Abstract
Purpose
Epilepsy is a disease of neurological character. Approximately one third of epileptic patients demonstrate a drug-resistant phenotype, which is associated with the development of drug-resistant epilepsy. The multidrug resistance protein 1 and glycoprotein P, encoded by MDR1, play a significant role in the transmembrane transport of anti-epileptic agents. Single nucleotide polymorphism C3435T (rs1045642) within MDR1 gene may be associated with an increased expression of P-gp which affects the levels of antiepileptic drugs in plasma. The presented studies analysed the association between C3435T polymorphism of MDR1 gene and the incidence of drug-resistant epilepsy in the population of Polish children.
Methods
C3435T polymorphism of MDR1 gene was analysed by the high resolution melting technique in a group of patients with drug-resistant (n = 106) and drug-responsive epilepsy (n = 67), as well as in non-epileptic children (n = 98) hospitalised at the Department of Neurology, Polish Mother’s Memorial Hospital in Lodz. Genotype and allele distributions were evaluated and their compatibility with the Hardy–Weinberg distribution was assessed by means of the χ2 test. Genotype and allele evaluation, regarding their relationship with a given feature, was supported by an analysis of odds ratio and 95 % confidence interval, calculated according to the logistic regression model.
Results
An association was observed between the incidence rate of DRE and the presence of C allele in C3435T polymorphism of MDR1 gene, which may enhance the risk of the disease. The T allele may then play a protective role. No differences were found in the studied groups, regarding either genotype or allele distribution in reference to patient’s gender or concomitant diseases.
Conclusion
Following the obtained results, C3435T polymorphism of MDR1 gene may be connected with the incidence of drug-resistant epilepsy in the population of Polish children.
ISRCTN ISRCTN73824458. Registered 28th September 2014.
Similar content being viewed by others
Background
Epilepsy is one of the most frequent diseases of the nervous system, with prevalence rate estimated at approx. 1 % of the population in the world. The incidence of epilepsy is slightly higher in men than in women, while presenting significantly higher rates in children and in subjects above 65 years. The average incidence rate in the world population amounts to 50–70/100,000/year. In Poland, the number of affected subjects approaches 400,000 with the incidence of 7/1000 inhabitants [1]. For comparison, in the Asian populations, e.g., in Japan, the incidence is 1.5/1000 and in India, 5.59/1000 [1, 2].
The basic treatment of epilepsy consists of a wide range of pharmacological substances—anticonvulsant medications. However, despite the constant development of new anticonvulsants with various molecular mechanisms of action, the fully effective pharmacological treatment of epilepsy has not yet been reached. In some of the patients, so-called, DRE may occur, as a serious clinical problem.
There are two main theories to explain the phenomenon of drug-resistance. The hypothesis of transporters assumes that the expression or function of multidrug transporters gets enhanced in the brain, thus suppressing the access of antiepileptic drugs to the central nervous system. The other hypothesis is based on the assumption that epilepsy-related changes induce lower sensitivity to anticonvulsant [3].
Despite the increasing knowledge of suspected risk factors of DRE, it still unclear why the responsiveness to pharmacological treatment may vary significantly between epilepsy patients with supposedly similar clinical manifestations of the disease. The suggested determinants of DRE include genetic factors and varying pharmacodynamic and pharmacokinetic features of administered drugs [4–7].
The to-date’s literature data indicate a significant role of MDR 1 and P-gp, encoded by MDR1, in the transmembrane transport of anti-epileptic agents [8–10]. The C3435T polymorphism (rs1045642) of MDR1 gene has been suggested as one of the genetic factors underlying DRE phenomenon [8–13]. It has been demonstrated that C3435T polymorphism determines the modified expression of MDR1 gene and thus the activity of P-gp, which plays a significant role in the transmembrane drug transport [10]. It has been demonstrated that P-gp participates in the transport of phenobarbital, carbamazepine, phenytoin, lamotrigine, felbamate and gabapentin through the blood–brain barrier, as well as through the cellular membranes of astrocytes and neurons in an epileptic focus. The results of the studies indicate participation of P-gp in the pathogenesis of DRE [10].
The MDR1 gene polymorphism, while structuring the variable expression of P-gp, may then control the efficacy of anti-epileptic therapy. An increased expression of this gene leads to higher volumes of P-gp in the endothelial cells of the blood–brain barrier and in astrocytes. This process results in decreasing the parenchymal concentrations of drugs, despite achieved therapeutic level in blood [10, 13]. Importantly, in patients with DRE, evaluated by van Vliet et al. and Lazarowski et al., the homozygotic CC genotype was associated with increased P-gp expression, which affected the concentration levels of anti-epileptic drugs in plasma [10, 13].
However, the currently available literature data lack an unequivocal response to the question about the exact role of the MDR1 gene C3435T polymorphism in the development of DRE in children [12–17].
The goal of the study was to analyse the association between C3435T polymorphism of MDR1 gene and DRE in the population of Polish children.
Methods
Patients
Whole blood samples (1 ml), used as material for molecular studies, were collected from children with DRE (n = 106) and drug-responsive (n = 67) epilepsy, as well as from non-epileptic children (n = 98), hospitalised at the Department of Neurology, Polish Mother’s Memorial Hospital, Research Institute, in the years 2010–2016. The group of children with DRE included 52 boys and 54 girls and the group of therapy responders included 35 boys and 32 girls, while the group of non-epileptic children comprised 52 boys and 46 girls. The demographic parameters of the study groups were not significantly different (see Table 1). All of the studied individuals, patients and controls were Caucasians from the same ethnic and geographical origins, living in the Lodz region of central Poland.
All the epilepsy patients were subjected to pharmacotherapy. The administered medicinal agents included: CBZ, topiramat, OXC, GBP, LTG and LEV—all in various doses and configurations, depending on patient’s health status. A formal consent was obtained from the Bioethical Committee of the Institute of the Polish Mother’s Memorial Hospital in Lodz (decision of 15th December 2010). Genetic studies of MDR1 gene C3435T polymorphism were performed at the Laboratory of Cancer Genetics, Department of Clinical Pathomorphology, Institute of the Polish Mother’s Memorial Hospital in Lodz.
HRM analyses
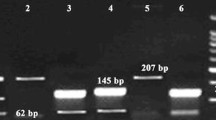
Genomic DNA was prepared, using DNeasy Blood & Tissue Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer instruction. PCR products for the analysed variants were analysed by HRM analysis. Real-time PCR cycling and conditions and primers for HRM analysis of all the examined MDR1 SNPs are summarized in Table 2. The analysis of the SNPs was performed with support of a Light Cycler® 480 High Resolution Melting Master Kit (Roche, Mannheim, Germany), according to the manufacturer’s recommendations. A non-template control contained water, instead of genomic DNA, as a negative control. Additionally, positive controls (DNA samples with known genotype) were employed in each run of HRM analysis. PCR amplification was performed in a LightCycler® 96 (Roche, Mannheim, Germany) Thermocycler. The collected data were analysed, using the LightCycler® 96 software version SW 1.1 (Roche, Mannheim, Germany).
Statistical analysis
Genotype and allele distributions were evaluated and their compatibility with HWE distribution was assessed by means of the χ2 test. Differences between distributions in particular groups were evaluated also by the χ2 test. All multivariate models were adjusted for age and use of variety of drugs. T test (for normal distribution) or Mann–Whitney test (for non-normal distribution) was used to compare each parameter between two groups. An ANOVA test was used to identify parameters that would make significant differences between more than two groups; Scheffe’s test was then used to test the significance of difference in each identified parameter between any two groups. Genotype and allele evaluation, regarding their relationship with a given feature, e.g., a risk for disease, was supported by an analysis of OR and 95 % CI, calculated according to the logistic regression model. The wild type of genotype and allele was a reference group. The Statistica v. 7.0 software package (StatSoft, Tulsa, OK, USA) was used.
Results
It was demonstrated that the MDR1 gene C3435T polymorphism may be associated with the incidence of DRE in Polish children.
See Table 3 for the distribution of genotypes and the incidence of alleles in the group of DRE and in the group of non-epileptic children. Statistically significant differences were demonstrated in genotype distribution between the study groups (p < 0.05). The CC genotype occurred in the patients with statistically significantly higher frequency, while the TT homozygote was found with statistically significantly lower frequency vs. the group of healthy controls (p < 0.05). It was demonstrated that the C allele in those children may be a risk factor for DRE, unlike the T allele which, by contrast, may play a protective role (OR 0.53; 95 % CI, 0.33–0.85; p = 0.012).
The observed genotype frequency of MDR1 C3435T in the controls group were in agreement with HWE (p > 0.05). In case of the C3435T polymorphism of MDR1 gene the distribution of the genotypes in the patients differed significantly from one expected from HWE (p < 0.05). It is caused by the very low abundance of the MDR1 TT genotype in the examined Polish population.
Similar results were observed when the group of epileptic non-responders was compared with the responders (see Table 4). The CC homozygote in the patients with DRE occurred with a statistically significantly higher frequency (33 %), while the TT homozygote demonstrated a statistically significantly lower frequency (30 %) vs. the group of children with therapy-responding epilepsy (CC-24 %, TT-49 %). The C allele in the patients with DRE occurred with a statistically significantly higher frequency (51 %), while the T allele demonstrated a statistically significantly lower frequency (49 %) vs. the group of children therapy-responding epilepsy (C-37 %, T-63 %). The C allele in those children may be a risk factor for DRE. The T allele may then play a protective role (OR 0.56 95 % CI 0.36–0.87; p = 0.014).
No statistically significant differences were demonstrated in the distribution of genotypes between the group of responders and healthy controls (p > 0.05) (see Table 5).
No differences were found in the study groups, either in genotype or allele distribution in reference to patient’s gender or concomitant diseases (p > 0.05).
Discussion
The MDR1 gene polymorphism, while structuring the variable expression of P-gp, may then control the efficacy of anti-epileptic therapy. An increased expression of this gene leads to higher volumes of P-gp in the endothelial cells of the blood–brain barrier and in astrocytes. This process results in decreased parenchymal concentrations of drugs, despite their achieved therapeutic levels in blood [18, 19]. It should be emphasised that the majority of data from world literature concerns SNPs of MDR1-C3435T.
In the presented study, a genetic evaluation was performed of the MDR1 gene C3435T polymorphism in order to define its association with DRE in the population of Polish children. This polymorphism was selected on the basis of the earlier mentioned literature data, which are suggestive of its correlations with DRE [8–11]. Studies of this type have been rather scarce in the world perspective, what raised our interest in this challenging issue.
The polymorphisms of MDR1 gene, including C3435T polymorphism, were analysed by Alpman et al. [17] in a group of 39 children with DRE vs. a control group (n = 92). A conclusion was drawn that the polymorphisms of MDR1 gene were not associated with multidrug resistance but that CC3435/GG2677 genotypes might affect the efficacy of administered therapies in cases of DRE.
Shaheen et al. [15] studied the functional importance of C3435T polymorphism in a population of epileptic children in the southern India. The risk of non-responsiveness to therapy was much higher in patients, being the carriers of TT genotype vs. CC homozygotic patients. The researchers suggested that C3435T polymorphism could be related to DRE, however, further studies in different ethnic populations are necessary to explain the role of the polymorphism in DRE patients.
However, there are also reports which do not confirm the relationship between C3435T polymorphism of MDR1 gene and DRE in children [12, 14, 16].
In Poland, two reports have been published on this issue, describing a study of C3435T polymorphism in a group of children from the Silesian region [20, 21]. The obtained results suggest no relationship between the occurrence of DRE and the above-mentioned polymorphism of MDR1 gene in the population of Polish children. Following the authors’ suggestions, further genetic studies are necessary to better understand the causes of DRE, which may help in developing a more effective therapy.
In contrast to those reports, in this reported study, the influence of C allele on the risk of DRE in children was found and confirmed. An increased incidence rate of CC genotype was found in the group of non-responders to therapy vs. both the responders and healthy controls. A decreased incidence rate of TT homozygote was found in the group of non-responders to therapy vs. both the responders and healthy controls. It suggests that the T allele may play a protective role.
It is possible that the presence of MDR1 allele remains in a linkage disequilibrium with another, so far unknown, mutation, which may be important, regarding P-gp concentrations in plasma.
A number of hypotheses try to explain the relationship between the MDR1 C3435T polymorphism and the activity of P-gp. The first hypothesis assumes some conjugation of 3435T allele, which correlates with decreased transporter activity, with G2677 (A, T) changes, leading to modifications in the sequence of amino acids (Val > Ser, Val > Thr). A consequence of these changes is different stability and activity of this protein [22, 23].
Since this conjugation is not complete and its degree differs in particular populations, the above-mentioned correlation was not observed in all the reported studies. The presence of other, missense type, polymorphisms, e.g., C1236T, may be an additional worrying factor [22]. Another theory assumes a correlation of the C3435T polymorphism with other, still not identified changes in the regulatory domains of this gene, which may affect its expression levels. However, there is no reliable literature data to support this hypothesis.
The obtained results demonstrate a possibility of a relationship between C3435T polymorphism and the incidence rate of DRE in the population of the Polish children. However, it requires further studies on much larger study groups. Still, one cannot exclude a possible influence of the studied polymorphism on epilepsy development in result of combined effects with other factors. For the process of epilepsy development is complicated and demanding a complex participation of multiple factors.
Drawing conclusions from obtained data should be done with a substantial level of caution due to the limitations affecting this study: test group and controls may be quantitatively unsatisfactory, SNPs linkage disequilibrium was not considered, circulating P-gp levels in patients were unknown and the relation between SNP C3435T in MDR1 and P-gp expression has not been stated. Among the abundance of genetic polymorphisms in MDR1 gene only one polymorphism of MDR1 was analysed without respect to linkage disequilibrium, which is a crucial feature of population genetics. A joint analysis of SNP C3435T of MDR1 and other SNPs in MDR1 could shed a new light on the role of SNPs in DRE.
Abbreviations
- CBZ:
-
carbamazepine
- CI:
-
confidence interval
- DRE:
-
drug-resistant epilepsy
- GBP:
-
gabapentin
- HRM:
-
high resolution melting
- HWE:
-
Hardy-Weinberg equilibrium
- LEV:
-
levetiracetam
- LTG:
-
lamotrigine
- MDR1:
-
multidrug resistance protein 1
- OR:
-
odds ratio
- OXC:
-
oxcarbazepine
- P-gp:
-
P-glycoprotein
- SNP:
-
single nucleotide polymorphism
References
Jędrzejczak J, Zwoliński P. Padaczka. In: Kozubski W, Liberski PP, editors. Choroby układu nerwowego. Warszawa: Wydawnictwo Lekarskie PZWL; 2004. p. 442–66.
Udani V. Pediatric epilepsy - an Indian perspective. Indian J Pediatr. 2005;72:309–13.
Remy S, Beck H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain. 2006;129:18–35.
Löscher W, Potschka H. Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J Pharmacol Exp Ther. 2002;301:7–14.
Spear BB. Pharmacogenetics and antiepileptic drugs. Epilepsia. 2001;42:31–4.
Patsalos PN. Antiepileptic drug pharmacogenetics. Ther Drug Monit. 2000;22:127–30.
Marroni M, Marchi N, Cucullo L, Abbott NJ, Signorelli K, Janigro D. Vascular and parenchymal mechanism in multiple drug resistance: a lesson from human epilepsy. Curr Drug Targets. 2003;4:297–304.
Siddiqui A, Kerb R, Weale ME, Brinkmann U, Smith A, Goldstein DB, Wood NW, Sisodiya SM. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003;348:1442–8.
Tan NC, Heron SE, Scheffer IE, Pelekanos JT, McMahon JM, Vears DF, Mulley JC, Berkovic SF. Failure to confirm association of a polymorphism in ABCB1 with multidrug-resistant epilepsy. Neurology. 2004;63:1090–2.
Lazarowski A, Czornyj L. Potential role of multidrug resistant proteins in refractory epilepsy and antiepileptic drugs interactions. Drug Metabol Drug Interact. 2011;26:21–6.
Ponnala S, Chaudhari JR, Jaleel MA, Bhiladvala D, Kaipa PR, Das UN, Hasan Q. Role of MDR1 C3435T and GABRG2 C588T gene polymorphisms in seizure occurrence and MDR1 effect on anti-epileptic drug (phenytoin) absorption. Genet Test Mol Biomarkers. 2012;16:550–7.
Saygi S, Alehan F, Atac FB, Erol I, Verdi H, Erdem R. Multidrug resistance 1 (MDR1) 3435C/T genotyping in childhood drug-resistant epilepsy. Brain Dev. 2014;36:137–42.
van Vliet EA, Zibell G, Pekcec A, Schlichtiger J, Edelbroek PM, Holtman L, Aronica E, Gorter JA, Potschka H. COX-2 inhibition controls P-glycoprotein expression and promotes brain delivery of phenytoin in chronic epileptic rats. Neuropharmacology. 2010;58:404–12.
Sun G, Sun X, Guan L. Association of MDR1 gene C3435T polymorphism with childhood intractable epilepsy: a meta-analysis. J Neural Transm (Vienna). 2014;121:717–24.
Shaheen U, Prasad DK, Sharma V, Suryaprabha T, Ahuja YR, Jyothy A, Munshi A. Significance of MDR1 gene polymorphism C3435T in predicting drug response in epilepsy. Epilepsy Res. 2013;108:251–6.
Seven M, Batar B, Unal S, Yesil G, Yuksel A, Guven M. The drug-transporter gene MDR1 C3435T and G2677T/A polymorphisms and the risk of multidrug-resistant epilepsy in Turkish children. Mol Biol Rep. 2014;41:331–6.
Alpman A, Ozkinay F, Tekgul H, Gokben S, Pehlivan S, Schalling M, Ozkinay C. Multidrug resistance 1 (MDR1) gene polymorphisms in childhood drug-resistant epilepsy. J Child Neurol. 2010;25:1485–90.
Marchi N, Hallene KL, Kight KM, Cucullo L, Moddel G, Bingaman W, Dini G, Vezzani A, Janigro D. Significance of MDR1 and multiple drug resistance in refractory human epi-leptic brain. BMC Med. 2004;2:37.
Tishler DM, Weinberg KI, Hinton DR, Barbaro N, Annett GM, Raffel C. MDR1 gene expression in brain of patients with medically intractable epi-lepsy. Epilepsia. 1995;36:1–6.
Emich-Widera E, Likus W, Kazek B, Niemiec P, Balcerzyk A, Sieroń AL, Zak I. CYP3A5*3 and C3435T MDR1 polymorphisms in prognostication of drug-resistant epilepsy in children and adolescents. Biomed Res Int. 2013;2013:526837.
Emich-Widera E, Likus W, Kazek B, Sieroń AL, Urbanek K. Polymorphism of ABCB1/MDR1 C3435T in children and adolescents with partial epilepsy is due to different criteria for drug resistance - preliminary results. Med Sci Monit. 2014;20:1654–61.
Tanabe M, Ieiri I, Nagata N, Inoue K, Ito S, Kanamori Y, Takahashi M, Kurata Y, Kigawa J, Higuchi S, Terakawa N, Otsubo K. Expression of P-glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)-1 gene. J Pharmacol Exp Ther. 2001;297:1137–43.
Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S, Lan LB, Schuetz JD, Schuetz EG, Wilkinson GR. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–99.
Authors’ contributions
Conceived and designed the experiments: MS, BS, HR. Performed the experiments – case group: BS, HR. Case group design and collect: MM, KP, MC, DS. Performed the experiments – control group: BS, HR. Analysed data: MS, BS, HR. Contributed reagents/materials/analysis tools MS. Contributed to the writing of manuscript: BS, HR, MS. All authors read and approved the final manuscript.
Acknowledgements
Authors acknowledge the financial support provided by the Institute of Polish Mother’s Memorial Hospital, Lodz, Poland, to conduct the study.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
The datasets supporting the conclusions of this article are included within the article.
Ethics approval
All the study participants gave a written informed consent. A formal consent was also issued by the Bioethical Committee of the Institute of the Polish Mother’s Memorial Hospital in Lodz (Approval number, 15.12.2010).
Funding
This work was supported by the Institute of Polish Mother’s Memorial Hospital, Lodz, Poland from the Statutory Development Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Stasiołek, M., Romanowicz, H., Połatyńska, K. et al. Association between C3435T polymorphism of MDR1 gene and the incidence of drug-resistant epilepsy in the population of Polish children. Behav Brain Funct 12, 21 (2016). https://doi.org/10.1186/s12993-016-0106-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12993-016-0106-z