Abstract
Background:
Orthokeratinized odontogenic cyst (OOC) is a rare developmental odontogenic cyst of the jaw. It was originally believed to be a variant of odontogenic keratocyst (OKC) but is now considered to be a distinct entity. OOC usually presents as a single lesion and recurs infrequently. On the other hand, OKC often presents with multiple lesions and displays locally aggressive behavior and a high recurrence rate associated with the protein patched homolog 1 (PTCH1) gene mutation. Multiple OOC cases are extremely rare and seem to be aggressive, but their pathogenesis is not fully understood. This study aimed to determine the clinical, pathological, and genetic characteristics of multiple OCC.
Methods:
Three cases of multiple OOC were evaluated for clinical and histological findings, and immunohistochemical expression of Ki-67 and Bcl-2. Furthermore, PTCH1 mutations were analyzed by next-generation sequencing using a custom panel to cover the entire exon of PTCH1.
Results:
The three cases of multiple OOC included two men and one woman with a mean age of 25.3 years old (range, 18–38 years old). Each case had two or three OOCs (total of seven OOCs), all of which were simultaneously detected. Of the seven OOCs that manifested as multiple jaw cysts, seven (100%) occurred in the posterior regions, four (57.1%) occurred in the mandible, and four (57.1%) were associated with an impacted tooth. Histological examination revealed cysts lined by orthokeratinized stratified squamous epithelium. Immunohistochemistry showed a low Ki-67 labeling index and no Bcl-2 expression in the seven OOCs. No pathogenic PTCH1 mutations were detected in any of the seven OOCs. None of the patients had any other symptoms or signs of recurrence at the last follow-up (6–60 months).
Conclusion:
Multiple OOCs appeared to occur more often in younger patients than solitary OOC. Both multiple and solitary OOCs may be related diseases within the entity of odontogenic cysts. Multiple OOCs are clinicopathologically and genetically distinct from OKC.
Similar content being viewed by others
Introduction
Orthokeratinized odontogenic cyst (OOC) is a developmental odontogenic cyst characterized by a lining of orthokeratinized stratified squamous epithelium [1]. In 1981, they were first described by Wright et al. [2], and were originally thought to be in the spectrum of odontogenic keratocysts (OKC). However, several studies have discussed the clinical and pathological differences between OOC and OKC [2,3,4,5,6,7,8,9]. The new edition of the World Health Organization classification of head and neck tumors published in 2022 has described OOC as a distinct entity from OKC [1, 10].
Histologically, OOC shows predominantly orthokeratinization with a granular cell layer, whereas OKC displays parakeratinization with corrugated surface keratin layers and palisaded basal cells [1]. Clinically, OOC recurs less frequently, in comparison to OKC, which has recurrence rate as high as 2.5–62% [1, 9, 11]. In addition, OKC is genetically associated with mutations in the protein patched homolog 1 (PTCH1) gene, which activates the Sonic hedgehog (SHH) signaling pathway and results in aberrant cell proliferation of the OKC epithelium [1, 10, 11]. The PTCH1 mutation was detected in up to 93% of sporadic OKCs [10]. In addition, 5% of all OKCs occur as part of nevoid basal cell carcinoma syndrome (NBCCS) caused by PTCH1 mutation [1, 11]. However, pathogenic PTCH1 mutations have not been detected in OOC [9, 12].
OOC usually presents as a single lesion, whereas about 10% of OKC present with multiple lesions [1, 9, 11]. Multiple OOC are extremely rare, and only 18 cases of multiple OOC have been reported in the literature to date [9, 13,14,15,16,17,18,19]. However, little is known about the differences in clinicopathological and genetic characteristics of multiple OOC between solitary OOC and OKC. Here, we report the results of the clinical, histological, immunohistochemical, and genetic analyses of a small series of multiple OOC cases.
Methods
Histological and immunohistochemical examination
Three cases of multiple OOC with formalin-fixed paraffin-embedded (FFPE) tissues were retrieved from the pathology files. The final diagnosis of OOC was made by two pathologists (SO and KH). This study was approved by the ethical review board of the Graduate School of Dentistry, Osaka University (IBR No. R1-E46 and R4-E1).
Resected tissue samples were fixed with 10% formalin, routinely embedded in paraffin, cut into 4 μm thick serial sections, and used for hematoxylin and eosin, and immunohistochemical staining. Immunohistochemical staining was performed using a Roche Ventana BenchMark GX autostainer (Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer’s instructions. Primary antibodies against Ki-67 and Bcl-2 were used.
Next-generation sequencing (NGS)
To examine PTCH1 mutational status, we performed next-generation sequencing (NGS) with a custom panel as previously described [20]. Genomic DNA was extracted from FFPE tissues using the QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The gene panel was designed using SureDesign [21] to cover the whole exon of PTCH1 gene (NM_000264.3). On average, 70 ng of the extracted DNA was fragmented using the SureSelect Fragmentation Enzyme (Agilent Technologies, Inc. Santa Clara, CA, USA) to 150–200 bp. Sequence libraries were prepared using a custom SureSelect Low Input Target Enrichment System (Agilent Technologies Inc. Santa Clara, CA, USA), according to the manufacturer’s instructions, and sequenced using Illumina MiSeq (Illumina, San Diego, CA, USA). SureCall ver4.0 [22] was used for variant calling. DNA in introns or non-cording DNA was excluded.
Results
Clinical findings
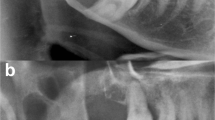
The three cases included two men and one woman, with a mean age of 25.3 years old (range, 18–38 years old). The patients had no relevant medical or family history. The presenting symptoms were swelling without pain (Cases 1 and 2) and with pain (Case 3). The preoperative clinical diagnoses were OKC (Cases 1 and 3) and odontogenic cysts (Case 2). Each case had two or three OOCs (total of seven OOCs), all of which were detected simultaneously on radiographic examination (Fig. 1a, d, g, h). Of the seven OOCs that manifested as multiple jaw cysts, seven (100%) occurred in the posterior regions, four (57.1%) occurred in the mandible, and four (57.1%) were associated with an impacted tooth. All patients were treated with enucleation and had no signs of recurrence at the last follow-up (6–60 months). At the last follow-up, no clinical features of NBCCS were noted. The clinical findings of multiple OOC in previously reported cases and in the present series are summarized in Table 1.
Radiographic and histological analyses of multiple OOC.
Panoramic radiographs of Case 1 (a), 2 (d), and 3 (g), and coronal CT of Case 3 (h). The OOCs show a well-demarcated, unilocular lesion (dotted line). Representative histological findings in Cases 1 (b, c), 2 (e, f), and 3 (i-k). The epithelial lining of OOC shows orthokeratinization with prominent granular cells. The lumen was filled with keratin material (insets i and k). OOC, orthokeratinized odontogenic cysts; CT, computed tomography. Scale bars: 100 μm in Figs. b, c, e, f, i, j and k
Histological findings
All seven OOCs showed a fibrous cyst wall lined by stratified squamous epithelium of variable thickness (Cyst #1–7) (Fig. 1b, c, e, f, i-k). An orthokeratinized epithelial lining with a prominent granular cell layer was observed (Cyst #1–5) (Fig. 1b, c, e, f, i). In two cysts (Cyst #6, 7) (Fig. 1i, k), broad non-keratinization was detected in association with inflammation, but the lumen of the cyst was filled with keratin material (insets of Fig. 1i and k). Histological findings of OKC, including corrugated surface keratin layers, palisaded basal cells, or small satellite cysts in the wall, were not observed. In addition, there were no findings suggestive of other odontogenic lesions.
Immunohistochemical findings
Immunohistochemical examination of Ki-67 and Bcl-2 expression was performed. The Ki-67 labeling index, indicating the proliferative activity, was 9.43% (range, 6–13%) (Fig. 2a). Bcl-2 expression was not detected in any of the seven OOCs (Fig. 2b). The results of the immunohistochemical analysis are summarized in Table 2.
Molecular genetic findings
PTCH1 mutations were evaluated in all seven OOCs. PTCH1 mutations were detected in six lesions collected in the three analyzed cases. Of the seven OOCs, PTCH1 mutations (p.P1315L) were identified in five OOCs (Cyst #2–5, 7), and PTCH1 mutations (p.G1212S) were identified in three OOCs (Cyst #5–7). None of the PTCH1 mutations were detected in one OOC (Cyst #1). The results of the molecular genetic analyses are summarized in Table 2.
Discussion
Multiple OOCs are an extremely rare developmental odontogenic cyst of the jaw. The present study is the first to report a series of clinical, histological, immunohistochemical, and genetic analyses of synchronous multiple OOC cases. To date, a total of 21 cases with multiple OOC have been reported, including the present study (Table 1) [9, 13,14,15,16,17,18,19]. Wang et al. [9] reported eight cases of multiple OOC, but did not provide detailed clinical data, including that regarding age, sex, cyst location, number of cysts, and medical history. After removing these eight cases, a total of 13 cases were analyzed (Table 1). The mean age was 24.4 years old (range, 18–38 years old), and the male-to-female ratio was 10:3. The mandible (66.7%, 20/30) was 2 times more frequently affected than maxilla (33.3%; 10/30). All OOCs were located in the posterior regions of the jaw (100%, 30/30), and 22 OOCs of which were associated with impacted teeth (73.3%). No association with NBCCS was reported in any patient and no evidence of recurrence was noted (Table 1). Compared with solitary OOC and OKC in previous studies [2, 12, 19, 23, 24], the mean age of patients with multiple OCC was about 10 years younger (Multiple OOC, 24.4 years old vs. Solitary OOC, 31.5–38.9 years old vs. OKC, 32.8–33.1 years old). Both multiple and solitary OOC were predominant in men compared with OKC (Male-to-female ratio; Multiple OOC, 3.3:1 vs. Solitary OOC, 2.6:1 vs. OKC, 1.05–1.76) [19].
Histologically, OOC is characterized by an orthokeratinized stratified squamous epithelium lining with prominent granular cell [1, 2, 19]. OOC often shows varying degrees of inflammation in the cyst wall and non-keratinization is detected in association with inflammation [19]. Multiple OOC in previously reported cases and the present series showed typical histological features of OOC, but no features of OKC have been reported [13,14,15,16,17,18,19]. Previous studies have shown that the immunohistochemical expression of various markers related to biological behavior varies between solitary OOC and OKC [3,4,5,6,7,8]. The present series was in general agreement with previous immunohistochemical findings, and a lower proliferative activity in OOC linings was confirmed (Ki-67: Multiple OOC, 9.43% vs. Solitary OOC, 6-8.6% vs. OKC 10.7–53%) (Fig. 2a) [5, 7, 8, 11]. Unlike OKC, Bcl-2 expression was not detected in multiple OOC or in previously reported solitary OOC (Fig. 2b) [6, 9]. Bcl-2, regulated by the SHH downstream protein Gli1, encodes a protein that prevents apoptosis. Lower expression of Bcl-2 in OOC may result in mild biological behavior and a lower tendency to recur [6, 9, 11]. Therefore, a low Ki-67 labeling index and lack of Bcl-2 expression in multiple OOC indicated mild biological behavior of odontogenic cysts. As a result, multiple OOC never recur and solitary OOC rarely recurs, whereas OKC displays locally aggressive behavior and a high recurrence rate [1, 9, 11]. Considering the clinical and pathological findings, multiple OOC and solitary OOC are probably the same entity as odontogenic cysts, instead of OKC.
The PTCH1 mutation was detected in more than 80% of OKCs, both syndromic and sporadic [1, 9,10,11, 25]. We detected PTCH1 p.P1315L (c.3944 C > T) mutation in all 3 cases, and p.G1212S (c.3634G > A) mutation in 1 case. The PTCH1 p.P1315L mutation has also been identified in solitary OOC and reported to be unrelated to the risk of basal cell carcinoma, which is one of the symptoms of NBCCS [9, 26]. Several studies have indicated that PTCH1 p.P1315L is a nonpathogenic mutation [9, 27]. Moreover, the interpretation of PTCH1 p.P1315L is “Benign” in ClinVar [28]. The identified PTCH1 p.G1212S mutation is reported in the COSMIC (Catalogue of Somatic Mutations in Cancer) database, however the function of this mutation is not investigated. The interpretation of PTCH1 p.G1212S is “Likely Benign” in ClinVar [28]. Taken together, our targeted NGS did not identify any pathogenic PTCH1 mutations common to multiple OOC cases. Two previous multiple OOC cases failed to detect pathogenic PTCH1 mutations by genetic testing using DNA from one blood sample or one OOC lesion [9, 17]. Thus, abnormalities related to pathogenic PTCH1 mutations frequently detected in OKC were not observed in multiple OOC cases. The absence of pathogenic PTCH1 mutations in multiple OOC, which was in sharp contrast to the results for OKC, suggested a different entity of odontogenic cysts.
Conclusion
In conclusion, the present study reports a small series of multiple OOC cases and confirms their consistent clinicopathological and genetic characteristics. Multiple OOCs occurred more often in younger patients than solitary OOCs. Both multiple and solitary OOCs may be related diseases within the entity of odontogenic cysts. Multiple OOC are clinicopathologically and genetically distinct from OKC.
Data Availability
The surgical materials and datasets analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- FFPE:
-
formalin-fixed paraffin-embedded.
- NBCCS:
-
nevoid basal cell carcinoma syndrome.
- NGS:
-
next-generation sequencing.
- OOC:
-
orthokeratinized odontogenic cyst.
- OKC:
-
odontogenic keratocyst.
- PTCH1:
-
protein patched homolog 1.
- SHH:
-
Sonic hedgehog.
References
El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO Classification Head and Neck Tumours. 4th ed.: International Agency for Research on Cancer; 2017.
Wright JM. The odontogenic keratocyst: orthokeratinized variant. Oral Surg Oral Med Oral Pathol. 1981;51:609–18.
Li TJ, Kitano M, Chen XM, Itoh T, Kawashima K, Sugihara K, et al. Orthokeratinized odontogenic cyst: a clinicopathological and immunocytochemical study of 15 cases. Histopathology. 1998;32:242–51.
da Silva MJ, de Sousa SO, Corrêa L, Carvalhosa AA, De Araújo VC. Immunohistochemical study of the orthokeratinized odontogenic cyst: a comparison with the odontogenic keratocyst. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:732–7.
Koizumi K. Odontogenic keratocyst, orthokeratinized odontogenic cyst and epidermal cyst: An immunohistochemical study including markers of proliferation, cytokeratin and apoptosis related factors. Int J Oral-Med Sci. 2004;2:14–22.
Rangiani A, Motahhary P. Evaluation of bax and bcl-2 expression in odontogenic keratocysts and orthokeratinized odontogenic cysts: A comparison of two cysts. Oral Oncol. 2009;45:e41-4.
Dong Q, Pan S, Sun LS, Li TJ. Orthokeratinized odontogenic cyst: a clinicopathologic study of 61 cases. Arch Pathol Lab Med. 2010;134:271–5.
Tsuji K, Wato M, Hayashi T, Yasuda N, Matsushita T, Ito T, et al. The expression of cytokeratin in keratocystic odontogenic tumor, orthokeratinized odontogenic cyst, dentigerous cyst, radicular cyst and dermoid cyst. Med Mol Morphol. 2014;47:156–61.
Wang YJ, Zhang JY, Dong Q, Li TJ. Orthokeratinized odontogenic cysts: A clinicopathologic study of 159 cases and molecular evidence for the absence of PTCH1 mutations. J Oral Pathol Med. 2022;51:659–65.
Vered M, Wright JM. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Odontogenic and Maxillofacial Bone Tumours. Head Neck Pathol. 2022;16:63–75.
Li TJ. The odontogenic keratocyst: a cyst, or a cystic neoplasm? J Dent Res. 2011;90:133–42.
Diniz MG, Galvão CF, Macedo PS, Gomes CC, Gomez RS. Evidence of loss of heterozygosity of the PTCH gene in orthokeratinized odontogenic cyst. J Oral Pathol Med. 2011;40:277–80.
Premalatha BR, Roopa RS, Jude J, Prasad K. Bilateral orthokeratinized odontogenic cyst: an unusual presentation and review. Int J Contemp Dent. 2012;3:73–6.
Pereira FAC, Vidal MTA, Campos PSF, Neto AAPV, Fernandes A, Santos JND. Orthokeratinized odontogenic cyst: a report of two cases in the mandible. Rev Odonto Ciência. 2012;27:174–8.
Pimpalkar RD, Barpande SR, Bhavthankar JD, Mandale MS. Bilateral orthokeratinized odontogenic cyst: A rare case report and review. J Oral Maxillofac Pathol. 2014;18:262–6.
Cheng YS, Liang H, Wright J, Teenier T. Multiple orthokeratinized odontogenic cysts: a case report. Head Neck Pathol. 2015;9:153–7.
Crane H, Da Forno P, Kyriakidou E, Speight PM, Hunter KD. Multiple Orthokeratinized Odontogenic Cysts: A Report of Two Cases and Review of the Literature. Head Neck Pathol. 2020;14:381–5.
Joseph J, Dungarwalla MM, Jones J. A case report and literature review of multiple orthokeratinizing odontogenic cysts: The great mimicker. Oral Surg. 2021;14:407–11.
Oh KY, Kim JE, Cho SD, Yoon HJ, Lee JI, Hong SD. Orthokeratinized odontogenic cyst: A large series and comprehensive literature review with emphasis on synchronous multiple occurrence and neoplastic transformation. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133:e72–82.
Hori Y, Hirose K, Ozeki M, Hata K, Motooka D, Tahara S, et al. PIK3CA mutation correlates with mTOR pathway expression but not clinical and pathological features in Fibro-adipose vascular anomaly (FAVA). Diagn Pathol. 2022;17:19.
SureDesign. https://earray.chem.agilent.com/suredesign. Accessed 3 May 2022.
SureCall. https://www.agilent.com/en/download-software-surecall (2022). Accessed 30 June 2022.
González-Alva P, Tanaka A, Oku Y, Yoshizawa D, Itoh S, Sakashita H, et al. Keratocystic odontogenic tumor: a retrospective study of 183 cases. J Oral Sci. 2008;50:205–12.
Jung HD, Lim JH, Kim HJ, Nam W, Cha IH. Appropriate follow-up period for odontogenic keratocyst: a retrospective study. Maxillofac Plast Reconstr Surg. 2021;43:16.
Sim YC, Kim GH, Choi SW, Ahn KM. Novel PTCH1 Gene Mutation in Nevoid Basal Cell Carcinoma Syndrome. J Craniofac Surg. 2018;29:e252-5.
Liboutet M, Portela M, Delestaing G, Vilmer C, Dupin N, Gorin I, et al. MC1R and PTCH gene polymorphism in French patients with basal cell carcinomas. J Invest Dermatol. 2006;126:1510–7.
Nakase Y, Hamada A, Kitamura N, Hata T, Toratani S, Yamamoto T, et al. Novel PTCH1 mutations in Japanese familial nevoid basal cell carcinoma syndrome. Hum Genome Var. 2020;7:38.
ClinVar. NCBI. 2021. https://www.ncbi.nlm.nih.gov/clinvar/. Accessed 30 June 2022.
Acknowledgements
Not applicable
Funding
This study was funded by the Japan Society for Promotion of Science (JSPS) KAKENHI Grant-in-Aid forScientific Research (No. 21K17114).
Author information
Authors and Affiliations
Contributions
The authors have contributed to this work. S.O. and K.H. conceptualized this study and wrote the manuscript. S.O., K.H., S.N., D.M., Y.H., K.O., Y.F. and S.T. performed the experiments and assembled the data. S.S. and Y.I. reviewed the clinical and radiological data. S.O. and K.H. critically revised the manuscript for intellectual content. All authors have read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethical review board of the Graduate School of Dentistry, Osaka University (IRB No. R1-E46 and R4-E1), and were performed in accordance with the committee guidelines and regulations.
Consent for publication
Written informed consent for the publication of clinical details and clinical images was obtained from all patients. A copy of the consent form is available for review by the editor of the journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ono, S., Hirose, K., Sukegawa, S. et al. Multiple orthokeratinized odontogenic cysts: clinical, pathological, and genetic characteristics. Diagn Pathol 17, 82 (2022). https://doi.org/10.1186/s13000-022-01261-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-022-01261-0