Abstract
Background
The research aimed to evaluate the optimal Single-Photon Emission Computed Tomography (SPECT) cut-off value in differentiating condylar growth activeness, to observe 3-dimensional (3D) mandibular growth pattern, and to explore the potential correlation between 3D measurement parameters and SPECT uptake ratios in Chinese unilateral condylar hyperplasia (UCH) patients.
Methods
Data of fifty-four Chinese UCH patients were analyzed retrospectively. All patients underwent SPECT within 1 month before or after the first CT examination (CT1); and received a second CT examination at least 12 months later (CT2). Data from CT scans were analyzed by comparing bilateral differences between CT1 and CT2. The sensitivity and specificity of SPECT were calculated by the receiver operating characteristic (ROC) curve. Pearson’s correlation analysis was performed to investigate whether the mandibular growth was correlated with SPECT value.
Results
SPECT had a sensitivity of 68.00% and a specificity of 72.41%, with an area under the ROC curve being 0.709. The optimal SPECT cut-off value for evaluating condylar activity has been determined to be 13%. In patients with an active growing condyle, there was a significant increase in Co-Gn and Co-Go, but not in Go-Gn, Go-MF, or MF-Gn. Pearson’s correlation analysis revealed no correlation between 3D measurement parameters and differences in relative condylar uptake ratios.
Conclusion
SPECT showed good diagnostic performance in UCH with the cut-off value of 13%. For those with an active growing condyle, the mandible grows diagonally and vertically, while the relative condylar uptake ratio was not directly related to mandibular growth.
Similar content being viewed by others
Background
Unilateral condylar hyperplasia (UCH) is characterized by unilaterally excessive condyle growth, resulting in an inconsistency in the size, shape, and position of the mandible, eventually leading to dental abnormalities, asymmetry deformities, and temporomandibular joint (TMJ) disorders [1, 2]. Based on Obwegeser [3], Wolford et al. defined it as condylar hyperplasia type 1B with an accelerated and prolonged growth aberration of the ‘normal’ mandibular condylar growth pattern, inducing a predominantly horizontal growth vector, resulting in prognathism unilaterally [4]. Even though the etiology remains unclear, mandibular growth of UCH usually ceases at a certain point after a progressive unbalanced growth [5]. Consequently, treatment for UCH depends on the growth activity of the condyle. Thus, it is of utmost importance to choose an appropriate diagnostic method to accurately evaluate the condylar growth potential to determine the appropriate time and method for treatment.
A rapid diagnostic tool known as Single-Photon Emission Computed Tomography (SPECT) has been developed in recent decades to evaluate condylar growth potential, which allows for the acquisition of precise ROIs (Region of Interests) based on anatomical contours and will thus provide higher accuracy in ROI definition [6]. Though the uptake of the radiopharmaceutical in the choice of ROI could be influenced by the size, excellent intra- and inter-observer agreement of SPECT scans was reported when drawing an ROI in the condyles without using a CT scan [7]. Nevertheless, there is no standardized threshold for SPECT relative uptake ratios in assessing condylar growth activity, as different studies exhibited a variation of 5-12% in uptake ratios between normal condyles, with a variation of 10% being mostly reported [8,9,10,11]. While some researchers recommended a lower criterion [12] and others recommended a greater one [13], we assume the discrepancy in the diagnostic threshold might be due to different sample sizes, diverse assessment methods, or possible racial differences. Notably, the SPECT criterion for UCH in the Chinese population has yet to be established, and the mandibular growth pattern of Chinese UCH patients with various condylar growing conditions has not been investigated. According to our clinical experience, it is also not uncommon for condyles to remain inactive whereas the uptake ratio is 11% or 12%, and different growing conditions might lead to diverse growing patterns.
Considering the prediction threshold for mandibular growth activity and the growing tendency of UCH patients in the Chinese population has not yet been studied profoundly, this study aims to evaluate the SPECT value to differentiate the condylar growth activeness, to observe the 3-dimensional (3D) mandibular growth pattern and to explore the potential correlation between 3D measurement parameters and SPECT uptake ratios in Chinese UCH patients.
Methods
Study design
This retrospective study was conducted at Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine from May 2015 to March 2018. The study was designed under the Declaration of Helsinki and the protocol was approved by the Institutional Ethical Review Board of Shanghai Ninth People’s Hospital (SHDC12013103). Informed consent was obtained from all patients or guardians (for patients who were under 18 but above 16).
Data of fifty-four patients (28 male and 26 female) diagnosed with UCH were analyzed retrospectively in this study, with an average age of 19.87 ± 2.76 years old. The cases had been evaluated clinically and radiologically by experienced maxillofacial surgeons. The diagnosis was made by at least two experts through a combination of the patient’s history and serial assessment of the clinical and radiological findings according to Wolford’s criterion. All of them underwent two visits at an interval of more than one year and an average of 14.43 ± 1.92 months, with complete baseline and follow-up documentation including dental casts, clinical and 3D pictures, X rays and CT scans.
Patients underwent SPECT within 1 month before or after the first CT examination (CT1); and received a second CT examination at least 12 months after the first CT examination (CT2). Patients were excluded if they had a condylar tumor, a history of craniofacial trauma, acquired facial deformity, osteoarthritic diseases or congenital syndromes which may affect the growth and morphology of the mandible and condyle.
The reference criterion was defined according to Chan et al. [14] that if any of the clinical or radiological examinations revealed any changes during the one-year interval, growth was considered active; otherwise, the subject was considered inactive for growth. Thus, patients were classified into three populations based on growth status only or combined with relative condylar uptake ratios. Briefly, those with active condyles were defined as population A, while the subjects with inactive condyles were defined as population I. Patients in population A whose difference in relative condylar uptake ratio was greater than 23%, were defined as population S.
SPECT acquisition and interpretation
The acquisition method of SPECT was similar to previous description [8]. Briefly, 99mTc-labeled methylene diphosphonate (99mTc-MDP) 555 to 851MBq (14.8MBq/Kg) was injected intravenously four hours before imaging. SPECT was conducted with a SPECT scanner (GE Healthcare, USA) supplied with a low-energy high resolution collimator (LEHR). 140 keV and a 20% symmetrical window were set as the photopeak, and a circular orbit was used for obtaining emission data in the supine position with a matrix of 128 × 128. Each head circulated 180° with 30 stops, with each stop setting at 6°. Coronal, sagittal, and trans-axial tomograms were reconstructed by a Butterworth filter and ordered-subset expectation maximization iterative reconstruction. An ROI of 16 pixels\(\times\)3 slices was drawn over bilateral condylar heads. The individual condylar counts within the ROIs were calculated and the clivus bone was used as a control [14, 15], and calculation of the uptake ratio was performed by the following formula:
Condylar uptake =\(\frac{\text{c}\text{o}\text{n}\text{d}\text{y}\text{l}\text{e} \text{c}\text{o}\text{u}\text{n}\text{t}\text{s}}{\text{L}\text{e}\text{f}\text{t} \text{c}\text{o}\text{u}\text{n}\text{t}\text{s}+\text{r}\text{i}\text{g}\text{h}\text{t} \text{c}\text{o}\text{u}\text{n}\text{t}\text{s}}\times 100\)
Condylar uptake of at least 55% with a difference of at least 10% between both sides, was regarded as active unilateral condylar hyperplasia on the reference standard [8]. All ROI analyses and image interpretations were acquired by a Xeleris 3 workstation (GE Healthcare, USA).
Computed tomography scan reconstruction and 3D measurement
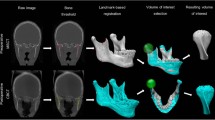
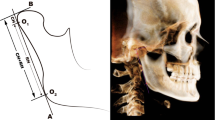
Parameters of the CT scan (GE Healthcare, USA) were 120 kV and 178 mAs. The slice thickness of the CT image was 1.25 mm, and CT scans were reconstructed and brought into 3D measurements with Proplan Software (Edition 1.4, Materialise, Leuven, Belgium) as we previously described [16]. Landmarks and measurement parameters are shown in Fig. 1; Table 1.
Statistical analysis
Data from 3D measurements were presented as mean ± SD and the bilateral difference between two CT scans (△CT2-CT1) was analyzed with ANOVA. The sensitivity and specificity of SPECT were calculated based on the 3D measurements of CT scans, and the receiver operating characteristic (ROC) curve was drawn, with the area under the ROC curve (AUC) calculated to reflect the diagnostic capability of SPECT. Pearson’s correlation analysis was performed on the 3D measurement parameters and difference in SPECT uptake ratios to investigate whether the mandibular growth was correlated with SPECT value. To ensure the reliability of the measurement data, the 3D measurement was repeated by the same measurer at an interval of two weeks and the average value of the two measurements was taken as the final value. Intraclass correlation coefficient (ICC) was used to assess intra-investigator reliability. IBM SPSS software (New York, USA) was used for statistical analysis. A level of significance of 5% was applied. P < 0.05 was considered to demonstrate statistically significant differences.
Results
Data of fifty-four patients (28 male and 26 female) diagnosed with UCH were analyzed retrospectively in this study, with an average age of 19.87 ± 2.76 years old. Demographic characteristics and basic diagnostic information are shown in Fig. 2. The left condyle was affected in 20 cases, while the right condyle was affected in 34 cases. The difference in relative condylar uptake ratios was recorded, ranging from 1 to 28%, with a median uptake ratio of 13.11 ± 7.66%. No significant difference was observed between male and female patients or between patients over 18 years old and under 18 years old (Tables 2 and 3).
The sensitivity of SPECT was 68.00%, and the specificity was 72.41% (Table 4). The ROC curve in Fig. 3 illustrated that SPECT could be used to determine whether the condyle is active or inactive based on the difference in relative uptake ratios, with 13% as the optimal cut-off value (Table 4). The AUC was 0.709, and the 95% confidence interval (95% CI) was 0.569 to 0.835. In the case where the difference in relative condylar uptake ratios was greater than 23%, the specificity was 100%, which indicated that all the patients were in an active condylar growth state, and this group of UCH patients was defined as population S (Table 5).
The average intra-operator ICC for 3D-measurement of the CT scan was 0.956. The results in Fig. 4 demonstrated that there was a significant increase in Co-Gn (△CT2-CT1) in population A and S when compared to population I (△=1.208 ± 0.159 mm, p<0.05; △=1.065 ± 0.353 mm, p<0.05), indicating unbalanced growth unilaterally in the diagonal direction of the mandible. Meanwhile, as for Co-Go (△CT2-CT1), which indicates growth in the ramus height of the mandible, the increase was also significantly greater in population A compared to population I (△=0.884 ± 0.229 mm, p<0.05), as well as in population S compared to population I (△=0.901 ± 0.320 mm, p<0.05). Furthermore, changes in parameters related to mandibular body length, including Go-Gn, Go-MF, and MF-Gn, were observed in population I, A, and S. However, no significant difference was detected. Notably, with all P values greater than 0.05, Pearson’s correlation analysis revealed no correlation between 3D measurement parameters and the difference in relative condylar uptake ratios, which indicated that SPECT and radiological 3D measurement may both be needed in the evaluation of the UCH activeness in clinical practices (Table 6).
Changes in 3D measurement of the mandible in population I, A, and S. Changes in CT scan of the follow-up visit (CT2) compared with initial CT scan (CT1) of Co-Gn, Co-Go, Go-Gn, Go-MF, and MF-Gn in population I, A and S. Data were calculated by comparing changes in bilateral difference (affected side vs. unaffected side). *p < 0.05, NS: no significant difference
Discussion
It’s critical to distinguish the mandibular condyle’s active and inactive stages in managing UCH. Various methods have been used to evaluate the growth of the mandible, including clinical examination, dental casts, X-rays, and cephalometric analysis [12, 17,18,19]. Nevertheless, factors like body posture and mannerism can conceal the growth imbalance, which might lead to incorrect treatment decisions [20]. Hence, alternative diagnostic procedures such as 99mTc-MDP bone scintigraphy and SPECT should be used in conjunction with clinical examination [21,22,23,24]. In recent decades, evaluation of condylar growth activeness of UCH with SPECT is increasingly recommended, with an optimal diagnostic threshold of 10% difference in relative uptake ratios [25]. Hodder et al. [17] used SPECT as an aid to diagnosis and confirmed that an uptake ratio of more than 10% was considered as active condylar hyperplasia, together with appropriate clinical and plain radiographic features. On the other hand, Kajan et al. [12] determined that the variation in growth activity in normal mandibular condyles was less than 6.2%. Similiarly, Fernandes et al. [1] concluded that the variation in condylar uptake was less than 5% in 37 out of 44 TMJ ‘normal’ patients, and in none was it 9% or more. However, the condylar uptake difference greater than 10% was not always acknowledged as the standard reference for predicting active condyle growth [12,13,14]. We assume the discrepancies in the diagnostic threshold of relative uptake ratios among studies might be due to different sample sizes, diverse assessment methods, and possible racial differences. Crucially, the difference in relative uptake ratios of UCH in the Chinese population has not been identified before. Based on the ROC curve drawn by SPECT assessment, this study determined that 13% is the optimal cut-off value for evaluating condyle activity in Chinese UCH patients for the first time. In ROC curve analysis, we discovered that SPECT had a sensitivity of 68.00% and a specificity of 72.41%, yielding an AUC of 0.709, indicating that SPECT is a valuable tool in measuring condylar growth activity. We also discovered that when the relative condylar uptake ratios differed by more than 23%, all the patients were defined as active, suggesting a ‘definite’ active condition.
Our results could explain why some studies concluded that SPECT was not a suitable diagnostic tool for evaluating condylar growth, as the criterion was set at 10% or the assessment was conducted with an improper method or based on a 2D imaging approach [14, 26]. Xiao et al. [26] adopted 3D measurement results as the gold standard, defining a difference of more than 5% in Co-Go, Go-Pg, Co-B, or condylar volume between two visits as active in assessing condylar growth status, and thus concluded that SPECT had poor sensitivity and specificity. However, this gold standard may have disproportionately increased the number of cases diagnosed as active UCH due to the large uncertainty and manual variance in the measurement of condylar volume. As Gateno et al. [27] observed that UCH could expand a hemimandible with or without considerable condylar enlargement and there was no correlation between condylar enlargement and overgrowth direction. The specificity of SPECT would inevitably diminish if the number of ‘active’ instances increased excessively.
UCH can occur at any age [28]. In our study, the mean age of the patients was 19.87 ± 2.76 years old, ranging from 16 to 29 years old, with the right condyle being more frequently affected (63%). Interestingly, Martin-Granizo et al. [29] reported the similar proportion. Meanwhile, some researchers revealed the same tendency regarding the side of laterognathia [30], while others suggested that difference in the affected side was not statistically significant [10, 31]. Female patients accounted for 26 (48.1%) and male patients accounted for 28 (51.9%) herein, which appears to counter earlier findings indicating UCH mostly affects women [27]. The discrepancy might be explained by the fact that patients in our research only represent a biased sample with active hyperplasia condyles and follow-up visits, also suggesting that male patients were more likely to require follow-up visits. Females were found to present with UCH at a younger age, and as a consequence, they ceased their growth activity earlier, whereas male patients were found to present with UCH later, and as a result, they ceased their growth activity later [14]. However, the distribution pattern of SPECT values among UCH patients has not been studied. Our study first investigated the differences in SPECT uptake ratios by age and sex in a Chinese population of UCH patients and observed no significant differences between male and female patients, or between patients over 18 years old and under 18 years old.
In 1986, Obwegeser first proposed that UCH is consistent with two independent conditions: hemimandibular hyperplasia and hemimandibular elongation. In hemimandibular hyperplasia, the volume of half the mandible increases, since the condyle expands, the neck widens and lengthens, and the ramus and body heighten. In hemimandibular elongation, one side of the mandible lengthens in a horizontal plane, shifting the chin to the unaffected side, as the condyle remains unchanged or slightly expands. The neck can lengthen, however, this is not always the case [3]. The following three assumptions guide the above classification: First, UCH overgrowth is bimodal, developing vertical or horizontal in some cases, and diagonally in others; Second, hyperplasia can be either global or linear, with global hyperplasia enlarging the condyle and the rest of the hemimandible, and linear hyperplasia lengthening one side of the jaw while maintaining the condyle’s previous size; Third, the direction of overgrowth is determined by the type of hyperplasia, as vertical growth is produced by global hyperplasia, whereas horizontal growth is produced by linear hyperplasia [32]. Herein, we found a significantly unbalanced growth unilaterally in the diagonal direction of the mandible and considerably greater growth in the ramus height of the mandible in population A compared to population I. Furthermore, no significant difference was detected in parameters related to mandibular body length in either group. Similar findings were found in the recent scientific literature on mandibular structure measurement of patients with UCH. Gateno et al. [27] investigated that the growth vectors in UCH are unimodally distributed, with the majority expanding diagonally. Lopez et al. [33] indicated that patients with UCH displayed larger condylar length and volume on the affected side. Evangelista et al. [34] concluded that the mandibular body length was more correlated to mandibular rotation than condylar differences, which might explain our study’s undifferentiated mandibular body length.
Furthermore, according to our study, the relative condylar uptake ratio was not directly related to mandibular growth. Contrary to CT scans and X-rays that require greater changes in bone density for the anatomical alteration to be evident, bone scans are sensitive examinations of the entire skeleton dependent on blood flow and absorption into hydroxyapatite crystals; as a result, SPECT could detect early-stage alterations without observable anatomical changes in radiographs or CT scans [35, 36]. This could be the explanation why there is no statistically significant association between positive SPECT results and changes in CT scans. Additionally, in cases of active condylar hyperplasia, alterations can occur in the condylar volume with anatomical modification of the joint cavity without necessarily projecting the entire modification in a sagittal or transverse direction at the mandibular level. As Lopez et al. [33] reported a positive correlation between the increased dimensions of the articular eminence and the more posterior position of the glenoid fossa in the affected side. According to their latest research which is contrary to our results, Lopez et al. [36] concluded that there is a significant correlation between the magnitude of mandibular deviation quantified on CT and metabolic findings obtained by SPECT in patients with UCH. Further studies on the correlation between SPECT value and mandibular structure should be carried out in the subsequent research.
Finally, the following are the main limitations of this study: (1) We acknowledge that the study’s principal limitation was the retrospective nature of the research, which might lead to biases. (2) The study was conducted at a single center with a limited sample of the population. As a result, extending the findings from this study to a different ethnic group may not be entirely accurate. For further evaluation, multicenter and larger-sample studies should be conducted. (3) There was still an average inaccuracy in the repeated 3D measurements. (4) With larger samples, studies of growth prediction in UCH patients using cluster and discriminant function analysis should be further performed.
Conclusion
In conclusion, the sensitivity and specificity of SPECT for identifying active condylar growth were 68.00% and 72.41%, respectively, demonstrating good diagnostic performance in UCH with the cut-off value of 13%. For Chinese UCH patients with an active growing condyle, the mandible of the vast majority grows diagonally and vertically, while the relative condylar uptake ratio was not directly related to mandibular growth.
Data Availability
Datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fernandes AR, Faria MT, Oliveira A, Barata Coelho P, Pereira JG. Assessment of relative uptake by mandibular condyles in a “normal” population. Br J Oral Maxillofac Surg. 2019;57(3):251–54.
Leung MY, Leung YY. Three-dimensional evaluation of mandibular asymmetry: a new classification and three-dimensional cephalometric analysis. Int J Oral Maxillofac Surg. 2018;47(8):1043–51.
Obwegeser HL, Makek MS. Hemimandibular hyperplasia — hemimandibular elongation. J Maxillofac Surg. 1986;14:183–208.
Wolford LM, Movahed R, Perez DE. A classification system for conditions causing condylar hyperplasia. J Oral Maxillofac Surg. 2014;72(3):567–95.
Arora KS, Bansal R, Mohapatra S, Pareek S. Review and classification update: unilateral condylar hyperplasia. BMJ Case Rep. 2019;12(2)
Anzola FL, Moron AS, Jaramillo CM, Quijano GD, Hernandez HN, et al. Quantitative bone SPECT analysis of mandibular condyles in an asymptomatic population: an approach to normal reference values. Int J Oral Maxillofac Surg. 2021;50(6):733–39.
Karssemakers LHE, Raijmakers PG, Nolte JW, Tuinzing DB, Becking AG. Interobserver variation of single-photon emission computed tomography bone scans in patients evaluated for unilateral condylar hyperactivity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(3):399–405.
Liu P, Shi J. Is Single-Photon Emission computed Tomography/Computed Tomography Superior to Single-Photon Emission Computed Tomography in assessing unilateral Condylar Hyperplasia? J Oral Maxillofac Surg. 2019;77(6):1279. e1-79 e7.
Kao YH, Magsombol BM, Ng DC. The potential of hybrid SPECT/CT fusion imaging to improve diagnostic accuracy in the scintigraphic quantitative functional assessment of suspected unilateral mandibular hyperactivity. Oral Maxillofac Surg. 2012;16(1):89–93.
Lopez BD, Corral SC. Comparison of planar bone scintigraphy and single photon emission computed tomography for diagnosis of active condylar hyperplasia. J Craniomaxillofac Surg. 2016;44(1):70–4.
Rushinek H, Tabib R, Fleissig Y, Klein M, Tshori S. Evaluation of three analysis methods for (99m)tc MDP SPECT scintigraphy in the diagnosis of unilateral condylar hyperplasia. Int J Oral Maxillofac Surg. 2016;45(12):1607–13.
Kajan ZD, Motevasseli S, Nasab NK, Ghanepour H, Abbaspur F. Assessment of growth activity in the mandibular condyles by single-photon emission computed tomography (SPECT). Aust Orthod J. 2006;22(2):127–30.
Saridin CP, Raijmakers PGHM, Tuinzing DB, Becking AG. Comparison of planar bone scintigraphy and single photon emission computed tomography in patients suspected of having unilateral condylar hyperactivity. Oral surgery, oral medicine, oral Pathology. Oral Radiol Endodontology. 2008;106(3):426–32.
Chan BH, Leung YY. SPECT bone scintigraphy for the assessment of condylar growth activity in mandibular asymmetry: is it accurate? Int J Oral Maxillofac Surg. 2018;47(4):470–79.
Fahey FH, Abramson ZR, Padwa BL, Zimmerman RE, Zurakowski D, et al. Use of (99m)Tc-MDP SPECT for assessment of mandibular growth: development of normal values. Eur J Nucl Med Mol Imaging. 2010;37(5):1002–10.
Zhao H, Li X, Jiang T, Zhang J, Yu H. Computer-aided autogenous coronoid process graft combined with median and unilateral Sagittal Split Osteotomy for Late Reconstruction of Condylar fracture and occlusion after Trauma. J Craniofac Surg. 2022.
Hodder SC, Rees JI, Oliver TB, Facey PE, Sugar AW. SPECT bone scintigraphy in the diagnosis and management of mandibular condylar hyperplasia. Br J Oral Maxillofac Surg. 2000;38(2):87–93.
Pogrel MA. Quantitative assessment of isotope activity in the temporomandibular joint regions as a means of assessing unilateral condylar hypertrophy. Oral surgery, oral medicine, and oral pathology. 1985;60(1):15–7.
Bohuslavizki KH, Brenner W, Kerscher A, Fleiner B, Tinnemeyer S, et al. The value of bone scanning in pre-operative decision-making in patients with progressive facial asymmetry. Nucl Med Commun. 1996;17(7):562–7.
Sandor GK, McGuire TP, Ylikontiola LP, Serlo WS, Pirttiniemi PM. Management of facial asymmetry. Oral Maxillofac Surg Clin North Am. 2007;19(3):395–422. vi.
Alexander JM. Radionuclide bone scanning in the diagnosis of lesions of the maxillofacial region. J Oral Surg. 1976;34(3):249–56.
Reuland P, Werz R. Investigations on skeletal growth zones via bone scans as base of determination of optimal time for surgery in mandibular asymmetry. Nuklearmedizin. 2000;39(5):121–6.
Saridin CP, Raijmakers P, Becking AG. Quantitative analysis of planar bone scintigraphy in patients with unilateral condylar hyperplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(2):259–63.
Fahey FH, Abramson ZR, Padwa BL, Zimmerman RE, Zurakowski D, et al. Use of (99m)Tc-MDP SPECT for assessment of mandibular growth: development of normal values. Eur J Nucl Med Mol Imaging. 2010;37(5):1002–10.
Surendran KSB, Cobb MA, Keith DJW, Banks RJ, Gannon M. Choosing the best method to utilise single positron emission computed tomography (SPECT) scans in the management of unilateral condylar hyperplasia. Br J Oral Maxillofac Surg. 2020;58(9):1193–96.
Xiao J, Wu Z, Ye W. Using 3D Medical Modeling to Evaluate the Accuracy of Single-Photon Emission Computed Tomography (SPECT) Bone Scintigraphy in Diagnosing Condylar Hyperplasia. J Oral Maxillofac Surg. 2022;80(2):285.e1-85.e9.
Gateno J, Coppelson KB, Kuang T, Poliak CD, Xia JJ. A better understanding of unilateral condylar hyperplasia of the Mandible. J Oral Maxillofac Surg. 2021;79(5):1122–32.
Liu P, Shi J. Growth trends analysis of unilateral condylar hyperplasia followed up with planar scintigraphy: retrospective overview of 249 cases. Medicine. 2021;100(51):e28226.
Martin-Granizo R, Garcia-Rielo JM, De la Sen O, Maniegas L, Berguer A, et al. Correlation between single photon emission computed tomography and histopathologic findings in condylar hyperplasia of the temporomandibular joint. J Craniomaxillofac Surg. 2017;45(6):839–44.
Karssemakers LHE, Nolte JW, Rehmann C, Raijmakers PG, Becking AG. Diagnostic performance of SPECT-CT imaging in unilateral condylar hyperplasia. Int J Oral Maxillofac Surg. 2022.
Nitzan DW, Katsnelson A, Bermanis I, Brin I, Casap N. The clinical characteristics of condylar hyperplasia: experience with 61 patients. J Oral Maxillofac Surg. 2008;66(2):312–8.
Obwegeser HL, Obwegeser JA. New clinical-based evidence for the existence of 2 growth regulators in mandibular condyles: hemimandibular elongation in hemifacial microsomia mandible. J Craniofac Surg. 2010;21(5):1595–600.
López DF, Botero JR, Muñoz JM, Cardenas-Perilla RA. Mandibular and temporomandibular morphologic characteristics of patients with suspected unilateral condylar hyperplasia: a CT study. Dent Press J Orthod. 2020;25(2):61–8.
Evangelista K, Valladares-Neto J, Garcia Silva MA, Soares Cevidanes LH, de Oliveira Ruellas AC. Three-dimensional assessment of mandibular asymmetry in skeletal class I and unilateral crossbite malocclusion in 3 different age groups. Am J Orthod Dentofac Orthop. 2020;158(2):209–20.
Koppula BR, Morton KA, Al-Dulaimi R, Fine GC, Damme NM, et al. SPECT/CT in the evaluation of suspected skeletal Pathology. Tomography. 2021;7(4):581–605.
López DF, Ríos Borrás V, Muñoz JM, Cardenas-Perilla R, Almeida LE. SPECT/CT correlation in the diagnosis of unilateral Condilar Hyperplasia. Diagnostics (Basel). 2021;11(3)
Acknowledgements
No Acknowledgements.
Funding
This work was supported by the National Natural Science Foundation of China (82101047, 81571022), the Research Fund of Medicine and Engineering of Shanghai Jiao Tong University (YG2017MS06), Multi-center Clinical Research Project of Shanghai Jiao Tong University, School of Medicine (DLY201808), and Clinical Research Program of Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (JYLJ023).
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. All authors contributed to the study conception and design. Ningjuan Ouyang and Chenlong Zhang: methodology, analysis, and interpretation of data and writing original draft preparation Tiantian Chen: data curation Feng Xu: investigation Guofang Shen: supervision Jiawen Si and Hongbo Yu: conceptualization, writing, reviewing, and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All the participants’ or guardians’ (for participants who were under 18 but above 16) informed consents were obtained. The study was designed under the Declaration of Helsinki, approved by the Institutional Ethical Review Board of Shanghai Ninth People’s Hospital (SHDC12013103), and all methods were carried out in accordance with relevant guidelines and regulations. No subjects were under 16/illiterate/dead.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ouyang, N., Zhang, C., Xu, F. et al. Evaluation of optimal single-photon emission computed tomography reference value and three-dimensional mandibular growth pattern in 54 Chinese unilateral condylar hyperplasia patients. Head Face Med 19, 18 (2023). https://doi.org/10.1186/s13005-023-00365-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13005-023-00365-2