Abstract
Background
Clear cell adenocarcinoma of the lower urinary tract (CCACLUT) is a rare primary malignant neoplasm with heterogenous morphology. There is a paucity of data in the literature regarding its immunohistochemical profile.
Methods
The immunohistochemical features (extent and intensity) of a multinational cohort of CCACLUT were evaluated with comparison between clear cell adenocarcinoma of the female genital tract (CCACFGT, tissue microarray) and nephrogenic adenoma (NA).
Results
33 CCACLUT (24 female, 9 male; mean age 59 years) were collected. CCACLUT most commonly arose from the urinary bladder (26/33, 78%), particularly from the trigone (10/33, 30.3%) followed by the urethra (8/33, 22%). All 12 NA cases were located at the urinary bladder, whereas the most common CCACFGT location was the ovary (29/56, 52%). None of the CCACLUT patients had, intestinal metaplasia, NA, or urothelial carcinoma. One patient had concurrent endometriosis of the sigmoid colon. Most frequently observed morphology in CCACLUT was papillary/tubulocystic (9/3; 27.3%), followed by papillary/tubular (6/33; 18.2%) and papillary/solid (5/33; 15.2%). GATA3 expression was significantly higher in CCACLUT (18/33, 54.5%) and NA (6/12, 50%), when compared to CCACFGT cases 6/56, 11.7%)(p = 0.001 and p = 0.022, respectively). The extent of GATA3 was significantly higher in CCACLUT group (19.2 ± 16.6%) than the other groups (9.6 ± 22.5% in NA and 2.6 ± 9% in CCACFGT group) (p = 0.001). 4/33 patients (12.1) had weak, 10/33 patients (30.3%) had moderate, and 4/33 patients (12.1%) had strong GATA3 intensity in CCACLUT group. In NA group, one patient (8.3%, 1/12) had weak, one patient (8.3%, 1/12) had moderate and 4 patients (33.3%, 4/12) had strong GATA3 intensity. Most cases (CCACLUT 29/33, 88%; NA 11/12, 92%; CCACFGT 46/56, 82.1%) had positive Napsin A expression, by which CCACLUT had significantly more cases with Napsin A expression (p = 0.034). p63 was consistently negative in all cases (30/33 (91.9%) CCACLUT; 12/12 (100%) NA; 42/56 (75%) CCACFGT. Ki67 (MIB) proliferation index was significantly higher in CCACLUT group (54.6 ± 21%) when compared to NA group (4.5 ± 2.7%) and CCACFGT group (35.5 ± 25.8%) (p = 0.001).
Conclusion
CCACLUT has consistent GATA3 expression, which may cause challenge in the diagnosis of urothelial carcinoma but can be used to distinguish CCACLUT from CCACFGT.
Highlights
Clear cell adenocarcinoma of the lower urinary tract is rare, with heterogenous morphology.
GATA3 expression can be seen in these tumors, causing diagnostic challenge in the urinary tract or in the metastatic setting.
Along with morphologic clues, immunohistochemical panel of PAX8, GATA3, and p63 might be useful to distinguish these tumors from urothelial carcinoma.
Similar content being viewed by others
Introduction
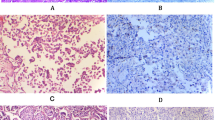
Clear cell adenocarcinoma (CCAC) is a malignant neoplasm arising predominantly in the female genital tract (FGT), particularly from the ovary [1] as well as a variant of endometrial carcinoma [2]. CCAC can rarely be seen primarily in the lower urinary tract (LUT)[3], and most commonly occurs in the urethra (particularly in diverticula) and trigone or posterior wall of the urinary bladder [4], with significant female predominance (female-to-male ratio = 3:1) [3, 4], although occurrence in male LUT is well documented [5]. CCAC may demonstrate tubulocystic (Fig. 1 A), papillary (Fig. 1B), or diffuse growth patterns (Fig. 1 C) [3]. Occasionally nephrogenic adenoma-like (NA-like) morphology (Fig. 1D) may be present[3]. These growth patterns are often observed in the same tumor with varying proportions (Fig. 1E), and high-grade cytologic atypia is almost always present (with descriptive cellular “hobnailing”; Fig. 1 F). Concurrent endometriosis and/or urothelial carcinoma (UCa) in a subset of cases are reported [6]. This morphologic heterogeneity along with additional neoplastic and non-neoplastic findings cause challenge in the differential diagnosis, mimicking UCa, particularly of glycogen rich (“clear cell”) subtype [7] and/or NA. The current view of the World Health Organization (WHO) positions these tumors under “tumors of the Müllerian type” [3], due to its morphologic features resembling its FGT counterpart as well as the persistent expression of PAX8; accompanied by keratin 7, napsin A, and HNF1B, further supporting the Müllerian differentiation. However, there is conflicting evidence whether these tumors originate from non-urothelial cell type such as some embryologic Mullerian remnants or derivation from urothelial carcinoma [8].
Clear cell adenocarcinoma of the lower urinary tract demonstrates various morphologic patterns including tubulocystic (A; 100X), papillary (B; 100X), diffuse (C; 100X), or nephrogenic adenoma-like morphology (D; 100X); multiple patterns are often present (E; 40X). High-grade cytologic atypia is almost always observed, with characteristic “hobnailing” of the tumor cells (F; 200X)
GATA3 is one of the six members of the GATA gene family of transcription factors and is first identified in the hematopoietic system, particularly in T-helper type 2 cells [9]. GATA3 takes part in the development of various tissue types such as T-cells, skin, and breast parenchyma [10]. GATA3 expression is vastly utilized in the breast and the urothelial carcinomas due to its high specificity; and many other benign and malignant lesions arising from skin, kidney, uterus, testis, ovary, and pancreas [11]. UCa shares its GATA3 expression with NA [12] and paraganglioma arising from the urinary tract, although GATA3 expression in CCAC of the urinary tract has not been evaluated.
Methods
Case acquisition
After approval of the local institutional review boards, multi-insitutional cohort of CCAC of the LUT cases (CCACLUT) were collected. Patients’ gender, age at the diagnosis, procedure (transurethral resection (TUR) and/or cystectomy/cystoprostatectomy), tumor site, and gross tumor size (cm) were recorded. Hematoxylin and eosin (HE) stained slides of the tumor sections were reviewed by pathologists with genitourinary pathology expertise (M.A., S.M., A.O., L.C., G.T.M, A.S., G.Q.X., A.B.) and the diagnoses were confirmed. Predominant morphologic features were noted. Similarly, NA and CCAC of the FGT (CCCFGT) cases were collected from the archives of department of pathology and laboratory medicine at University of Iowa were gathered. For CCACFGT cases, tissue microarrays (TMAs) were constructed with Manual Tissue Arrayer MTA-1 (Beecher Instruments Inc.) utilizing 1 mm punches (Estigen OÜ), with each tumor sampled in triplicate. Immunohistochemical (IHC) assays with appropriate controls including GATA3, p63, keratin 7, napsin A, and MIB1 (Ki-67) were selected for evaluation. Intensity of the IHC was quantified in the spectrum of 0–3 (0 = no expression; 1 = weak; 2 = moderate; 3 = strong), whereas tumor extent was quantified as percentage of tumor cells expressing the biomarker. The H-score was determined by adding the results of multiplication of the percentage of cells with staining intensity ordinal value (scored from 0 for “no signal” to 3 for “strong signal”) with 300 possible values.
Statistical analysis
Data analysis was performed using SPSS (Statistical Package for Social Sciences; SPSS Inc., Chicago, IL) version 25.0. Descriptive data were shown as numbers (n) and percentage (%) in categorical data and mean ± standard deviation (mean ± SD) in continuous data. Pearson Chi-square test was used to compare categorical variables between groups. Conformity of continuous variables to normal distribution was evaluated with Kolmogorov-Smirnov test. Mann Whitney U-test was used to compare normally distributed variables in two groups. One Way ANOVA test was used for parametric variables and Kruskal Wallis test was used for nonparametric variables when comparing more than two groups. Bonferroni correction was used for pair-group comparison in post-hoc analyses. Spearman correlation analysis was used to compare two continuous variables. p < 0.05 was accepted as statistically significant.
Results
Cohort characteristics
A total of 101 patients were included in this study, including 33 patients with CCAC, 12 with NA, and 56 with CCACFGT. In the CCACLUT group, 24 (73%) patients were female and 9 (27%) were male. 4 of 12 patients (%33) in NA group were female. The mean age of the patients in CCACLUT group was 59 years, while it was 64 years in NA group, and 64 years in CCACFGT. In 4/33 (12.1%) patients with CCACLUT, tumor exclusively arose from the urethra, whereas 26/33 (78.7%) patients tumor arose from the urinary bladder. In 3/33 (9%) patients, both urethra and urinary bladder were involved. Tumor size was available from 27 patients (range 1.7–9 cm) in the CCACLUT group and 50 patients (1.2–27.4 cm) in the CCACFGT group. CCACLUT most commonly arose from trigone (10/33, 30.3%). CCACFGT mainly arose from ovary (29/56; 52%), followed by endometrium (23/56; 41) and cervix (4/56; 7%). Most frequently observed morphology in CCACLUT was papillary/tubulocystic (9/33; 27.3%), followed by papillary/tubular (6/33; 18.2%) and papillary/solid (5/33; 15.2%). No cases had history of the endometriosis in the CCACLUT cases, one case had concurrent endometriosis identified in the sigmoid colon. None of the CCACLUT cases had intestinal metaplasia, NA, or UCa in the urinary tract. Table 1 includes cohort characteristics.
GATA3 expression
More than half of the CCACLUT (18/33, 54.5%) and half of the NA cases (6/12, 50%) had GATA3 expression. Four of 33 (12.1%) CCACLUT patients had strong GATA3 expression (Fig. 2A-2B). About one-third (10/33, 30.3%,) of the patients in CCACLUT group had moderate GATA3 expression (Fig. 2C-D), while four patients had weak (4/33, 12.1%) expression (Fig. 2E-F). In contrast, most of the CCACFGT cases were negative (50/56, 89.3%) for GATA3 (Fig. 3A-3B). There was significant difference between CCACFGT and NA groups (p = 0.022), and between CCACLUT and CCACFGT groups (p = 0.001) in terms of number of cases with GATA3 expression, although there was no statistically significant difference between NA and CCACLUT groups (p = 0.254) (Table 2). The extent of GATA3 was significantly higher in CCACLUT group (19.2 ± 16.6%) than the other groups (9.6 ± 22.5% in NA and 2.6 ± 9% in CCACFGT group) (p = 0.001). Finally, in CCAFGT group, 1 patient (1.7%, 1/56) had weak, 2/56 patients (3.4%) had moderate, and 3/56 patients (5.1%) had strong GATA3 intensity. In terms of intensity, the difference was statistically significant between CCACFGT and NA groups (p = 0.022), and between CCACLUT and CCACFGT groups (p = 0.001) while the difference was not significant between NA and CCACLUT groups (p = 0.254).
PAX8 expression is strong and diffuse in clear cell adenocarcinoma of lower urinary tract (CCACLUT; 4 A-4B; 200X), nephrogenic adenoma (4E-4 F; 200X), and clear cell adenocarcinoma of the female genital tract (CCACFGT; ovarian primary; 4I-4 J; 200X). Somewhat patchy but persistent Napsin A was present in CCACLUT (4 C; 200X), NA (4G; 200X), and CCACFGT (4 K; 200X). p63 was all negative in CCACLUT (4D; base of the urothelium as control; 200X) and NA (4 H; 200X); as well as all but one CCACFGT cases (4 L; 200X).
The mean H score of GATA3 was statistically significantly higher in the CCACLUT group (28.9 + 36.1) than in the CCACFGT group (6.9 + 26.5) but was similar with NA group (27.1 + 67.8) (p: 0.001).
PAX8 expression
All CCACLUT and NA cases diffusely express PAX8 with predominantly strong (26/28; 92.9%; Fig. 4A-4B) and exclusively strong intensity (12/12; 100%; ), respectively. Except for one case (55/56; 98%), all CCACFGT cases had diffuse and strong PAX8 expression.
Napsin A expression
Most cases in all groups (CCACLUT 29/33, 87.9%; NA 11/12, 91.7%; CCACFGT 46/56, 82.1%) had positive Napsin A expression. Napsin A extent was 42.2 ± 30.7% in CCACLUT group; 36.6 ± 30% in NA group; and 25.8 ± 30.2 in CCACFGT group. Napsin A extent was significantly higher at CCACLUT group than the other groups (p = 0.034). Strong Napsin A expression was seen in 15/33 (45.5%), 11/12 (91.7%), and 38/56 (67.9%) in CCACLUT, NA, and CCACFGT, respectively, which reached statistical significance between CCACLUT and NA (p:0,041) and between CCACLUT and CCACFGT groups (p = 0.007) (Table 2).
P63 expression
Only 3/33 (9.1%) CCACLUT cases were positive for p63 with moderate intensity, and none of the NA cases expressed p63 (0/12). 14/56 (25%) of CCACFGT cases were positive for p63 with mostly weak intensity (8/56; 14.2%). There was no significant difference between CCACLUT, NA and CCACFGT groups in terms of p63 intensity (p: 0.101).
MIB1 (Ki67) expression
MIB extent, also known as proliferation index, was highest at CCACLUT group (54.6 ± 21%) which significantly higher than NA group (4.5 ± 2.7%) and CCACFGT group (35.5 ± 25.8%) (p = 0.001).
Other biomarkers
Keratin 7, AMACR, and WT1 biomarkers were only examined in CCACLUT group. Keratin 7 expression was present in almost all available (28/29, 97%) CCACLUT cases, with 22/25 cases having more than 50% positive cells (88%) and strong expression in 20/29 cases (69%). Similarly, AMACR was positive in most of the available CCACLUT cases (25/27, 93%). WT1 was available in 22/33 CCACLUT cases and all were negative.
Discussion
Few CCACLUT cases have been documented in the literature with scarce data on the immunoprofile. Young and Scully reported 3 CCAC of the urinary bladder with review of 16 CCACLUT in the pre-IHC era, followed by Oliva and Young’s [13] report on exclusively urethral CCAC of 19 mostly female (n = 18) patients, establishing morphologic features and drawing close association to the urethral diverticulum and lack of endometriosis. Early reports on CCACLUT IHC highlighted the striking overlap between top differential entities including UCa, NA, and CCACFGT; all expressing non-specific pancytokeratin, CAM5.2, keratin 7, and EMA markers [4]. Although there has been lack of dedicated large CCACLUT studies on practical markers; case reports and small series showed that CCACLUT express AMACR [8], PAX8 [14], and napsin A [12]. Our study not only contains the largest multi-institutional CCACLUT cohort but also claims to be the first report evaluating GATA3 IHC expression in these tumors. GATA3 expression was present in more than half of the CCACLUT cases with significantly higher extent and intensity when compared to CCACFGT and NA, which proves the presence of the concerning overlap between CCACLUT and UCa. Morphologic heterogeneity coupled with persistent GATA3, and keratin 7 expression may cause the misdiagnosis of CCACLUT as UCa. Our study also found persistent lack of p63 expression in CCACLUT, which may be helpful in differentiating from UCa, as p63 is strongly positive in most of the UCa [15].
The controversy on the cellular origin of CCACLUT remains despite several reports dedicated solely to address the issue [8, 16]. Oliva et al [6] reported four CCACLUT with concurrent UCa, with additional five cases demonstrating “pseudostratified epithelium reminiscent of transitional epithelium”, presenting as supporting evidence of the urothelial origin of CCACLUT. However, there were also four CCACLUT in the same cohort with concurrent Müllerian-type tissue. Sung et al [8] found chromosome number alterations (gains on chromosome 3, 7, and 17 in all 12 CCACLUT cases; 9p21 loss in 3/12 cases) using UroVysion fluorescence in situ hybridization (FISH) assay, similar to UCa. In contrast, Ortiz-Bruchle found ARID1A gene mutations in 5/11 CCACLUT cases with no TERT gene promoter alterations [17]. Similarly, our recent report on comprehensive next generation sequencing (NGS) in one of the cases in the current cohort [18] identified ARID1A, PBRM1, ERBB4, and SMARCA4 mutations. Another hypothesis is the malignant transformation of NA to CCACLUT, suggested in two separate case reports [19, 20] [1]. The lack of concurrent NA, endometriosis in the urinary bladder, or UCa, and consistent expression of biomarkers associated with the Mullerian origin in our cohort further support that CCAC of the urinary tract does not arise from the urothelium, which is in line with the current WHO designation of the CCAC of the urinary tract as non-urothelial (under tumors of the Mullerian origin).
There are several limitations in our study. Multiple labs and different clones were involved in the IHC evaluation of this multinational cohort, causing comparison of potentially different assay performances. We did not include a group of UCa in this study, although morphologic and biomarker characteristics of UCa and its subtypes are well established [15]. CCACFGT cohort was included in the form of TMA that prevented evaluation of more tissue. Finally, we did not include the outcome data of these patients in our study, therefore, we have no data with regards to GATA3 expression and its impact on the survival in CCACLUT or CCACFGT.
In conclusion, CCACLUT has consistent GATA3 expression is a subset of cases, which may cause misinterpretation as UCa, but can be used to distinguish CCACFGT or NA. A limited panel of IHC including PAX8, GATA3, and p63 in suspected cases may prevent potential misdiagnosis. Studies with comprehensive molecular interrogation is needed to further characterize these tumors.
Availability of data and materials
Study data is available upon request. Please contact the corresponding author.
References
Peres LC, Cushing-Haugen KL, Anglesio M, Wicklund K, Bentley R, Berchuck A, et al. Histotype classification of ovarian carcinoma: A comparison of approaches. Gynecol Oncol. 2018;151(1):53–60.
DeLair DF, Burke KA, Selenica P, Lim RS, Scott SN, Middha S, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol. 2017;243(2):230–41.
Netto GJ, Amin MB, Berney DM, Compérat EM, Gill AJ, Hartmann A, et al. The 2022 World Health Organization Classification of Tumors of the Urinary System and Male Genital Organs-Part B: Prostate and Urinary Tract Tumors. Eur Urol. 2022.
Adeniran AJ, Tamboli P. Clear cell adenocarcinoma of the urinary bladder: a short review. Arch Pathol Lab Med. 2009;133(6):987–91.
Grosser D, Matoso A, Epstein JI. Clear Cell Adenocarcinoma in Men: A Series of 15 Cases. Am J Surg Pathol. 2021;45(2):270–6.
Oliva E, Amin MB, Jimenez R, Young RH. Clear cell carcinoma of the urinary bladder: a report and comparison of four tumors of mullerian origin and nine of probable urothelial origin with discussion of histogenesis and diagnostic problems. Am J Surg Pathol. 2002;26(2):190–7.
Zhou Z, Kinslow CJ, Wang P, Huang B, Cheng SK, Deutsch I, et al. Clear Cell Adenocarcinoma of the Urinary Bladder Is a Glycogen-Rich Tumor with Poorer Prognosis. J Clin Med. 2020;9(1).
Sung MT, Zhang S, MacLennan GT, Lopez-Beltran A, Montironi R, Wang M, et al. Histogenesis of clear cell adenocarcinoma in the urinary tract: evidence of urothelial origin. Clin Cancer Res. 2008;14(7):1947–55.
Ho IC, Pai SY. GATA-3 - not just for Th2 cells anymore. Cell Mol Immunol. 2007;4(1):15–29.
Chou J, Provot S, Werb Z. GATA3 in development and cancer differentiation: cells GATA have it! J Cell Physiol. 2010;222(1):42–9.
Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, et al. GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol. 2014;38(1):13–22.
Sharifai N, Abro B, Chen JF, Zhao M, He H, Cao D. Napsin A is a highly sensitive marker for nephrogenic adenoma: an immunohistochemical study with a specificity test in genitourinary tumors. Hum Pathol. 2020;102:23–32.
Oliva E, Young RH. Clear cell adenocarcinoma of the urethra: a clinicopathologic analysis of 19 cases. Mod Pathol. 1996;9(5):513–20.
Tong GX, Weeden EM, Hamele-Bena D, Huan Y, Unger P, Memeo L, et al. Expression of PAX8 in nephrogenic adenoma and clear cell adenocarcinoma of the lower urinary tract: evidence of related histogenesis? Am J Surg Pathol. 2008;32(9):1380–7.
Akgul M, MacLennan GT, Cheng L. The applicability and utility of immunohistochemical biomarkers in bladder pathology. Hum Pathol. 2020;98:32–55.
Ortiz-Brüchle N, Wucherpfennig S, Rose M, Garczyk S, Bertz S, Hartmann A, et al. Molecular Characterization of Muellerian Tumors of the Urinary Tract. Genes (Basel). 2021;12(6).
Günes C, Wezel F, Southgate J, Bolenz C. Implications of TERT promoter mutations and telomerase activity in urothelial carcinogenesis. Nat Rev Urol. 2018;15(6):386–93.
Abdelwahab H, Friedman D, Lightle A, Mian B, Fisher H, Akgul M. Distinct mutational status in GATA3-Positive clear cell adenocarcinoma of the urinary tract: A CASE report. Urol Case Rep. 2021;39:101793.
Hartmann A, Junker K, Dietmaier W, Schröder S, Lopez D, Hofstädter F, et al. Molecular evidence for progression of nephrogenic metaplasia of the urinary bladder to clear cell adenocarcinoma. Hum Pathol. 2006;37(1):117–20.
Suttmann H, Holl-Ulrich K, Peter M, Kausch I, Doehn C, Jocham D. Mesonephroid adenocarcinoma arising from mesonephroid metaplasia of the urinary bladder. Urology. 2006;67(4):846.e7-8.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MA, RH, AO, AS, and AOO were responsible for study design, manuscript writing, and data acquisition. SY and ENK performed statistical analyses. The remainder of the authors provided cases with clinicopathologic data. All authors read and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Ethical committees, Internal Review Boards and guidelines of each authors’ institutions were followed accordingly.
Competing interests
N/A.
Declarations of interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akgul, M., Humble, R., Osme, A. et al. GATA3 expression in clear cell adenocarcinoma of the lower urinary tract: a potential diagnostic pitfall. Diagn Pathol 17, 87 (2022). https://doi.org/10.1186/s13000-022-01269-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-022-01269-6