MY, Jiang Q, Xu ZS, Wang F, Xiong AS. High-throughput sequencing of small RNAs and anatomical characteristics associated with leaf development in celery. Sci Rep. 2015;5:11093.
Wang R, Yang X, Wang N, Liu X, Nelson RS, Li W, Fan Z, Zhou T. An efficient virus-induced gene silencing vector for maize functional genomics research. Plant J. 2016;86:102–15.
Tzean Y, Lee MC, Jan HH, Chiu YS, Tu TC, Hou BH, Chen HM, Chou CN, Yeh HH. Cucumber mosaic virus-induced gene silencing in banana. Sci Rep. 2019;9:11553.
Li HG, Zhang DF, Xie K, Wang Y, Liao QS, Hong YG, Liu YL. Efficient and high-throughput pseudorecombinant-chimeric Cucumber mosaic virus-based VIGS in maize. Plant Physiol. 2021;187:2865–76.
Tuo D, Yan P, Zhao G, Cui H, Zhu G, Liu Y, Yang X, Wang H, Li X, Shen W, Zhou P. An efficient papaya leaf distortion mosaic potyvirus vector for virus-induced gene silencing in papaya. Hortic Res. 2021;8:144.
Liang YC, Reid MS, Jiang CZ. Controlling plant architecture by manipulation of gibberellic acid signalling in petunia. Hortic Res. 2014;1:14061.
Griffiths J, Murase K, Rieu I, Zentella R, Zhang ZL, Powers SJ, Gong F, Phillips AL, Hedden P, Sun TP, Thomas SG. Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell. 2006;18:3399–414.
Illouz-Eliaz N, Ramon U, Shohat H, Blum S, Livne S, Mendelson D, Weiss D. Multiple gibberellin receptors contribute to phenotypic stability under changing environments. Plant Cell. 2019;31:1506–19.
Ori N, Cohen AR, Etzioni A, Brand A, Yanai O, Shleizer S, Menda N, Amsellem Z, Efroni I, Pekker I, Alvarez JP, Blum E, Zamir D, Eshed Y. Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nat Genet. 2007;39:787–91.
D’Ario M, Griffiths-Jones S, Kim M. Small RNAs: big impact on plant development. Trends Plant Sci. 2017;22:1056–68.
Koyama T, Sato F, Ohme-Takagi M. Roles of miR319 and TCP transcription factors in leaf development. Plant Physiol. 2017;175:874–85.
Jones-Rhoades MW, Bartel DP. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell. 2004;14:787–99.
Liu D, Song Y, Chen Z, Yu D. Ectopic expression of miR396 suppresses GRF target gene expression and alters leaf growth in Arabidopsis. Physiol Plant. 2009;136:223–36.
Das Gupta M, Nath U. Divergence in patterns of leaf growth polarity is associated with the expression divergence of miR396. The Plant Cell Online. 2015;27:2785–99.
Rodriguez RE, Mecchia MA, Debernardi JM, Schommer C, Weigel D, Palatnik JF. Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development. 2010;137:103–12.
Hofgen R, Willmitzer L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 1988;16:9877–9877.
Geng C, Cong QQ, Li XD, Mou AL, Gao R, Liu JL, Tian YP. DEVELOPMENTALLY REGULATED PLASMA MEMBRANE PROTEIN of Nicotiana benthamiana contributes to potyvirus movement and transports to plasmodesmata via the early secretory pathway and the actomyosin system. Plant Physiol. 2015;167:394–410.
Acknowledgements
We thank Jingjie PTM BioLab (Hangzhou) Co. Ltd and all the other members of plant virology laboratory at Yangzhou University for their helpful suggestions and constructive criticism. We thank Dr. Xianbing Wang (China Agricultural University, Beijing, China) for grifting of the35S-CMV FNY infectious clones. We also thank Dr. XiaoRong Tao (Nanjing Agricultural University, Nanjing, China) for kindly providing the pCB301 vector.
Funding
This work was supported by grants from the Jiangsu Seed Industry Revitalization ‘Jie Bang Gua Shuai’ project (JBGS [2021] 017), the China Agriculture Research System (CARS-24), the National Natural Science Foundation of China (No. 32272485), the key intergovernmental special projects for international scientific and technological innovation cooperation (2022YFE0130900), the Natural Science Foundation of the Jiangsu Province (BK20211323) and the High-Level Talent Support Program of Yangzhou University, Yangzhou University interdisciplinary high-level young talent Cultivation project.
Author information
Authors and Affiliations
Contributions
ZH, KZ and LJL conceived the experiments of proteomic analysis. LQW and TTD carried out experiments. ZH, SYS and LQW wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. Construction of pCB301-CMV-RNA2△2b. (A) Map of pCB301-RNA2 and pCB301-RNA2△2b. (B) Inverse-PCR detection of CMV-RNA2. Figure S2. The CMV-Fny 2b deletion mutant infected N. benthamiana by agro-infection. (A) Symptoms of the CMV-Fny 2b deletion mutant in N. benthamiana. Scale bars are 2 cm. (B) Detection of CMV-Fny 2b deletion mutant-infected N. benthamiana by agro-infection (8 dpi). The vector plasmid with the CMV RNA3 insert was amplified as the positive (lane P) control. Figure S3. Silencing of the NbPDS gene in N. benthamiana using the CMV VIGS vector. (A) Construct diagram of infectious clones of pCB301CMV-NbPDS. (B) RT‒PCR detection of the PDSC of N. benthamiana. (C) Phenotypes of pCB301CMV-NbPDS in N. benthamiana (14 dpi). Scale bars are 2 cm. (D) Detection of pCB301CMV-NbPDS-infected N. benthamiana by agro-infection (14 dpi). (E) RT‒qPCR analysis of NbPDS expression levels in CMV△2b- or CMV-NbPDS-infected plants. Figure S4. Identification of PDS gene in O. javanica. (A) Phylogenetic analysis of PDS of O. javanica. (B) Map of PDS gene of O. javanica. (C) Silencing Sequence of PDS gene in O. javanica. Figure S5. Identification of GID1 gene in O. javanica. (A) Phylogenetic analysis of GID1 of O. javanica. (B) Map of GID1 gene of O. javanica. (C) Silencing Sequence of GID1 gene in O. javanica. Figure S6. Silencing of the OjGID1 gene in O. javanica using the CMV VIGS vector. (A) Phenotypes of pCB301CMV-OjGID1 in O. javanica (30 dpi). Scale bars are 2 cm. (B) Systematic leaf morphology analysis of pCB301CMV-OjGID1 in ‘Fq1’ (30 dpi). (C) Detection of pCB301CMV-OjGID1-infected ‘Fq1’ by agro-infection (30 dpi). (D) RT‒qPCR analysis of OjGID1 expression levels in CMV△2b- or CMV-OjGID1-infected plants. (E) RT‒qPCR analysis of GA-related genes expression in CMV△2b- or CMV-OjGID1-infected plants. Figure S7. RT‒qPCR analysis of 6 OjGID1 genes expression in CMV△2b- or CMV-OjGID1-infected O. javanica. (A) RT‒qPCR analysis of 6 OjGID1 genes expression in CMV△2b- or CMV-OjGID1-infected ‘Yzcbq’. (B) RT‒qPCR analysis of 6 OjGID1 genes expression in CMV△2b- or CMV-OjGID1-infected ‘Fq1’. Figure S8. Silencing of the NbGID1 gene in N. benthamiana using the CMV VIGS vector. (A) Phenotypes of pCB301CMV-NbGID1 in N. benthamiana (17 dpi). Scale bars are 2 cm. (B) Systematic leaf morphology analysis of pCB301CMV-NbGID1 in N. benthamiana (17 dpi). (C) Detection of pCB301CMV-NbGID1-infected N. benthamiana by agro-infection. (D) RT‒qPCR analysis of NbGID1 expression levels in CMV△2b- or CMV-NbGID1-infected plants. (E) RT‒qPCR analysis of NbGID1 expression levels in CMV△2b- or CMV-NbGID1-infected plants. Figure S9. Silencing of miR319 in O. javanica using CMV-based VbMS vector. (A) Phenotypes of plants inoculated with CMV-STTM319 at 30 dpi. Scale bars are 2 cm. (B) Systematic leaf morphology analysis of pCB301CMV-STTM319 in ‘Yzcbq’. (C) Detection of pCB301CMV-STTM319-infected ‘Yzcbq’ by agro-infection. (D) Stem‒loop RT‒qPCR analysis of miR319 expression levels in CMV△2b- or CMV-STTM319-infected plants. (E) Detection of the relative expression levels of the miR319 target genes TCP2 and TCP4. Figure S10. Silencing of miR319 in N. benthamiana using CMV-based VbMS vector. (A) Phenotypes of plants inoculated with CMV-STTM319 at 15 dpi. Scale bars are 2 cm. (B) Systematic leaf morphology analysis of pCB301CMV-STTM319 in N. benthamiana. (C) Detection of pCB301CMV-STTM319-infected N. benthamiana by agro-infection. (D) Stem‒loop RT‒qPCR analysis of miR319 expression levels in CMV△2b- or CMV-STTM319-infected plants. (E) Detection of the relative expression levels of the miR319 target genes TCP2 and TCP4. Figure S11. Silencing of miR396 in O. javanica using CMV-based VbMS vector. (A) Phenotypes of plants inoculated with CMV-STTM396 at 30 dpi. Scale bars are 2 cm. (B) Systematic leaf morphology analysis of pCB301CMV-STTM396 in ‘Yzcbq’. (C) Detection of pCB301CMV-STTM396-infected ‘Yzcbq’ by agro-infection. (D) Stem‒loop RT‒qPCR analysis of miR396 expression levels in CMV△2b- or CMV-STTM396-infected plants. (E) Detection of the relative expression level of the miR396 target gene Growth-Regulating Factor 9-like. Figure S12. Silencing of miR396 in N. benthamiana using CMV-based VbMS vector. (A) Phenotypes of plants inoculated with CMV-STTM396 at 15 dpi. Scale bars are 2 cm. (B) Systematic leaf morphology analysis of pCB301CMV-STTM396 in N. benthamiana. (C) Detection of pCB301CMV-STTM396-infected N. benthamiana by agro-infection. (D) Stem‒loop RT‒qPCR analysis of miR396 expression levels in CMV△2b- or CMV-STTM396-infected plants. (E) Detection of the relative expression level of the miR396 target gene polyamine oxidase 1. Figure S13. Naturally infected of water dropwort by CMV. (A) Symptoms of CMV in O. javanica. (B) RT-PCR and western blot detection of the water dropwort samples.
Additional file 2:
Table S1. The silencing efficiency of PDS genes in N. benthamiana. Table S2. The silencing efficiency of PDS genes in water dropwort. Table S3. RT‒qPCR analysis of OjPDS expression levels in CMV-OjPDSN- or CMV-OjPDSC-infected ‘Fq1’. Table S4. RT‒qPCR analysis of OjPDS expression levels in CMV-OjPDSN- or CMV-OjPDSC-infected ‘Yzcbq’. Table S5. The silencing efficiency of GID1 genes. Table S6. The silencing efficiency of miRNA319 genes. Table S7. The silencing efficiency of miRNA396 genes. Table S8. Primers used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, Z., Sheng, S., Wang, L. et al. Cucumber mosaic virus-induced gene and microRNA silencing in water dropwort (Oenanthe javanica (Blume) DC). Plant Methods 20, 6 (2024). https://doi.org/10.1186/s13007-023-01129-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13007-023-01129-4