Abstract
We had recently reported that linalool odor exposure induced significant analgesic effects in mice and that the effects were disappeared in olfactory-deprived mice in which the olfactory epithelium was damaged, thus indicating that the effects were triggered by chemical senses evoked by linalool odor exposure. However, the peripheral neuronal mechanisms, including linalool receptors that contribute toward triggering the linalool odor-induced analgesia, still remain unexplored. In vitro studies have shown that the transient receptor potential ankyrin 1 (TRPA1) responded to linalool, thus raising the possibility that TRPA1 expressed on the trigeminal nerve terminal detects linalool odor inhaled into the nostril and triggers the analgesic effects. To address this hypothesis, we measured the behavioral pain threshold for noxious mechanical stimulation in TRPA1-deficient mice. In contrast to our expectation, we found a significant increase in the threshold after linalool odor exposure in TRPA1-deficient mice, indicating the analgesic effects of linalool odor even in TRPA1-deficient mice. Furthermore, intranasal application of TRPA1 selective antagonist did not alter the analgesic effect of linalool odor. These results showed that the linalool odor-induced analgesia was triggered by a TRPA1-independent pathway in mice.
Similar content being viewed by others
Introduction
We had recently demonstrated that odor exposure of linalool (3,7-dimethylocta-1,6-dien-3-ol), one of the monoterpene alcohols found in lavender extracts, induces analgesic effects in mice [1]. We observed that the effects were disappeared in olfactory-deprived mice in which the olfactory epithelium was damaged, thus indicating that the effects were triggered by chemical senses evoked by linalool odor exposure. However, the peripheral neuronal mechanisms, including linalool receptors that contribute toward triggering the linalool odor-induced analgesia, have not yet been explored.
Odorous volatile compounds inhaled into the nostril are detected by two sensory systems, the main olfactory system and the trigeminal sensory system. In the main olfactory system, olfactory sensory neurons expressing a given odorant receptor is translated into electrical signals. These signals are then transmitted to the main olfactory bulb, the first relay station of the main olfactory system, and further to the olfactory cortices to perceive the olfactory input [2, 3]. In addition to the classical main olfactory pathway, the trigeminal nerve pathway contributes to the detection of odorous compounds in the nostril [4]. The ethmoidal nerve arising from the ophthalmic division of the trigeminal nerve projects into the nasal cavity and makes free end terminals in the epithelium [5,6,7,8]. The central projections of the ethmoidal nerve terminate on the superficial laminae of the medullary dorsal horn [9, 10] with collateral branches which reach directly to the olfactory bulb [7, 11]. In spite of the individual peripheral receptors, the olfactory and trigeminal input mutually affect each other in the perception of odors [12,13,14,15], indicating that the information detected by the two chemosensory systems is functionally integrated in our central nervous system. The exact location of the interaction between the two systems is not yet determined. But several anatomical areas such as the olfactory epithelium, the olfactory bulb, the mediodorsal thalamus, the piriform cortex, the orbitofrontal cortex and the insula cortex have been proposed as the site [5, 7, 11, 16,17,18].
Chemosensors expressed on the free end terminals can be activated by the chemical compounds that are inhaled into the nostril and drive the trigeminal pathway [19]. Among the chemosensors, the transient receptor potential ankyrin 1 (TRPA1), which was first identified as the thermosensor [20], detects a range of odorous compounds [21], and plays a key role in triggering the allyl isothiocyanate odor-induced bradypnea in mice [22]. In addition to pungent odorous compounds, linalool activates the TRPA1 in dissociated dorsal root ganglia neurons and in human embryonic kidney 293 cells expressing the wild-type TRPA1 channels [23, 24]. These observations raise a hypothesis that the TRPA1 on the trigeminal nerve terminal in the nasal cavity detects the inhaled linalool and triggers the analgesic effects induced by linalool odor. To investigate this hypothesis, we measured the threshold for the behavioral pain response immediately after linalool odor exposure and compared the threshold between TRPA1-deficient (KO) mice and wild-type mice.
Materials and methods
Animals and housing conditions
Male wild-type (WT) mice (C57BL/6, weighing 26.6–34.6 g, n = 77) and TRPA1 KO mice (weighing 22.7–27.4 g, n = 43) were used in this study. The mutant mice were originally purchased from the Jackson Laboratory and genotyped as previously described [25]. They were maintained as heterozygotes in our facility and crossed to obtain null mutants and WT littermates. The mutant mice were backcrossed with C57BL/6J mice (Clea Japan Inc., Tokyo, Japan) for more than 10 generations. All animals were maintained under a constant temperature (24 °C ± 1 °C) with free access to food and water. The animals were housed with lights on at 7:00 A.M. and off at 7:00 P.M. All experiments were conducted during the light cycle, between 1:00 P.M. and 5:00 P.M. The animals were naive to linalool odor, and each mouse was used only once to avoid carryover effects.
Chemicals
Linalool (CAS#: 78-70-6) was purchased from Tokyo Chemical Industry (Tokyo, Japan), stored at 4 °C, and dispensed into a glass vial during each trial to prevent degradation. A TRPA1 selective antagonist AP18 ((Z)-4-(4-chlorophenyl)-3-methylbut-3-en-2-oxime, gifted from Prof. Mori at Kyoto University [26]) was dissolved in polyethylene glycol (PEG#400, Nacalai, Kyoto, Japan) at 60 mM.
Linalool odor exposure
We used a custom-made olfactometer for linalool odor exposure as described previously [1]. Briefly, 0.5 mL of linalool (> 96 %) was dispensed into an uncapped glass vial (diameter: 27.5 mm, content: 20 mL). The vial was placed in an odor chamber (0.32 L), and then linalool was vaporized at room temperature (24 °C ± 1 °C). Clean air deodorized using a charcoal filter and double-distilled water was introduced into the odor chamber (top diameter: 8 cm, base diameter: 11.5 cm, height: 15 cm, content: 1 L) at a constant flow rate (1 L/min). After 20 min of pre-ventilation of the linalool odor, a mouse was placed in the observation chamber and exposed to linalool odor for 5 min. Because the humidity of the carrier gas and the temperature of the odor chamber were maintained constant, the concentration of linalool odor was considered to be constant.
Acclimatization for pain assay
For acclimatization to the experimental condition, the animals were moved to the experiment room for 2 h, handled and gently touched for 3 min, and their head and body were covered with a towel and loosely restrained for 3 min. This series of acclimatization procedures was repeated for 6 days. On the experiment day, the mice were moved to the experiment room 2 h before the experiment.
Tail pincher test
For evaluating the analgesic effects of linalool odor, we measured the mechanical nociceptive threshold for tail pinch using calibrated forceps (Rodent Pincher-analgesia meter, Bioseb, Pinellas Park, USA) [27, 28]. Immediately after 5 min of odor exposure, we gently restrained the mouse with a towel and pressured on the marking of the tail using the calibrated forceps. We recorded the latency of the flicking, tail withdrawal, or struggling of the mouse in the cotton towel. We repeatedly measured the threshold for five times (trial interval: 10–15 s). We then excluded the maximum and minimum values from the sets of five measurements and used the average value of another three trials as the threshold value [28]. To prevent the mouse from being injured, a cut-off pressure point was set at 500 g. Each animal was used only once to prevent hyperalgesia.
Hot plate test
To assess the thermal nociceptive threshold, we performed a classical hot plate test with electronically controlled hot plate apparatus (#7280; Ugo Basile, Italy) as previously reported [1]. We set the hot plate temperature at 54.5 °C. Immediately after 5 min odor exposure, we placed a mouse on the hot plate. The latency before the animal licked, shock, or fluttered its hind paw, or jumped on the hot plate was recorded. To prevent the mouse from being injured, a cutoff time was set to 1 min.
Intranasal application of TRPA1 antagonist
To prevent the function of TRPA1 in the nostril, we administered the TRPA1 antagonist into the nasal cavity as previously described [21]. A small ball of the TRPA1 solution (10 µL) was attached to the nostril and was aspirated into the nasal cavity with spontaneous breathing. For the negative control experiment, a same amount of vehicle solution was administered. The prevention of TRPA1 was confirmed by the behavioral odor preference/avoidance test. 10 min after the intranasal application, further behavioral pain tests were performed.
Data analyses
GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA) was used for statistical analyses. Bartlett’s test was applied to examine the equal variances among groups. The difference between each pair of groups was compared using Welch’s ANOVA, followed by Dunnett’s T3 multiple comparison test. To avoid false-negative results caused by the effects of meaningless pairs ((WT) / control (CON) vs. TRPA1-deficient mice (KO) / linalool (LIN) and WT / LIN vs. KO / CON)), we selected four pairs (WT / CON vs. WT / LIN, WT / CON vs. KO / CON, KO / CON vs. KO / LIN, WT / LIN vs. KO / LIN) among the possible six pairs. Differences with p values < 0.05 were considered to be significant. We also calculated Cohen’s d for comparison of two groups as the effect size. The effect size was considered as large in case of d > 0.8, medium in case of d > 0.5, and small in case of d > 0.2. The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Results
Linalool odor-induced analgesia in TRPA1 KO mouse
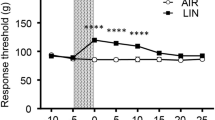
To investigate the contribution of TRPA1 to the linalool odor-induced analgesia, we measured the behavioral response threshold for mechanical nociceptive stimulation using the tail pincher test [27, 28] immediately after linalool odor exposure (Fig. 1). Bartlett’s test revealed the significant difference in variances among the four group (WT / CON, WT / LIN, KO / CON, KO / LIN) (χ2 = 13.63, p = 0.0035). Welch’s ANOVA, followed by Dunnett’s T3 multiple comparison test, was applied to compare the difference in each pairs as an alternative method of ordinary two-way ANOVA followed by Sidak’s test. The results of Welch’s ANOVA showed that a significant difference existed among the groups (W3, 22.29 = 20.87, p < 0.0001). The post hoc Dunnett’s T3 multiple comparison test indicated that linalool exposure induced a significant increase in the response threshold (θ) in wild-type mice (θWT/CON = 92.92 ± 3.846 g, θWT/LIN = 128.8 ± 3.741 g, p < 0.0001, d = 2.909) and also in TRPA1-deficient (KO) mice with large effect sizes (θKO/CON = 118.5 ± 7.482 g, θKO/LIN = 157.1 ± 9.503 g, p = 0.0171, d = 1.302). The threshold of KO mice after odorless air exposure was significantly larger than that of WT mice with a large effect size (p = 0.0297, d = 1.235), indicating that the basal response threshold for mechanical nociception was higher in KO mice. Moreover, the threshold after linalool odor exposure was slightly (but not significantly) increased in KO mice (p = 0.0557, d = 1.103).
Linalool odor-induced analgesia for mechanical nociception in TRPA1-deficient mice. Behavioral response threshold for mechanical nociceptive stimulation to tail was plotted. WT: wild-type mice; KO: TRPA1-deficient mice; CON: odorless air-exposed control mice; LIN: linalool odor-exposed experimental mice. Bars represent mean ± SEM. nWT/CON = 11, nWT/LIN = 10, nKO/CON = 12, nKO/LIN = 12, ****p < 0.0001, *p < 0.05 (post hoc Dunnett’s T3 multiple comparison test)
Next, we examined the analgesic effects in thermal nociceptive stimulation with the hot plate test in TRPA1 KO mice (Fig. 2). The hot plate test revealed that the thermal nociceptive threshold was significantly increased in both wild type mice and the KO mice after linalool odor exposure as in the tail pincher test. Bartlett’s test revealed that there were relatively large (but not significant) differences in variances among the four group (WT / CON, WT / LIN, KO / CON, KO / LIN) (χ2 = 7.305, p = 0.0614). The results of Welch’s ANOVA showed that a significant difference existed among the groups (W3, 18.94 = 9.118, p < 0.0006). The post hoc Dunnett’s T3 multiple comparison test indicated that linalool exposure induced a significant increase in the response latency (λ) in wild type mice (λWT/CON = 13.22 ± 0.704 s, λWT/LIN = 20.21 ± 1.481 s, p < 0.0023, d = 1.741) and also in TRPA1 KO mice (λKO/CON = 13.34 ± 1.347 s, λKO/LIN = 20.95 ± 1.973 s, p = 0.0251, d = 1.490) with large effect sizes. The response latency of KO mice after odorless air exposure was not significantly larger than that of WT mice with a small effect size (p > 0.9999, d = 0.036), indicating that the basal response latency for thermal nociception was not different between phenotypes. In addition, the latency after linalool odor exposure was not significantly increased in KO mice (p = 0.9966, d = 0.135). These results indicate that linalool odor exposure induced significant analgesic effects even in TRPA1 KO mice.
Linalool odor-induced analgesia for thermal nociception in TRPA1-deficient mice. Behavioral response latency for thermal nociceptive stimulation to hind paw was plotted. WT: wild-type mice; KO: TRPA1-deficient mice; CON: odorless air-exposed control mice; LIN: linalool odor-exposed experimental mice. Bars represent mean ± SEM. nWT/CON = 12, nWT/LIN = 12, nKO/CON = 10, nKO/LIN = 9, **p < 0.005, *p < 0.05 (post hoc Dunnett’s T3 multiple comparison test)
Linalool odor-induced analgesia under pharmacological prevention of intranasal TRPA1
To examine the possibility that the other receptor(s) may compensate the linalool response in TRPA1 KO mice, we accessed the linalool odor analgesia by pharmacological prevention of intranasal TRPA1 in wild type mice (Fig. 3). Intranasal application of AP18, a TRPA1 selective antagonist, revealed that the mechanical nociceptive threshold was not significantly altered after the TRPA1 antagonist administration. Bartlett’s test revealed the significant difference in variances among the four group (VEH / CON, VEH / LIN, AP / CON, AP / LIN) (χ2 = 8.250, p = 0.0411). The results of Welch’s ANOVA showed that a significant difference existed among the groups (W3, 14.85 = 9.282, p < 0.0011). The post hoc Dunnett’s T3 multiple comparison test indicated that linalool exposure induced a significant increase in the response threshold (θ) in vehicle-treated mice (θVEH/CON = 96.62 ± 3.639 g, θVEH/LIN = 130.5 ± 9.464 g, p < 0.0314, d = 1.672) and also in AP18-treated mice (θAP/CON = 105.1 ± 3.887 g, θAP/LIN = 135.5 ± 7.170 g, p = 0.0125, d = 1.867) with large effect sizes. The threshold of AP18-treated mice after odorless air exposure was not significantly different from that of WT mice (p = 0.4157, d = 0.794), indicating that the basal response threshold was not affected by the intranasal AP18 treatment. In addition, the threshold after linalool odor exposure was not significantly altered in AP18-treated mice (p = 0.987, d = 0.210).
Linalool odor-induced analgesia after intranasal application of TRPA1-selective antagonist. Behavioral response threshold for mechanical nociceptive stimulation to tail was plotted. VEH: intranasal vehicle-treated mice; AP: intranasal AP18 (TRPA1 selective antagonist)-treated mice; CON: odorless air-exposed control mice; LIN: linalool odor-exposed experimental mice. Bars represent mean ± SEM. nVEH/CON = 8, nVEH/LIN = 8, nAP/CON = 8, nAP/LIN = 8, *p < 0.05 (post hoc Dunnett’s T3 multiple comparison test)
Altogether, we concluded that TRPA1 did not contribute toward triggering the linalool odor-induced analgesia, and thus the analgesia was triggered by a TRPA1-independent pathway in mice.
Discussion
We first hypothesized that the TRPA1 expressed on the trigeminal nerve in the nasal epithelium detects the linalool inhaled into the nostril and triggers the linalool odor-induced analgesia. To explore this hypothesis, we conducted a mechanical (tail pincher test) and a thermal (hot plate test) pain test immediately after linalool odor exposure in TRPA1KO mice. Our results, in contrast to our prediction, demonstrated that the KO mice exhibited a significant analgesic effect on both mechanical and thermal nociception. Furthermore, intranasal application of TRPA1 selective antagonist did not impair the linalool odor-induced analgesia. These results imply that the analgesic effect is triggered by a TRPA1-independent pathway.
We first intended to conduct the ordinary two-way ANOVA (genotype × odor treatment), followed by Sidak’s multiple comparison test. However, the results of Bartlett’s test revealed unequal variances among the examined groups, indicating that the ordinary two-way ANOVA was not applicable to our dataset. Therefore, we applied Welch’s ANOVA, followed by Dunnett’s T3 multiple comparison test [29]. Using these statistical methods, we could not examine the major effects (genotype, odor treatment, and its interaction), but we could compare the difference in each group pair.
In addition to TRPA1, other three types of TRP family channels (transient receptor potential vanilloid 1 (TRPV1), transient receptor potential vanilloid 2 (TRPV2) and transient receptor potential melastatin 8 (TRPM8)) are expressed on trigeminal ganglion cells (TRPV1 [30,31,32], TRPV2 [33, 34], TRPM8 [30, 35, 36]). In vitro studies have suggested that linalool could be also detected by TRPM8 [37], though the half maximal effective concentration (EC50) of TRPM8 is sixty times higher than that of TRPA1 [23, 37]. Therefore, the trigeminal system could contribute toward triggering the linalool odor-induced analgesia through TRPM8. Further studies are required to evaluate the contribution of TRPM8.
In our experimental condition, the KO mice exhibited a higher response threshold to mechanical pain stimulation. TRPA1 is expressed in not only trigeminal ganglion cells but also dorsal root ganglion cells with small diameter [20, 30] and could affect the noxious mechanical transduction [38]. Our result is consistent with previous studies indicating that TRPA1-deficient mice had a higher threshold to mechanical pain stimuli than that of wild type mice [25, 39].
In this study, we examined the contribution of TRPA1 to analgesic effects of linalool odor that is vaporized in room temperature with simple odor chamber system. However, the contribution of TRPA1 at higher linalool concentration has not yet untried. Previous studies have shown that the trigeminal system could be generally activated in higher odor concentrations in human [40, 41] and mouse [42]. Therefore higher concentration linalool might affect to the analgesic effects via trigeminal TRPA1 system. In addition to the concentration, the duration of linalool odor exposure could affect the results. Yousem and colleagues reported that repetitive odor stimulation of trigeminal nerve enhances the cortical responses in contrast to the desensitization of olfactory nerve input on human subjects [43]. Therefore the longer exposure could make increase the number of inhalation of linalool odor, which in turn might drive the trigeminal activation via TRPA1 and affect to the analgesic effects. Further studies are required to assess these points.
Except for the trigeminal system, linalool can also be detected by classical odorant receptors expressed on olfactory sensory neurons. To our knowledge, at least one odorant receptor (hOR1C1) has been reported to detect linalool [44,45,46]. However, hOR1C1 is present only in the genome of humans and not mice [47]. Therefore, it is possible that other unknown odorant receptors could detect linalool odor and trigger analgesic effects in mice.
Availability of data and materials
The dataset supporting the conclusions of this is available from the corresponding author on reasonable request.
References
Tashiro S, Yamaguchi R, Ishikawa S, Sakurai T, Kajiya K, Kanmura Y, et al. Odour-induced analgesia mediated by hypothalamic orexin neurons in mice. Sci Rep. 2016;6:37129.
Shepherd GM. The synaptic organization of the brain. 5th ed. Oxford: Oxford University Press; 2004. p. 719.
Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631.
Brand G. Olfactory/trigeminal interactions in nasal chemoreception. Neurosci Biobehav Rev. 2006;30(7):908–17.
Maurer M, Papotto N, Sertel-Nakajima J, Schueler M, De Col R, Möhrlen F, et al. Photoactivation of olfactory sensory neurons does not affect action potential conduction in individual trigeminal sensory axons innervating the rodent nasal cavity. PLoS One. 2019;14(8):e0211175.
Daiber P, Genovese F, Schriever VA, Hummel T, Möhrlen F, Frings S. Neuropeptide receptors provide a signalling pathway for trigeminal modulation of olfactory transduction. Eur J Neurosci. 2013;37(4):572–82.
Genovese F, Bauersachs HG, Gräßer I, Kupke J, Magin L, Daiber P, et al. Possible role of calcitonin gene-related peptide in trigeminal modulation of glomerular microcircuits of the rodent olfactory bulb. Eur J Neurosci. 2017;45(4):587–600.
Finger TE, St Jeor VL, Kinnamon JC, Silver WL. Ultrastructure of substance P- and CGRP-immunoreactive nerve fibers in the nasal epithelium of rodents. J Comp Neurol. 1990;294(2):293–305.
Anton F, Peppel P. Central projections of trigeminal primary afferents innervating the nasal mucosa: a horseradish peroxidase study in the rat. Neuroscience. 1991;41(2–3):617–28.
Anton F, Herdegen T, Peppel P, Leah JD. c-FOS-like immunoreactivity in rat brainstem neurons following noxious chemical stimulation of the nasal mucosa. Neuroscience. 1991;41(2–3):629–41.
Schaefer ML, Böttger B, Silver WL, Finger TE. Trigeminal collaterals in the nasal epithelium and olfactory bulb: a potential route for direct modulation of olfactory information by trigeminal stimuli. J Comp Neurol. 2002;444(3):221–6.
Cain WS, Murphy CL. Interaction between chemoreceptive modalities of odour and irritation. Nature. 1980;284(5753):255–7.
Livermore A, Hummel T, Kobal G. Chemosensory event-related potentials in the investigation of interactions between the olfactory and the somatosensory (trigeminal) systems. Electroencephalogr Clin Neurophysiol. 1992;83(3):201–10.
Kobal G, Hummel T. Olfactory and intranasal trigeminal event-related potentials in anosmic patients. Laryngoscope. 1998;108(7):1033–5.
Hummel T, Doty RL, Yousem DM. Functional MRI of intranasal chemosensory trigeminal activation. Chem Senses. 2005;30(suppl_1):i205–6.
Inokuchi A, Kimmelman CP, Snow JB. Jr. Convergence of olfactory and nasotrigeminal inputs and possible trigeminal contributions to olfactory responses in the rat thalamus. Eur Arch Otorhinolaryngol. 1993;249(8):473–7.
Boyle JA, Frasnelli J, Gerber J, Heinke M, Hummel T. Cross-modal integration of intranasal stimuli: a functional magnetic resonance imaging study. Neuroscience. 2007;149(1):223–31.
Gao L, Hu J, Zhong C, Luo M. Integration of CO2 and odorant signals in the mouse olfactory bulb. Neuroscience. 2010;170(3):881–92.
Viana F. Chemosensory properties of the trigeminal system. ACS Chem Neurosci. 2011;2(1):38–50.
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–29.
Yonemitsu T, Kuroki C, Takahashi N, Mori Y, Kanmura Y, Kashiwadani H, et al. TRPA1 detects environmental chemicals and induces avoidance behavior and arousal from sleep. Sci Rep. 2013;3:3100.
Inui K, Chen C, Pauli JL, Kuroki C, Tashiro S, Kanmura Y, et al. Nasal TRPA1 mediates irritant-induced bradypnea in mice. Physiological reports. 2016;4(24):e13098.
Riera C, Menozzi-Smarrito C, Affolter M, Michlig S, Munari C, Robert F, et al. Compounds from Sichuan and Melegueta peppers activate, covalently and non‐covalently, TRPA1 and TRPV1 channels. Br J Pharmacol. 2009;157(8):1398–409.
Fothergill LJ, Callaghan B, Rivera LR, Lieu T, Poole DP, Cho HJ, et al. Effects of food components that activate TRPA1 receptors on mucosal ion transport in the mouse intestine. Nutrients. 2016;8(10):623.
Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang D-S, Woolf CJ, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50(2):277–89.
Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, et al. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol. 2011;7(10):701–11.
Luis-Delgado OE, Barrot M, Rodeau JL, Schott G, Benbouzid M, Poisbeau P, et al. Calibrated forceps: a sensitive and reliable tool for pain and analgesia studies. J Pain. 2006;7(1):32–9.
Kashiwadani H, Kanmura Y, Kuwaki T. Application of calibrated forceps for assessing mechanical nociception with high time resolution in mice. PLoS One. 2017;12(2):e0172461.
Dunnett CW. Pairwise multiple comparisons in the unequal variance case. J Am Stat Assoc. 1980;75(372):796–800.
Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493(4):596–606.
Rigoni M, Trevisani M, Gazzieri D, Nadaletto R, Tognetto M, Creminon C, et al. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br J Pharmacol. 2003;138(5):977–85.
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24.
Sato M, Sato T, Yajima T, Shimazaki K, Ichikawa H. The transient receptor potential cation channel subfamily V members 1 and 2, P2X purinoceptor 3 and calcitonin gene-related peptide in sensory neurons of the rat trigeminal ganglion, innervating the periosteum, masseter muscle and facial skin. Arch Oral Biol. 2018;96:66–73.
Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–41.
Nealen ML, Gold MS, Thut PD, Caterina MJ. TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. J Neurophysiol. 2003;90(1):515–20.
Abe J, Hosokawa H, Okazawa M, Kandachi M, Sawada Y, Yamanaka K, et al. TRPM8 protein localization in trigeminal ganglion and taste papillae. Mol Brain Res. 2005;136(1):91–8.
Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R. Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol. 2004;141(4):737–45.
Vilceanu D, Stucky CL. TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PloS one. 2010;5(8):e12177-e.
Zappia KJ, O’Hara CL, Moehring F, Kwan KY, Stucky CL. Sensory neuron-specific deletion of TRPA1 results in mechanical cutaneous sensory deficits. eNeuro. 2017;4(1):ENEURO.0069-16.2017.
Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav. 1978;20(2):175–85.
Cometto-MuñIz JE, Cain WS, Abraham MH, Kumarsingh R. Trigeminal and olfactory chemosensory impact of selected terpenes. Pharmacol Biochem Behav. 1998;60(3):765–70.
Carlson KS, Xia CZ, Wesson DW. Encoding and representation of intranasal CO2 in the mouse olfactory cortex. J Neurosci. 2013;33(34):13873–81.
Yousem DM, Williams SC, Howard RO, Andrew C, Simmons A, Allin M, et al. Functional MR imaging during odor stimulation: preliminary data. Radiology. 1997;204(3):833–8.
Mainland JD, Keller A, Li YR, Zhou T, Trimmer C, Snyder LL, et al. The missense of smell: functional variability in the human odorant receptor repertoire. Nature Neurosci. 2014;17(1):114.
Pietraszewska-Bogiel A, van Weeren L, Goedhart J. Seeing cells smell: dynamic optical measurements of Ca2+ and cAMP signaling from olfactory receptors transiently expressed in HEK293TN cells. bioRxiv. 2019. https://doi.org/10.1101/771261.
Trimmer C, Keller A, Murphy NR, Snyder LL, Willer JR, Nagai MH, et al. Genetic variation across the human olfactory receptor repertoire alters odor perception. Proc Natl Acad Sci USA. 2019;116(19):9475–80.
Gonzalez-Kristeller DC, do Nascimento JBP, Galante PAF, Malnic B. Identification of agonists for a group of human odorant receptors. Front Pharmacol. 2015. https://doi.org/10.3389/fphar.2015.00035.
Acknowledgements
We thank the members of the Department of Physiology and Dental Anesthesiology for useful discussion. We also acknowledge the Joint Research Laboratory and Laboratory of Animal Science at the Kagoshima University Graduate School of Medical and Dental Sciences for use of their facilities.
Funding
This work was supported by JSPS KAKENHI Grant Number 26670290 (HK) and Smoking Research Foundation (to TK and HK).
Author information
Authors and Affiliations
Contributions
YH and HK designed, conducted the study, and analyzed the data. MS and TK supervised the study, and YH, MS, TK, and HK wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experiments were conducted in accordance with guiding principles for the care and use of animals in the field of physiological sciences published by the Physiological Society of Japan (2015) and were approved by the institutional Experimental Animal Research Committee of Kagoshima University (MD13007, MD16092, MD17105).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kashiwadani, H., Higa, Y., Sugimura, M. et al. Linalool odor‐induced analgesia is triggered by TRPA1-independent pathway in mice. Behav Brain Funct 17, 3 (2021). https://doi.org/10.1186/s12993-021-00176-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12993-021-00176-y