Abstract
Background
Clinical advice may suggest discontinuing breastfeeding after the diagnosis of phenylketonuria in infants as the only effective way to monitor the newborn's intake and accurate measurement of phenylalanine (Phe). This study aims to investigate the prevalence and duration of breastfeeding, as well as its effect on serum Phe levels in infants with phenylketonuria at Education and Therapy Medical Center, Be'sat Hospital, Iran.
Methods
We conducted a cross-sectional study of 34 children under two years old diagnosed with phenylketonuria between September 2018 and December 2022. Infants were categorized as breastfed and non-breastfed (bottle-fed) based on their feeding method after diagnosis. Data on age at diagnosis, medical records, demographic information, and anthropometric indices were collected, and infants with incomplete data or mixed feeding (formula + breast milk) were excluded from the study.
Results
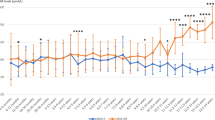
Of 94 infants managed in our hospital, 34 had complete medical records. Among the all patients 13 (38%) continued to be breastfed combined with phenylalanine-free amino acid-based protein substitute, while 21 (62%) were did not receive breast milk. The mean duration of breastfeeding was 2.57 ± 0.59 (1–3) months. The mean age at diagnosis was 22.6 ± 18.4 days. Phenylalanine concentrations at diagnosis were mean 10, SD 5.44; range 4–24 mg/dL [0.22–1.33 μmol/L] in the breastfed group and mean 14.3, SD 10.2; range 5–37 mg/dL [0.27–2.05 μmol/L] in the non-breastfed group.Non-breastfed infants had lower serum Phe levels than breastfed infants: mean 3.76, SD 2.10; range 1–7 mg/dL [0.05–0.38 μmol/L] and mean 4.89, SD 3.68; range 2–19 mg/dL [0.11–1.05 μmol/L], respectively, although not statistically significant [(t (34) = 118.0, P = 0.51]. Also we found no significant associations in body measurements for weight, height, and head circumference at birth and final assessment.
Conclusions
In conclusion, during treatment, there were no statistically significant associations between breastfeeding and serum Phe levels with growth in children with phenylketonuria.
Similar content being viewed by others
Background
Phenylketonuria (PKU) is an autosomal recessive inborn error of Inherited Metabolic Disorders (IMDs) that lead to enzymatic deficiencies within specific metabolic pathways caused by genetic mutations in the phenylalanine hydroxylase (PAH) gene encoding phenylalanine hydroxylase [1,2,3], which results in the inability to convert Phe to tyrosine, leading to increased phenylalanine concentrations in the blood and central nervous system [4]. Classic symptoms of untreated PKU include mental retardation, learning difficulties, spasticity, seizures, developmental delay, and congenital heart disease [5].
Comprehensive screening of newborns worldwide helps in the early identification and treatment of these metabolic disorders, subsequently reducing morbidity and mortality. A diagnostic method involving a heel prick test is conducted 24 h after birth to diagnose PKU [6]. Different methods for detecting PKU in dried blood spot sampling include fluorometric and colorimetric methods [7], enzymatic method [8], high-performance liquid chromatographic (HPLC) [9], and new techniques such as Tandem Mass Spectrometry [10]. Blood samples of all neonates in Iran are collected on days 3 to 5 after birth through a national program for screening and prevention of PKU established in 2007. Confirmation of PKU is done using by a colorimetric method, and if positive (phenylalanine levels of 4 mg/dL or higher [0.22 μmol/L]), infants are referred to be confirmed by the HPLC method [11]. Positive cases are referred to specialists for management and genetic counseling. Newborns with Phe levels equal to or greater than 4 mg/dL undergo regular follow-up, and if their Phe concentrations exceed 7 mg/dL (0.38 μmol/L), a restricted diet is initiated [12]. Since the inauguration of newborn screening in Iran, the incidence of PKU has been reported to be about one in 7000 live births, and this is noted to be more prevalent in some areas of the country [13]. Clinical sub-categories range from mild hyperphenylalaninemia (HPA) (Phe levels 120–360 μmol/ L) to the most common and severe form, classical PKU, defined as Phe > 1200 μmol/L [14]. Before newborn screening for PKU, Clinically, untreated disorder is characterized by irreversible intellectual disability, microcephaly, seizures, aberrant behavior, psychiatric symptoms, motor disturbances, and eczematous rash. Impairment of cerebral function had already occurred before newborn screening and treatment [15].
IMDs concerning lifelong management and treatment often focus on limiting substrate that cannot be metabolized from the diet and replacing other nutrients with supplements (medical foods) and drugs [16, 17]. Treatment of Infants diagnosed with PKU requires a unique low-phenylalanine formula, and it is recommended strict dietary therapy during their entire lifetime [18, 19]. However, in the management of infants with some IMDs, such as certain aminoacidopathies and urea cycle disorders, breastfeeding may be safely incorporated due to its lower protein and amino acid content than infant formula. Breastfeeding is included in management guidelines for some IMDs, including glutaric aciduria type I [20], maple syrup urine disease [21], phenylketonuria (PKU) [22, 23]; propionic acidemia [24], urea cycle disorders [25], and very long chain acyl-CoA dehydrogenase deficiency [26]. Since the Phe content in breast milk cannot be converted into tyrosine in the liver by the phenylalanine hydroxylase enzyme, unfortunately, exclusive feeding with breast milk in the first six months of life affects the cognitive-neural development of infants with PKU [27]. Severe cognitive impairments will be prevented with treatment [28]. In childhood, executive functions, such as working memory and reasoning/planning, attention, and processing speed, are mainly observed deficits [29]. Previously, the standard of care for patients with PKU was immediate cessation of breastfeeding to maintain adequate Phe levels with the combination of standard commercial infant formulas and amino acid-based protein substitutes without phenylalanine. In 1980, with the discovery of lower Phe levels in human breast milk compared to standard commercial infant formulas, breastfeeding began to replace the standard commercial formula in the protein-restricted diet of patients with PKU [30, 31]. Today, breastfeeding is encouraged in people with PKU [32, 33].
Previous studies reported that breastfed infants with PKU had no significant differences in weight gain, daily Phe intake, and mean serum Phe concentrations compared to bottle-fed infants with PKU [33,34,35]. On the other hand, a study by Banta-Wright et al. [32] showed that the mean serum Phe level in breastfed infants was lower than in bottle-fed infants with PKU.
In this study, we aimed to determine the prevalence and duration of breastfeeding, compare the effect of breastfeeding or non-breastfeeding on serum Phe levels, and anthropometric indices in infants with PKU. This is a subject for which limited results have been reported previously in the literature, and no study has been done to investigate this comparison in Iran.
Methods
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed in conducting this study [36], and are available as a supplementary file. This cross-sectional study surveyed children below two years old with phenylketonuria in Iran. The Kurdistan University of Medical Sciences reviewed and approved all study procedures on January 4, 2023 (IR.MUK.REC.1401.306). All participants provided written informed consent.
Medical records were analyzed from September 2018 to December 2022 for patients with classic PKU (serum Phe level above 6 mg/dl at diagnosis) admitted to Education and Therapy Medical Center, Be'sat Hospital, located in the capital city of Kurdistan. Patients with incomplete records or insufficient data were excluded from the study.
Study population
The medical records of 94 children with PKU admitted to our center were analyzed retrospectively. The study included only patients under two years of age (from September 2018 to December 2022) who were examined at least once a month. Infants with incomplete medical records (age at diagnosis, anthropometric data, feeding information, and serum Phe level), missing demographic data (parental consanguinity, gross annual household income, maternal education level, place of residence), and low adherence to diet (patients who were not fed according to diet list and patients who were fed both breast milk, commercial formula, and phenylalanine-free amino acid based protein substitute) were excluded from the study (Fig. 1). Thirty-four of the subjects had complete medical records and were enrolled in the study.
Setting
Infants were categorized as breastfed and non-breastfed (bottle-fed) according to the type of feeding after PKU diagnosis. Infants fed a combination of commercial formula and phenylalanine-free amino acid-based protein substitute after the diagnosis of PKU were included in the non-breastfed group. Infants who continued to be breastfed along with the phenylalanine-free amino acid-based protein substitute after the diagnosis of PKU were included in the breastfed group. After each breastfeeding, a phenylalanine-free amino acid-based protein substitute was given to the breastfed group. In the non-breastfed group, a combination of commercial formula and phenylalanine-free amino acid-based protein substitute was served at each feeding. All patients had their diet lists adjusted monthly and the volume of phenylalanine-free amino acid-based protein substitutes and commercial formulas were revised. Those whose consumptions were not monitored by their mothers (referred to as not adhering to the diet) were excluded. Also, infants who consumed mixed feedings (breast milk and formula) were not included in the study.
Data collection
Demographic data according to the information provided by the family and medical records (age at the time of diagnosis, gender, parental consanguinity, gross annual household income, maternal education level, place of residence) and clinical and laboratory findings (based on physical examination, clinical and dietitian's records of infants who were assessed at least once a month; birth and final assessment of anthropometric indices, duration of breastfeeding, serum Phe level) of the patients were documented. Missing data on medical records or absence of serum Phe values recorded at least once a month were defined as insufficient medical records and were determined as exclusion criteria. The HPLC method was used to measure phenylalanine concentrations in blood samples. In the analysis of mean Phe levels, data were collected from the medical records of the newborn screening program and confirmatory diagnostic serum Phe levels, which were ≥ 6 mg/dl (≥ 0.33 μmol/L) for each patient. All data for the study was obtained by physicians and dietitians who worked at Be’sat Hospital.
Data analysis
Statistical data analysis was performed using SPSS computer software version 15.0 (SPSS, Chicago, IL). To examine the normality of parameters, the Kolmogorov–Smirnov test was carried out. While categorical data were expressed as numbers and percentages (%), continuous data were expressed as mean ± standard deviation, median, full range, and 25th-75th centiles. Categorical variables (gender, parental consanguinity, gross annual household income, place of residence, maternal education level, breastfeeding experience of the mother) were assessed using chi-square. Continuous variables (serum Phe levels, anthropometric indices) were evaluated using the Independent Samples t Test and Mann–Whitney U Test, respectively. A p-value < 0.05 was considered significant.
Results
The study enrolled thirty-four infants diagnosed with PKU. Of these, 19 (56%) were female and 15 (44%) were male. The mean age at diagnosis was 22.5 ± 18.4 days (3–115). A comparison of breastfed and non-breastfed infants with PKU revealed no significant difference regarding gender and age at the time of diagnosis. There was a significant difference between the two groups regarding maternal education level t (3) = 34.4, P = 0.03 (Table 1).
Table 2 shows the anthropometric indices of patients for each group. No significant differences were seen in body measurements for weight, height, and head circumference at birth and final assessment. However, the average anthropometric indices of infants at birth and at the end of the evaluation in both groups were within the normal ranges of the Iranian standard growth centile charts [37] (Table 3). The weight, height and head circumference centiles of Iranian children were similar to UK and USA values [38].
Prevalence and duration of breastfeeding
After the diagnosis of PKU, breastfeeding was continued in 13 (38%) infants combined with a phenylalanine-free amino acid-based protein substitute. In 21 (62%) infants, mothers stopped breastfeeding and continued to feed with the combination of commercial formula and phenylalanine-free amino acid-based protein substitute. The mean duration of breastfeeding was 14.3 ± 0.59 (range 1–24) months (Table 1). No other relationship was detected between other demographic characteristics (gross annual household income, place of residence, first breastfeeding experience of the mother) and the duration of breastfeeding.
Analysis of serum Phe levels
Serum Phe concentrations of non-breastfed infants [mean 3.76, SD 2.10; range 1–7 mg/dL (0.05–0.38 μmol/L)] were lower than that of breastfed infants during the breastfeeding period [mean 4.89, SD 3.68; range 2–19 mg/dL (0.11–1.05 μmol/L)]. However, there was no significant difference in serum Phe levels between the two groups receiving breast milk and bottle t (34) = 118.0, P = 0.51.
Discussion
In this study of thirty-four infants with PKU, we found no differences in phenylalanine concentrations between the breastfed and non-breastfed infants and no differences in anthropometric indices.
Several studies have discussed using human milk or breastfeeding in the dietary management of infants with PKU. Adequate growth and development of infants with PKU in these studies supports the efficacy of using human milk [39,40,41]. Breastfeeding also emphasizes the benefits of strengthening the emotional bond between mother and child and accepting the disease. Maintaining regular feeding with breast milk as a source of Phe in the treatment of PKU means that phenylketonuria infants can receive all the benefits of breast milk, even if they receive it as part of mixed breastfeeding [41].
Lower serum Phe levels in most breastfed infants compared to non-breastfed infants have been investigated previously, which shows the effect of breastfeeding on serum Phe levels in patients with PKU [34, 42, 43]. On the other hand, a recent study revealed similar serum Phe levels in breastfed and non-breastfed patients with PKU [32]. Although no significant relationship was found in our research, the level of Phe concentrations was lower in the formula-receiving group. Even though no significant difference was detected in comparing both groups, the bottle-fed group kept all Phe values within the reference range of the United States and European guidelines. The treatment target of blood Phe concentrations is between 120–360 μmol/L [1.38 to 4.14 mg/dL] for all patients aged 0–12 years [44, 45]. It is worth emphasizing that breastfeeding in our population is performed without precise daily measurements, while daily feeding with commercial formula is measured accurately. This difference in the amount consumed and the inaccuracy of measuring breast milk can affect the serum level of phenylalanine.
We consider that the breastfeeding duration of children with PKU over the 24 months of follow-up was 2.57 ± 0.59 months. In this regard, in a study by Rijn et al. [43], breastfeeding duration was 2.5 months. However, Demirkol et al. [35], McCabe et al. [41], and Motzfeldt et al. [31] found mean breastfeeding duration in phenylketonuric patients of 6.1, 8.9, and 7.0 months, respectively. Although the breastfeeding duration of observation was below that recommended by the World Health Organization, one should take into account the fact that, when traditional treatment is used, children are definitively weaned during the first month of life. Maintaining breast milk in these phenylketonuria infants' diets benefits them for a more extended period.
The time for blood Phe levels to reach normal levels from treatment is crucial. In this study, we observed that the results of the two groups were statistically similar. No differences were found between the two groups for birth weight, age at initiation of dietary therapy, or blood Phe level. This suggests that the breastfed and non-breast-fed groups were comparable at the beginning of treatment. The time to achieve metabolic control was similar, proving that the breastfeeding strategy did not adversely affect initial control.
To the best of our knowledge, this is the first study of Iranian infants with PKU conducted to compare the serum levels of Phe in two groups. Also, previous studies on anthropometric indicators only examined body weight while we assessed the height growth in our patients. Height growth is a better indicator to assess long-term nutritional status.
Limitations
There are several limitations to the present study. Firstly, it is important to acknowledge the small sample size, which limits the ability to make an optimal comparison between the two groups. Of the 94 cases managed in our hospital, only 34 were analyzed. Additionally, only the most compliant families attended sufficiently regularly to be included. It is possible that monthly attendance signified compliance, which may have been reflected in adherence to diet and infant feeding recommendations. This self-selection of participants may have engendered both a selection and a collider bias [46]. It should also be noted that various parameters, such as infant medications, infections, treatment compliance, and socio-cultural conditions, which were not accounted for in our study, could have potentially influenced both growth and serum Phe levels. Finally, maternal factors such as maternal medicines, maternal age, employment, and smoking status, which are associated with breastfeeding, were not evaluated in our study. The small sample size precluded adjusted analyses. This is a single-site study, which limits generalization of findings.
Conclusion
Our conclusion was that PHE levels did not differ between breast milk and non-breast milk groups. Using breast milk as a source of Phe in PKU treatment can be achieved. with proper control of blood Phe levels and infant growth within normal limits. Positive reinforcement of the emotional bond between mother and baby is an inherent behavioral benefit of breastfeeding. This positively impacts the acceptance of the condition and compliance with treatment.
Availability of data and materials
The corresponding author confirms that authors will make the data (in de-identified form, if human data) used in the manuscript, code book, and analytic code available to editors and readers upon request either before or after publication for checking.
Abbreviations
- HPLC:
-
High-performance liquid chromatographic
- IMDs:
-
Inherited Metabolic Disorders
- Phe:
-
Phenylalanine
- PKU :
-
Phenylketonuria
References
Villoria JG, Pajares S, López RM, Marin JL, Ribes A. Neonatal screening for inherited metabolic diseases in 2016. Semin Pediatric Neurol. 2016;23(4):257–72.
Saudubray J-M, Garcia-Cazorla À. Inborn errors of metabolism overview: pathophysiology, manifestations, evaluation, and management. Pediatr Clin North Am. 2018;65(2):179–208.
Wasim M, Awan FR, Khan HN, Tawab A, Iqbal M, Ayesha H. Aminoacidopathies: prevalence, etiology, screening, and treatment options. Biochem Genet. 2018;56(1):7–21.
MacDonald A, Rocha J, Van Rijn M, Feillet F. Nutrition in phenylketonuria. Mol Genet Metab. 2011;104:S10–8.
Pourfarzam M, Zadhoush F. Newborn screening for inherited metabolic disorders; news and views. J Res Med Sci. 2013;18(9):801–8.
Alfadhel M, Al Othaim A, Al Saif S, Al Mutairi F, Alsayed M, Rahbeeni Z, et al. Expanded newborn screening program in Saudi Arabia: incidence of screened disorders. J Paediatr Child Health. 2017;53(6):585–91.
Yamaguchi A, Mizushima Y, Fukushi M, Shimizu Y, Kikuchi Y, Takasugi N. Microassay system for newborn screening for phenylketonuria, maple syrup urine disease, homocystinuria, histidinemia and galactosemia with use of a fluorometric microplate reader. Screening. 1992;1(1):49–62.
Elvers LH, Diependaal GAM, Blonk HJ, Loeber JG. Phenylketonuria screening using the Quantase phenylalanine kit in combination with a microfilter system and the dye Tartrazine. Screening. 1995;3(4):209–23.
Contreras J, Alonso E, Fuentes L. HPLC for confirmatory diagnosis and biochemical monitoring of Cuban patients with hyperphenylalaninemias. MEDICC Rev. 2015;17:23–8.
Schulze A, Kohlmueller D, Mayatepek E. Sensitivity of electrospray-tandem mass spectrometry using the phenylalanine/tyrosine-ratio for differential diagnosis of hyperphenylalaninemia in neonates. Clin Chim Acta. 1999;283:15–20.
Shirzadeh T, Saeidian AH, Bagherian H, Salehpour S, Setoodeh A, Alaei MR, et al. Molecular genetics of a cohort of 635 cases of phenylketonuria in a consanguineous population. J Inherit Metab Dis. 2018;41(6):1159–67.
Aghasi P, Setoodeh A, Sayarifard A, Rashidiyan M, Sayarifard F, Rabbani A, et al. Intellectual and developmental status in children with hyperphenylalaninemia and PKU who were screened in a national program. Iran J Pediatr. 2015;25(6):e3033.
Habib A, Fallahzadeh MH, Kazeroni HR, Ganjkarimi AH. Incidence of phenylketonuria in Southern Iran. Iran J Med Sci. 2010;35(2):137–9.
Waisbren SE, Noel K, Fahrbach K, Cella C, Frame D, Dorenbaum A, Levy H. Phenylalanine blood levels and clinical outcomes in phenylketonuria: a systematic literature review and meta-analysis. Mol Genet Metab. 2007;92(1):63–70.
de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99suppl1:S86–9.
Boyer SW, Barclay LJ, Burrage LC. Inherited metabolic disorders. Nutr Clin Pract. 2015;30(4):502–10.
Breilyn MS, Wasserstein MP. Established and emerging treatments for patients with inborn errors of metabolism. NeoReviews. 2020;21(10):e699–707.
Vallian S, Moeini H. A quantitative bacterial micro-assay for rapid detection of serum phenylalanine in dry blood-spots: application in phenylketonuria screening. J Appl Genet. 2006;47:79–83.
Lukacs Z, Santer R. Evaluation of electrospray-tandem mass spectrometry for the detection of phenylketonuria and other rare disorders. Mol Nutr Food Res. 2006;50(4–5):443–50.
Kölker S, Christensen E, Leonard JV, Greenberg CR, Boneh A, Burlina AB, et al. Diagnosis and management of glutaric aciduria type I – revised recommendations. J Inherit Metab Dis. 2011;34(3):677.
Frazier DM, Allgeier C, Homer C, Marriage BJ, Ogata B, Rohr F, Splett PL, Stembridge A, Singh RH. Nutrition management guideline for maple syrup urine disease: an evidence- and consensus-based approach. Mol Genet Metab. 2014;112(3):210–7.
Singh RH, Cunningham AC, Mofidi S, Douglas TD, Frazier DM, Hook DG, Jeffers L, McCune H, Moseley KD, Ogata B, et al. Updated, web-based nutrition management guideline for PKU: an evidence and consensus based approach. Mol Genet Metab. 2016;118(2):72–83.
van Wegberg AMJ, MacDonald A, Ahring K, Bélanger-Quintana A, Blau N, Bosch AM, et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet Journal of Rare Disease. 2017;12(1):162–162.
Jurecki E, Ueda K, Frazier D, Rohr F, Thompson A, Hussa C, Obernolte L, Reineking B, Roberts AM, Yannicelli S, et al. Nutrition management guideline for propionic acidemia: an evidence- and consensus-based approach. Mol Genet Metab. 2019;126(4):341–54.
Häberle J, Boddaert N, Burlina A, Chakrapani A, Dixon M, Huemer M, Karall D, Martinelli D, Crespo PS, Santer R, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012;7(1):32.
Van Calcar SC, Sowa M, Rohr F, Beazer J, Setlock T, Weihe TU, Pendyal S, Wallace LS, Hansen JG, Stembridge A, et al. Nutrition management guideline for very-long chain acyl-CoA dehydrogenase deficiency (VLCAD): an evidence- and consensus-based approach. Mol Genet Metab. 2020;131(1):23–37.
Kose E, Aksoy B, Kuyum P, Tuncer N, Arslan N, Ozturk Y. The effects of breastfeeding in infants with phenylketonuria. J Pediatr Nurs. 2018;38:27–32.
Blau N, Van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376(9750):1417–27.
Albrecht J, Garbade SF, Burgard P. Neuropsychological speed tests and blood phenylalanine levels in patients with phenylketonuria: a meta-analysis. Neurosci Biobehav Rev. 2009;33(3):414–21.
Ernest AE. Guide to breast feeding the infant with PKU. 1980.
Motzfeldt K, Lilje R, Nylander G. Breastfeeding in phenylketonuria. Acta Paediatr. 1999;88(s432):25–7.
Banta-Wright SA, Shelton KC, Lowe ND, Knafl KA, Houck GM. Breast-feeding success among infants with phenylketonuria. J Pediatr Nurs. 2012;27(4):319–27.
Kanufre VC, Starling AL, Leão E, Aguiar MJ, Santos JS, Soares RD, Silveira AM. Breastfeeding in the treatment of children with phenylketonuria. J Pediatr. 2007;83:447–52.
Ea VC, Ea VM, Colombo M, Mabe P, Ma MJ, De la Parra CA, Gc AV, Raimann E. Phenylketonuria diagnosed during the neonatal period and breast feeding. Rev Méd Chile. 2003;131:1280–7.
Demirkol M, Huner G, Kuru N, Donmez S, Baykal T, Seckin Y. Feasibility of breast-feeding in inborn errors of metabolism: experience in phenylketonuria. Ann Nutr Metab. 2001;45(Suppl 1):497–8.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Initiative ftS: the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18(6):800–4.
Heydari S, Fatemeh E, Amini M. Infants’ growth charts in Jahrom, Iran. Iran J Pediatr. 2009;25–34.
Amirhakimi GH. A longitudinal growth study from birth to maturity for weight, height and head circumference of normal Iranian children compared with western norms: a standard for growth of Iranian children. Iran J Med Sci. 2015;28(1):9–16.
Yannicelli S. Breastfeeding the infant with phenylketonuria: a practical approach. Top Clin Nutr. 1987;2(3):25–30.
McCabe L, Ernest AE, Neifert MR, Yannicelli S, Nord AM, Garry PJ, McCabe ER, et al. The management of breast feeding among infants with phenylketonuria. J Inherit Metab Dis. 1989;12(4): 467–74.
McCabe ER, McCabe L. Issues in the dietary management of phenylketonuria: breast-feeding and trace-metal nutriture. Ann N Y Acad Sci. 1986;477:215–22.
Huner G, Demirkol M. Breast-feeding and phenylketonuria. Istanbul: Turkish Society for PKU; 1996.
van Rijn M, Bekhof J, Dijkstra T, Smit PGPA, Moddermam P, van Spronsen FJ. A different approach to breast-feeding of the infant with phenylketonuria. Eur J Pediatr. 2003;162(5):323–6.
Jurecki ER, Cederbaum S, Kopesky J, Perry K, Rohr F, Sanchez-Valle A, et al. Adherence to clinic recommendations among patients with phenylketonuria in the United States. Mol Genet Metab. 2017;120(3):190–7.
van Spronsen FJ, van Wegberg AM, Ahring K, Bélanger-Quintana A, Blau N, Bosch AM, Burlina A, et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 2017;5(9):743–56.
Jordan S, Bromley R, Damase-Michel C, Given J, Komninou S, Loane M, et al. Breastfeeding, pregnancy, medicines, neurodevelopment, and population databases: the information desert. Int Breastfeed J. 2022;17:55.
Acknowledgements
Not applicable.
Funding
This research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
EB, and ZM designed research; LS analyzed data; EB, ZM, LS. and AF. wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures aligned with the ethical standards of the Kurdistan University of Medical Sciences (IR.MUK.REC.1401.306), which approved the protocol and informed consent form.
Consent for publication
All authors have read and approved the submission of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mohammadzadeh, Z., Sharifi, L., Fatholahpour, A. et al. The investigation of serum phenylalanine levels based on infant feeding method: a cross-sectional study of children less than two years old with phenylketonuria (PKU). Int Breastfeed J 19, 12 (2024). https://doi.org/10.1186/s13006-024-00617-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13006-024-00617-0