Abstract
Collision tumors of the stomach are rare. We report on a case of a collision tumor consisting of a gastrointestinal stromal tumor (GIST) and an inflammatory myofibroblastic tumor (IMT) of the stomach in a 16-year-old female. A polypoid mass located in the distal body of the stomach was observed on abdominal computed tomography. Laparoscopic wedge resection of the stomach and 4d lymph node biopsy was performed. On gross examination, a protruding submucosal mass, measuring 4 × 3.5 × 2.5 cm in size, was detected. Histological examination showed two distinct GIST and IMT component presenting a collision tumor. The small nodular area, composed of CD117-positive spindle cells, was typical of GIST, and the adjacent larger area, composed of myofibroblastic spindle cells with prominent chronic inflammatory cells infiltrate, mainly lymphocytes and plasma cells, had a characteristic appearance of IMT. The 4d lymph node showed metastatic inflammatory myofibroblastic tumor. To the best of our knowledge, this is the first case of a collision tumor consisting of a GIST and an IMT arising in the stomach.
Similar content being viewed by others
Background
Collision tumor is defined as co-existence of two adjacent but histologically distinct tumors with no histologic admixture at the interface. The synchronous occurrence of two different tumors in the stomach has been frequently reported [1–5]. However, collision tumors in the same gastric area are uncommon. Collision tumors consisting of adenocarcinomas coexisting with gastrointestinal stromal tumor (GIST) [6–9], neuroendocrine tumor [10, 11], leiomyoma [12], and schwannoma [13] have been reported. Diagnosis of collision tumors can be a challenge [14].
To the best of our knowledge, no case of a collision tumor consisting of a GIST and an inflammatory myofibroblastic tumor (IMT) has been previously reported in English literature. Herein we report the first case of a collision tumor consisting of a GIST and an IMT arising in the stomach of a 16 year-old female.
Case presentation
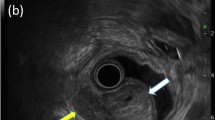
A 16-year-old female presented with abdominal pain for 2 weeks. Her medical and family history was nonspecific. Computed tomography of the abdomen showed a polypoid mass located in the distal body of the stomach (Fig. 1). Endoscopic ultrasound revealed a hypoechoic mass in the stomach. A polypoid mass located in the distal body with intact overlying mucosa was observed on esophagogastroduodenoscopy. The overlying mucosa was intact. The initial diagnosis was a gastric submucosal tumor. Laparoscopic wedge resection of the stomach and excisional biopsy of a 4d lymph node were performed.
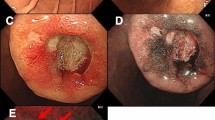
On gross examination, a well circumscribed, protruding submucosal mass was present, measuring 4 × 3.5 × 2.5 cm in size (Fig. 2). The cut surface was well circumscribed, yellow to red, and soft. Histologic examination of the mass showed the mass showed two distinct components in the muscularis propria (Fig. 3a, b). An area of palisaded spindle cells proliferation with uniform tapering nuclei and indistinct syncytial cytoplasm was observed. Perinuclear vacuoles were noted (Fig. 3c). Immunohistochemical staining showed that the cells of this component were positive for CD117 (Fig. 3d). The small area had characteristic appearance of GIST, spindle cell type, which measured 0.8 cm in size. The mitotic activity was 1 per 50 high-power fields (HPF). In mutational analysis for KIT performed from formalin-fixed and paraffin-embedded tissue, there were no KIT mutation in exon 9, 11, 13 and 17. The adjacent large area showed fascicular proliferation of myofibroblastic spindle-shaped tumor cells, admixed with prominent infiltrate of chronic inflammatory cells, mainly lymphocytes and plasma cells (Fig. 4a, b). The tumor cells had plump vesicular nuclei and eosinophilic cytoplasm (Fig. 4c). Some tumor cells had variably atypical nuclei. The immunohistochemical staining showed that the tumor cells of the large area were negative for CD117, DOG1, desmin, ALK, CD21, CD23, and S100 protein (Fig. 4d) and showed focal positivity for smooth muscle actin. In situ hybridization for Epstein-Barr virus (EBV)-encoded RNA did not show the presence of EBV. The majority of the infiltrating plasma cells were IgG-positive. The count of IgG4-positive plasma cells was less than 5 per high power field. Although the tumor cells were negative for ALK, the histologic appearance was characteristic of an inflammatory myofibroblastic tumor. A 4d lymph node showed a gray-white nodular lesion (Fig. 5a). Histologically, the lymph node was replaced by spindle cells proliferation and prominent lymphocytes and plasma cells (Fig. 5b, c, d). The spindle cells of the lymph nodes were negative for CD117 and ALK. The histologic findings of the lymph node were similar to those of the gastric IMT component. The lymph node was compatible with a metastatic inflammatory myofibroblastic tumor. The patient remains alive with no evidence of recurrence or metastasis 3 years after surgery.
Histologic findings of gastrointestinal stromal tumor (GIST) area. a At low-magnification view, gastrointestinal stromal tumor (GIST) and inflammatory myofibroblastic tumor (IMT) area are mapped (H&E stain, x2). b GIST and IMT components are present in a collision tumor (H&E stain, x40). c Spindle-shaped cells with perinuclear vacuoles are present (H&E stain, x200). d The cells of the GIST area are positive for CD117 (Immunohistochemical stain, x200)
Histologic findings of inflammatory myofibroblastic tumor (IMT) area. a Myofibroblastic spindle cells are arranged in a fascicular pattern with prominent chronic inflammatory cells infiltrate (H&E stain, x40). b Spindle-shaped cells are admixed with lymphocytes and plasma cells infiltrate (H&E stain, x100). c The tumor cells have plump vesicular nuclei and eosinophilic cytoplasm. Collagenous stroma and scattered chronic inflammatory cells are present (H&E stain, x200). d The tumor cells are negative for ALK (Immunohistochemical stain, x200)
Gross and histologic findings of the 4d lymph node. a A gray-white, nodular lesion is located within the lymph node. b Whole mount section of the lymph node shows a nodular lesion (HE stain, x1). c Low magnification view shows replacement of the lymph node by tumor (HE stain, x40). d Spindle-shaped cells and lymphoplasmacytic infiltrate are present (HE stain, x100)
Discussion
Collision tumors of the stomach are rare. Gastric collision tumors composed of gastrointestinal stromal tumor (GIST) are shown in Table 1. Six cases of adenocarcinoma [6–9, 15], 1 case of signet ring cell carcinoma [16], and 1 case of angiosarcoma [17] showed collision with GIST. To the best of our knowledge, a collision tumor containing a GIST and an IMT has not been previously reported in English literature. Therefore, our case is the first case of a collision tumor containing a GIST and an IMT.
Gastrointestinal stromal tumor (GIST) is the most common primary mesenchymal tumor of gastrointestinal tract [18]. It is generally immunohistochemically positive for CD117 (KIT), phenotypically paralleling Cajal-cell differentiation, and in most cases contains KIT- or PDGFRA-activating mutations. Approximately 60 % of GISTs arise in the stomach. GISTs may coexist with different types of cancer, either synchronously or metachronously [14].
IMT is a distinctive lesion composed of myofibroblastic spindle cells accompanied by an inflammatory infiltrate of plasma cells, lymphocytes, and eosinophils [19]. It primarily affects children and young adults, although the age range extends throughout adulthood. It occurs throughout the body, most frequently in mesentery, omentum, retroperitoneum, pelvis, and abdominal soft tissue. Immunohistochemically, IMTs are generally actin positive and may also show staining for desmin and cytokeratin. Cytoplasmic reactivity for ALK protein is detected in 50–60 % of cases [19]. Therefore, ALK positivity is helpful in diagnosis of IMT, however, its absence does not exclude the diagnosis of IMT [20, 21]. Although our case was negative for ALK, the histological features were typical of IMT. Gastric IMT is rare and may be confused with other submucosal lesions [20]. IMT should be considered, particularly if the young patient has a gastric submucosal lesion showing spindle cells accompanied by numerous chronic inflammatory cells infiltrates, mainly plasma cells [22].
In the current case, presence of a GIST and an IMT in the same site resulted in formation of a collision tumor. No transition was observed between the different components of the tumors. We consider that the occurrence of two distinct tumors may be a simple incidental coexistence. Further investigation of the relationship between tumors of these types is needed.
The differential diagnosis of gastric IMT includes GIST, inflammatory fibroid polyp, smooth muscle tumors, schwannoma, IgG4-related sclerosing disease, and inflammatory pseudotumor-like follicular dendritic cell (FDC) sarcoma. GIST typically does not have the inflammatory background seen in IMT. The palely eosinophilic, syncytial cytoplasm and cytological uniformity of GISTs contrast with the plump myofibroblasts, scattered ganglion-like cells and collagenous background seen in IMT [21, 23]. Immunohistochemically, GIST is typically positive for CD117 but negative for ALK. IMTs are consistently negative for CD117. Inflammatory fibroid polyps (IFP) are usually present in the submucosa [24]. Histologically, there is proliferation of spindle and stellate stromal cells, which tend to condense around blood vessels to form whorled, perivascular cuffs, which are absent in IMTs. Both IFP and IMT have an inflammatory background, but that of the former is rich in eosinophils. The majority of IFPs are positive for CD34. Gastric smooth muscle tumors, which typically do not have an inflammatory background, have fascicles of spindle cells with cigar-shaped nuclei and brightly eosinophilic cytoplasm and are diffuse positive for smooth muscle actin, desmin and caldesmon. Gastric schwannoma shows peripheral cuff-like lymphoid aggregates and immunoreactivity for S-100 protein. IgG4-related sclerosing disease is a recently described multisystemic disorder with histological appearance similar to that of IMT [25, 26]. The prominent IgG4-positive plasma cells infiltrate is characteristic in IgG4-related sclerosing disease. In our case, the number of IgG4-positive plasma cells was low. The distinction of IMT from inflammatory pseudotumor-like FDC sarcoma may be difficult. The latter occurs almost exclusively in the liver and spleen [27, 28]. In the present case, no expression for FDC markers such as CD21 and CD23 can exclude inflammatory pseudotumor-like FDC sarcoma. Careful histologic evaluation, immunohistochemistry, and clinical correlation are helpful for a correct diagnosis of gastric IMT.
Due to their rarity, it is difficult to determine the biological behavior of gastric collision tumors. In our case, the GIST area belonged to the very low risk group. Risk of aggressive behavior and metastasis appears to be increased for ALK-negative IMT [21, 23]. The current case was negative for ALK and lymph node metastasis was present.
Conclusions
We reported on a collision tumor consisting of a GIST and an IMT arising in the body of the stomach. This case is unique and the first report of a gastric collision tumor consisting of a GIST and an IMT. Awareness of this entity is important to distinguishing it from other submucosal lesions.
Consent
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
- GIST:
-
Gastrointestinal stromal tumor
- IMT:
-
Inflammatory myofibroblastic tumor
- IFP:
-
Inflammatory fibroid polyp
References
Maiorana A, Fante R, Maria Cesinaro A, Adriana FR. Synchronous occurrence of epithelial and stromal tumors in the stomach: a report of 6 cases. Arch Pathol Lab Med. 2000;124:682–6.
Andea AA, Lucas C, Cheng JD, Adsay NV. Synchronous occurrence of epithelial and stromal tumors in the stomach. Arch Pathol Lab Med. 2001;125:318–9.
Kaffes A, Hughes L, Hollinshead J, Katelaris P. Synchronous primary adenocarcinoma, mucosa-associated lymphoid tissue lymphoma and a stromal tumor in a Helicobacter pylori-infected stomach. J Gastroenterol Hepatol. 2002;17:1033–6.
Lee FY, Jan YJ, Wang J, Yu CC, Wu CC. Synchronous gastric gastrointestinal stromal tumor and signet-ring cell adenocarcinoma: a case report. Int J Surg Pathol. 2007;15:397–400.
Villias C, Gourgiotis S, Veloudis G, Sampaziotis D, Moreas H. Synchronous early gastric cancer and gastrointestinal stromal tumor in the stomach of a patient with idiopathic thrombocytopenic purpura. J Dig Dis. 2008;9:104–7.
Liu SW, Chen GH, Hsieh PP. Collision tumor of the stomach: a case report of mixed gastrointestinal stromal tumor and adenocarcinoma. J Clin Gastroenterol. 2002;35:332–4.
Katsoulis IE, Bossi M, Richman PI, Livingstone JI. Collision of adenocarcinoma and gastrointestinal stromal tumour (GIST) in the stomach: report of a case. Int Semin Surg Oncol. 2007;4:2.
Bi R, Sheng W, Wang J. Collision tumor of the stomach: gastric adenocarcinoma intermixed with gastrointestinal stromal tumor. Pathol Int. 2009;59:880–3.
Kleist B, Lasota J, Miettinen M. Gastrointestinal stromal tumor and gastric adenocarcinoma collision tumors. Hum Pathol. 2010;41:1034–9.
Unal B, Elpek GO, Gelen T, Gürkan A, Yildirim B. Gastric collision tumor: Case report of a rare adenocarcinoma and a typical carcinoid tumor. Oncol Lett. 2013;6:212–4.
Kadowaki Y, Nishimura T, Komoto S, Yuasa T, Tamura R, Okamoto T, et al. Gastroduodenal intussusception caused by a gastric collision tumor consisting of adenocarcinoma and neuroendocrine carcinoma. Case Rep Gastroenterol. 2014;8:89–94.
Tokunaga M, Ohyama S, Fujimoto Y, Hiki N, Fukunaga T, Yamamoto N, et al. Simultaneous adenocarcinoma and leiomyoma of the stomach presenting as a collision tumor. Clin J Gastroenterol. 2009;2:394–7.
Go JH. Collision of adenocarcinoma and schwannoma of the stomach: a case report. Korean J Pathol. 2012;46:373–6.
Agaimy A, Wünsch PH, Sobin LH, Lasota J, Miettinen M. Occurrence of other malignancies in patients with gastrointestinal stromal tumors. Semin Diagn Pathol. 2006;23:120–9.
Idema DL, Daryanani D, Sterk LM, Klaase JM. Collision tumor of the stomach: a case of an adenocarcinoma and a gastrointestinal stromal tumor. Case Rep Gastroenterol. 2008;2:456–60.
Trabelsi A, Stita W, Mokni M, Yacoubi T, Mestiri S, Korbi SY. Collision epithelial and stromal tumours of the stomach: a case report. Pathologica. 2008;100:18–20.
Adhikari M, Wu ML, Zhao X. Gastrointestinal stromal tumor colliding with angiosarcoma. Int J Surg Pathol. 2006;14:252–6.
Miettinen M, Fletcher CDM, Kindblom LG, Tsui WMS. Mesenchymal tumours of the stomach. In: Bosman FT, Carneiro F, Hrubban RH, editors. Theise ND: WHO classification of tumours of the digestive System. Lyon: IARC Press; 2010. p. 74–9.
Coffin CM, Fletcher JA. Inflammatory myofibroblastic tumour. In: Fletcher CDM, Bridge JA, Hogendoor PCW, Mertens F, editors. WHO Classification of tumours of soft tissue and bone. Lyon: IARC Press; 2013. p. 83–4.
Shi H, Wei L, Sun L, Guo A. Primary gastric inflammatory myofibroblastic tumor: a clinicopathologic and immunohistochemical study of 5 cases. Pathol Res Pract. 2010;206:287–91.
Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008;61:428–37.
Lee YK, Wang HY, Shyung LR, Chang CW, Chen MJ. Inflammatory myofibroblastic tumor: an unusual submucosal lesion of the stomach. Endoscopy. 2011;43 Suppl 2:E151–2.
Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509–20.
Liu TC, Lin MT, Montgomery EA, Singhi AD. Inflammatory fibroid polyps of the gastrointestinal tract: spectrum of clinical, morphologic, and immunohistochemistry features. Am J Surg Pathol. 2013;37:586–92.
Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–92.
Saab ST, Hornick JL, Fletcher CD, Olson SJ, Coffin CM. IgG4 plasma cells in inflammatory myofibroblastic tumor: inflammatory marker or pathogenic link? Mod Pathol. 2011;24:606–12.
Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001;25:721–31.
Choe JY, Go H, Jeon YK, Yun JY, Kim YA, Kim HJ, et al. Inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen: a report of six cases with increased IgG4-positive plasma cells. Pathol Int. 2013;63:245–51.
Acknowledgement
This work was supported by a grant from the Chunma medical research foundation, Korea, 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SHC, GMJ, and CJH participated in the histopathological evaluation. KSW participated in the clinical evaluation. KJW participated in the radiological evaluation. CJH wrote the manuscript. All authors have read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shin, H.C., Gu, M.J., Kim, S.W. et al. Coexistence of gastrointestinal stromal tumor and inflammatory myofibroblastic tumor of the stomach presenting as a collision tumor: first case report and literature review. Diagn Pathol 10, 181 (2015). https://doi.org/10.1186/s13000-015-0413-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13000-015-0413-y