Abstract
Background
Studies have shown that probiotics have an effect on reducing body fat on a strain-specific and dose–response bases. The purpose of this study was to evaluate the effect of a novel probiotic strain Lacticaseibacillus paracasei K56 on body fat and metabolic biomarkers in adult individuals with obesity.
Methods
74 adult subjects with obesity (body mass index ≥ 30 kg/m2, or percent body fat > 25% for men, percent body fat > 30% for women) were randomized into 5 groups and supplemented with different doses of K56 (groups VL_K56, L_K56, H_K56, and VH_K56: K56 capsules, 2 × 107 CFU/day, 2 × 109 CFU/day, 2 × 1010 CFU/day, 2 × 1011 CFU/day, respectively) or placebo (group Pla: placebo capsule) for 60 days. Subjects were advised to maintain their original dietary intake and physical activity. Anthropometric measurements, body composition assessment, and metabolic parameters were measured at baseline and after 60 days of intervention.
Results
The results showed that the L_K56 group had significant decreases in percent body fat (p = 0.004), visceral fat area (p = 0.0007), total body fat mass (p = 0.018), trunk body fat mass (p = 0.003), waist circumference (p = 0.003), glycosylated hemoglobin(p = 0.002) at the end of the study compared with baseline. There were non-significant reductions in Body weight and BMI in the L_K56, H_K56, VL_K56 groups, whereas increases were observed in the placebo and VH_K56 groups compared with baseline values. In addition, K56 supplementation modulated gut microbiota characteristics and diversity indices in the L-K56 group. However, mean changes in body fat mass, visceral fat area, weight, body mass index, waist circumference and hip circumference were not significantly different between groups.
Conclusions
The results suggest that supplementation with different doses of Lacticaseibacillus paracasei K56 has certain effect on reducing body fat and glycosylated hemoglobin, especially at a dose of 109 CFU/day.
Trial registration: clinicaltrials.gov Identifier: NCT04980599.
Similar content being viewed by others
Introduction
Obesity is a complex chronic disease defined as excessive or abnormal fat accumulation that adversely affects health [1]. In recent years, the increased prevalence of obesity has reached epidemic proportions, and presents a critical public health problem worldwide because of the substantial health risks associated with increased mortality from type 2 diabetes (T2D), hypertension, and cardiovascular diseases, as well as the incidence of some cancers [2]. However, safe and effective treatments for obesity are scarce and new strategies are needed to mitigate its substantial health effects.
Obesity is mostly a multifactorial disease due to obesogenic environments, psychosocial factors and genetic variants. In 2004, scientists have first reported that, gut microbiota as an important environmental factor affects energy harvest from the diet and energy storage in the host [3]. Since then, a large number of studies have explored the relationship between obesity and gut microbiota, and revealed that the changes in the gut microbial composition and function contribute to the pathophysiology of obesity [4,5,6] and that their modulation may aid in the prevention and treatment of this disease [7, 8].
Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host” [9]. Studies have shown potential therapeutic effects of probiotics on obesity and related metabolic disorders by influencing and maintaining the homeostasis of gut microbiota composition and function through various mechanisms of actions such as antimicrobial activity, enhancement of barrier function, immunomodulation [9, 10]. Lactic acid bacteria, specifically lactobacillus and Bifidobacterium are the most documented probiotics that appear to have beneficial effects of reducing fat mass, regulating glucolipid metabolism. Animal studies have shown that, Lactobacillus gasseri SBT2055 inhibits enlargement of visceral adipocytes, reduces body weight gain, improves glucose tolerance in rodents through anti-inflammatory effects and stimulation of energy expenditure [11, 12]. In another study, supplementation with Lactobacillus plantarum reduced fat mass and serum lipid profile concurrently with downregulation of lipogenic gene expression in the adipocytes, and modulated gut microbiota composition, resulting in reductions in the bodyweight of high fat diet (HFD) fed obese mice [13]. Similar results have been observed in other experiments in which probiotic Bifidobacterium longum supplemented to HFD-fed obese animals [14, 15]. In humans, supplementation with single species [16, 17] or multiple species of probiotics [18, 19] to overweight/obese subjects at various doses reduced abdominal adiposity, waist and hip circumference or improved glucolipid metabolism for varying extents. A recent meta-analysis highlighted a positive trend of probiotics supplementation in improving anthropometric measures of overweight and obese patients with associated metabolic diseases [20]. Interestingly, a strain-specific effect on body weight and metabolism of the probiotics has also been reported; Some clinical trials also suggest that the extent of anti-obesogenic effects of probiotics may depend on both the probiotic dose and viable form used [21, 22].
In a recently published animal study, a novel probiotic strain Lacticaseibacillus paracasei K56, isolated from the intestine of a healthy child, was treated by gavage at various doses to HFD-fed mice for 12 weeks. The results have shown that L. paracasei K56 significantly reduced body and fat mass and improved lipid metabolism [23]. In another animal study, administration of Lacticaseibacillus paracasei K56 effectively attenuated obesity parameters, such as body weight, insulin-resistance, plasma glucose and lipids; The beneficial effects may be related to the restored host gut microbiota [24]. This indicates that, K56 might be a promising probiotic strain for prevention and treatment of obesity and related metabolic disorders.
However, the beneficial effects of this novel probiotic strain have not been proved in humans, and the appropriate dosage for human administration needs to be evaluated. In this exploratory study, we aim to evaluate the metabolic effects of K56 and confirm the appropriate administration dose in humans preliminary.
Materials and methods
Test materials
The test materials were kindly provided by YILI industrial company Ltd. (China). The probiotic capsules contained different doses of L. paracasei K56 strain (very low dose: 1 × 107 colony forming units/capsule, low dose: 1 × 109 colony forming units/capsule, high dose: 1 × 1010 colony forming units/capsule, very high dose: 1 × 1011 colony forming units/capsule), and was standardized with maltodextrin and microcrystalline cellulose. The ingredients of the placebo capsule were similar to the probiotic capsule but without the addition of K56. The final products looked and tasted identical to each other. Participants were instructed to take two capsules per day before breakfast for 60 days.
Study participants
The participants were recruited for the study at the Huadong hospital affiliated to Fudan University, Shanghai, China. A total of 74 subjects with obesity were initially signed informed consent. The inclusion criteria were as follows: (1) Body mass index (BMI) ≥ 30 kg/m2, or percentage of body fat (PBF) assessed by electrical bioimpedance ≥ 25% formen and ≥ 30% for women; (2) Age >18 and ≤ 60 years. The selected subjects were excluded from the study if they had any of the following conditions: (1) Patients with severe chronic diseases (coronary heart disease, uncontrolled diabetes, hypertension, mental disorders, cancer, hepatic or renal dysfunctions, etc.) and their complications; (2) Patients with severe allergy, gastrointestinal diseases, immunodeficiency; (3) Hyperthyroidism or hypothyroidism, Cushing syndrome, or any other disease affecting the results of the study; (4) History of administration of drugs affecting body fat or functional foods/supplements for obesity improvement in the past two months; (5) Use of any weight control measures (diet, exercise, etc.) in the past month; (6) Participation in any other clinical trials within the previous 3 months; (7) Unable to maintain their current lifestyle during the study period. (8) Failure to take the study products as required, or failure to follow up on time.
Study design
This was a randomized, single blind, placebo controlled, pilot study and was approved by the Ethics Board Committee of Huadong Hospital (20200083), the protocol was registered at the U.S. National Institute of Health (clinicaltrials.gov Identifier: NCT04980599).
The recruitment was conducted through online enrollment questionnaires and telephone interviews, and subjects who met the inclusion criteria were scheduled for a baseline visit to assess their eligibility. Written informed consent was obtained from all eligible subjects who met the inclusion criteria and did not meet the exclusion criteria before enrollment. The subjects were then randomly assigned to one of the placebo group (Pla), very low dose K56 group (VL), low dose K56 group (L), high dose K56 group (H), and very high dose K56 group (VH) for a 60-day of intervention period. Randomization was performed using computer-generated random numbers by a statistician who had not participated in this study and group allocation was blinded to the participants.
The intervention period was lasted for 60 days, subjects were asked to take different doses of K56 or placebo capsules two capsules per day preferably before breakfast with the specific advice to maintain their previous dietary intake and physical activity, current treatments and lifestyles during the study period. At the first and second visit, investigators dispensed one bottle of test material (60capsules/bottle) to every participant, and to prevent any viability or shelf-life issues, capsules were delivered to participants in insulated bags with ice pack, and stored in refrigerator after delivery. During the intervention period, to make sure all participants to take capsules as we suggested, we made illustration about the usage of test material, and created a WeChat group in order to remind the participants to take capsules as we suggested every day. Compliance for the consumption of the test materials was assessed by counting the returned capsules at the second and the last visit. In addition, the investigators reviewed the questionnaires for missed doses submitted by the subjects every two weeks. The subjects also recorded about undesired adverse events and emergencies in the questionnaires. The Semi-quantitative food frequency questionnaire was used to monitor the changes of dietary habits and the daily walking step numbers recorded by motion recorder was used to monitor the changes of physical activity. Anthropometric measurements, body composition assessment and vital sign assessments were conducted at the day 0, day 30 and day 60 of the intervention period. Blood samples and fecal samples were collected for the biochemical and gut microbial analysis at the day 0 and day 60.
Outcomes
The primary outcomes were changes in body fat percentage (PBF) and visceral fat area (VFA) from baseline to day 60. Secondary outcomes were changes in BMI, body weight, waist circumference, muscle mass, and metabolic parameters from the baseline to day 60.
Body weight and body compositions, including body fat mass, percent body fat, visceral fat area, regional body fat mass, skeletal muscle mass were assessed using a bioelectrical impedance analysis machines (Inbody770, Biospace, Korea) while the subject was fasting and wearing only light underwear. BMI was calculated as body weight divided by the square of the height. Waist circumference was measured directly on the skin between the lowest rib margin and the iliac crest while the subject was in a standing position using a plastic measuring tape to the nearest 0.1 cm. After 10 min of rest, blood pressure was measured in a sitting position by a trained researcher using automatic BP monitor (U16, Omron,) on the left arm.
Blood samples were collected after 10–12 h overnight fasting, and were analyzed for serum total cholesterol (TCH), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), fasting blood glucose (FBG), insulin (INS), glycosylated hemoglobin (HbA1c), hepatic and renal functions, white blood cells by using routine laboratory methods at Hua Dong Hospital affiliated to Fudan University.
Fecal microbiome analysis
Sample collection and handling
Fecal samples were collected for microbiome analysis at the baseline and after 60 days of intervention. Participants were asked to use a fecal collection box and sterile fecal container which were provided by investigators prior to collection. Samples were transported to the laboratory on ice bags, after which they were frozen and stored at − 80 °C until use. Total genomic DNA from each sample was extracted using a Hipure Soil DNA Kit (Magen, Guangzhou, China) according to the manufacturer’s instructions and quantified with a Nanodrop spectrophotometer (Thermo Scientific, Waltham, MA, USA), A260/A280 ratios were measured to confirm the purity of DNA. DNA samples were snap frozen and stored at − 20 °C till used.
Real-time PCR analysis
The amplification, detection and melt curve analysis of DNA were performed on an ABI7900 Sequence Detection System (Applied Biosystems). The reaction mixture (10 μl) contained 5 μl 2 × Master mix, 0.2 μl of each of the forward and reverse primers, 1 μl of ROX, 1 μl of template DNA. The amplification program consisted of 1 cycle of 95 °C for 5 min; 40 cycles of 95 °C for 15 s, 60 °C for 1 min; followed by melting curve cycling. A standard curve from genomic DNA extracted from a pure K56 strain culture was used. Each plate was run with non-template control.
16S rDNA gene sequencing and bioinformatics analysis
The sequencing library was constructed using a MetaVX Library Preparation Kit (GENEWIZ, Inc., South Plainfield, NJ). Briefly, 20–30 ng of DNA was used to generate amplicons that cover V3 and V4 hypervariable regions of the 16 s rDNA gene of bacteria. The forward primer contains the sequence ‘CCTACGGRRBGCASCAGKVRVGAAT’ and the reverse primers contain the sequence ‘GGACTACNVGGGTWTCTAATCC’. The 25 μl PCR mixture was prepared with 2.5 μl of TransStart buffer, 2 μl of dNTPs, 1ul of each primer, 0.5 μl of TransStart Taq DNA polymerase and 20 ng template DNA. The PCR is performed by the following program: 3 min of denaturation at 94 °C, 24 cycles of 5 s at 95 °C, 90 s of annealing at 57 °C, 10 s of elongation at 72 °C, and a final extension at 72 °C for 5 min. Indexed adapters were added to the ends of the amplicons by limited cycle PCR. Finally, the library is purified with magnetic beads.
The concentration is detected by a microplate reader(Tecan, Infinite 200 Pro) and the fragment size is detected by 1.5% agarose gel electrophoresis which is expected at ~ 600 bp. Next generation sequencing was conducted on an Illumina Miseq Platform (Illumina, San Diego, USA). PE300 paired-end sequencing was performed according to the manufacturer’s instructions.
After sequencing, Illumina MiSeq raw data were sorted by sample using index sequences, and paired-end FASTQ files were generated for each sample. The sequencing adapter sequence and F/R primer sequence of the target gene region were removed, bases with Phred quality score lower than 20, and sequences less than 200 bp in length were removed using Cutadapt (v1.9.1, https://cutadapt.readthedocs.io/en/stable/). After sequencing, error-corrected paired-end sequences were assembled into one sequence, and sequences containing N and chimeric sequences were removed, resulting effective sequences for OUT clustering (The procedures were conducted by GENEWIZ, Inc., South Plainfield, NJ). VSEARCH (1.9.6) was used for clustering (sequence similarity is set to 97%) with reference data base Silva138. Then the representative sequences of OTUs were analyzed by RDP classifier (Ribosomal Database Program) Bayesian algorithm, and the community composition of each sample was counted at different species classification levels. Based on the obtained OTU analysis results, the α diversity information such as ACE, Shannon, Simpson and Chao1 indices were calculated to confirm the species diversity and uniformity of the microbial community in the sample using QIIME 1.9.1. Based on Bray–Curtis distance, beta diversity between samples (information about microbial community diversity between samples in comparison groups) was determined, and relationships between the samples were visualized using principal coordinate analysis (PCoA) plots. Linear discriminant analysis effect size (LEfSe) was performed using LEfSe software (v1.0, https://huttenhower.sph.harvard.edu/galaxy/).
Statistical analysis
Prism 8.0.1 (GraphPad, San Diego, CA, USA) was used for statistical analyses on body composition and blood parameters. For continuous variables, normality tests were performed using Shapiro–Wilk tests. Normally distributed data were expressed as mean ± standard deviation and were analyzed by one-way ANOVA test with multiple comparisons by controlling the false discovery rate (Benjamini, Krieger, & Yekutieli); data with skewed distribution were expressed as median (interquartile range) and were analyzed by Kruskal–Wallis test. To test the differences between the endpoint and baseline values, the paired t-test was conducted if the data were normally distributed or Wilcoxon signed rank test if the data distribution was skewed. Significant differences in the relative abundance of microbial phyla, genera, and alpha diversity were analyzed using R software. Kruskal–Wallis test was used for between group comparisons and Wilcoxon rank-sum test for within group comparisons. A false discovery rate (FDR) based on the Benjamini–Hoch-berg (BH) adjustment was applied for multiple comparisons. A p value < 0.05 was considered to be significant.
Results
Baseline characteristics of subjects
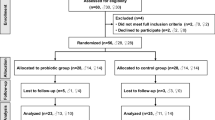
The study populations and reasons for exclusions are shown in Fig. 1. Seventy-four eligible subjects were enrolled in this study and randomized into five groups for 60-day intervention. Two subjects were dropped-out during the intervention period for personal reasons (one in the L_K56 group and one in the H_K56 group). A total of seventy-two subjects completed the 60-day intervention; however, one subject with < 85% treatment compliance, five subjects who administered antibiotics within two weeks before sample collection were excluded from the analysis. Therefore, a total of 66 subjects were included in the data analysis. No adverse events were reported as reasons for dropout. The baseline characteristics of the subjects who completed the study without major protocol violations are summarized in Table 1. The demographics of the subjects were similar among the different groups. There were no significant differences between groups in anthropometric variables, lipid profiles, glycosylated hemoglobin, fasting blood glucose and parameters of liver, renal functions at the baseline.
Food intake and activities
Subjects were advised to maintain their original dietary pattern and activity level throughout the intervention period. According to the questionnaires feedback and recorded step counts, most of the subjects were able to maintain the required consistency of dietary and activity habits throughout the intervention period. There were no significant differences between groups in dietary intake and habitual activity at the baseline and end of study.
Adverse events and safety parameters
The adverse events reported by participants during the intervention period included loose stools, feeling of incomplete evacuation, or flatulence, which were potentially product-related. Adverse events are summarized in Table 2. The symptoms were generally mild and of short duration, and there were no any dropouts occurred due to the adverse events. There were no significant abnormal changes in measured safety parameters: vital signs, renal and hepatic function markers (Table 3).
Efficacy analysis
K56 controls body and visceral fat, reduces waist circumference
The relative change in PBF from baseline to the end of the intervention period was the primary outcome of our study. After 60 days of probiotic intake, the mean value of PBF in L_K56 and H_K56 groups decreased compared with baseline values, especially the change in L_K56 group was statistically significant (− 0.867%, p = 0.004). In the placebo and VH_K56 groups, there were non-significant increases in PBF from baseline to 60 days (0.29%, 0.47%), resulting in significant differences in the mean value of changes in L_K56, H_K56 groups compared to placebo and VH_K56 groups. The total body fat mass was significantly reduced in the probiotic L_K56 group (− 0.72 kg, p = 0.018) at the end of the study compared with baseline. There were observations of non-significant reductions in BFM in the VL_K56, H_K56 groups, and non-significant increases in placebo and VH_K56 groups. Changes in body fat mass were most pronounced in the trunk area and a similar pattern was observed in the visceral fat area (Fig. 2). Body weight and BMI were not significantly reduced in L_K56, H_K56, VL_K56 groups, whereas there were increases in placebo and VH_K56 groups compared with baseline values, the change was statistically significant in group VH_K56. The results also indicate that, in L_K56 and H_K56 groups, there was a trend towards an increase in skeletal muscle mass. Regarding to waist and hip circumferences, the reduction in waist circumference from baseline (− 1.7 cm, p = 0.01) in L_K56 group and the increase in hip circumference from baseline (0.86 cm, p = 0.003) in VH_K56 group were statistically significant, while the changes in other groups were not significantly different. However, the mean change in BFM, VFA, weight, BMI, waist circumference and hip circumferences were not significantly different between groups.
Results of anthropometric and body composition variable measurements. The graphs show a percent body fat, b body fat mass, c body fat mass of trunk, d visceral fat area, e weight, f body mass index, g hip circumferences, h waist circumference, i skeletal muscle mass. The data points correspond to the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, comparison of baseline and 60 days values within groups (paired t-test); #p < 0.05, ##p < 0.01, ###p < 0.001, differences in changes in mean value from baseline to 60 days between groups (one-way ANOVA)
K56 decreased glycosylated hemoglobin compared to baseline, but did not affect lipid profiles
Table 4 shows blood lipid profile, insulin, glycosylated hemoglobin, glycated albumin, fasting blood glucose at baseline and after intervention. Serum total cholesterol, triglyceride, HDL-Cholesterol, LDL-Cholesterol were didn’t change significantly after 60 days intervention compared with baseline in all groups, and there were no significant differences in changes from baseline to 60 days between groups. A statistically significant reduction in glycosylated hemoglobin of L_K56 group was observed at the end of intervention compared with baseline, while the changes in other groups were not significant. In addition, the glycated albumin levels in placebo and VH_K56 groups were elevated from baseline with statistically significance, and the changes in other groups were not significant. However, there were not significant differences in changes of the abovementioned variables among all groups. Insulin concentration and C-peptides were not change significantly within groups, and did not differ significantly between groups.
Microbiome analyses
60 days supplementation with Lacticaseibacillus paracasei K56 increased fecal K56 levels determined by qPCR.
Table 5 shows the number of detected positive samples and average quantity of fecal K56 determined by qPCR at the baseline and after 60-day intervention. There were no significant differences in number of positive samples and average quantity of K56 between all groups at baseline. After 60-day of intervention, the number of samples with increased fecal K56 levels in all probiotic supplemented groups were significantly higher compared with the placebo group, where there were no elevated K56 levels in any subject.
60 days supplementation with Lacticaseibacillus paracasei K56 modulated gut microbial diversity and composition
Based on the results of the 16 rDNA sequences (V3–V4 region) using MiSeq performed in all groups, the bacterial group was dominated by the phyla Bacteroidota, Firmicutes, Proteobacteria, Actinobacteriota and Fusobacteriota (Fig. 3A).
Bacterial abundance at phylum and genus level, alpha diversity, beta diversity. A Bacterial abundance at the phylum level at baseline and end of study in placebo and probiotic groups. B bacterial abundance at the genus level at baseline and end of study in placebo and probiotic groups. Boxplots show the alpha diversity of bacterial communities at baseline and after intervention in placebo and probiotic groups for C Shannon, D Simpson, E ACE, F Chao1 indices. G Principal coordinate analysis (PCoA) showing the microbial community distance between baseline and end of study in each group
A small portion of the phyla Desulfobacterota, Verrucomicrobiota, Cyanobacteria, Synergistetes, Patescibacteria Patescibacteria and campilobacterota appeared in probiotic and control groups. At baseline, bacterial phylum did not differ significantly between groups, and there were no significant changes in the abundance of bacterial phylum from baseline to end of intervention in all groups. Considering the Firmicutes to Bacteroides ratio, the H_K56 group had higher F/B ratio than other groups at the baseline, but there were no significant differences among all groups (median for Pla, VL_K56, L_k56, H_K56, VH_K56 groups were 0.65, 0.90, 0.75, 1.43, 0.99 respectively, p > 0.05). The changes of B/F ratio from baseline to end-of-study were not differed significantly among all groups (median of changes − 0.05, 0.01, 0.17, − 0.20, − 0.17 for Pla, VL_K56, L_K56, H_K56, VH_K56 respectively).
At the genus level (Fig. 3B), the abundance of genus Bacteroides at baseline was lower and the abundance of genus Prevotella was higher in L_K56 group than other groups, but they didn’t differ significantly between groups. After 60 days of intervention, the abundance of genus Parabacteroides in L_K56 group increased significantly from baseline (p = 0.01, Wilcoxon test). The genus Bacteroides, Alistipes, Parasutterella in L_K56 group experienced increases in abundance and genus Prevotella in L_K56 group experienced decrease in abundance through the intervention period. The genus Bacteroides was increased slightly in VH_K56 group. Agathobacter in H_k56 group was decreased significantly (p = 0.035).
Alpha diversity indices (Fig. 3C–F), including ACE index, Chao1 index, Shannon index and Simpson index, indicate the richness and evenness of gut microbial community. At the end of the intervention, there were significant increases in ACE, Shannon, Chao1 and Simpson indices from baseline in the L_K56 group; And significant decreases in ACE and Chao1 indices in the H_K56 group. The increases in ACE and Shannon indices in the L_K56 group were differed significantly from other groups. The β diversity of each group was examined by principal coordinate analysis (PCoA), the results showed that there were no significant differences from baseline to end of study in each group except for a slight separation in L_K56 group (Fig. 3G).
The differentially abundant taxa between baseline and end of study in each group was identified by Linear discriminant analysis Effect Size (Fig. 4). At phylum level, there were no significantly differentiated bacteria among top ten phyla in all groups. The abundance of class Coriobacteria and its members (order Coriobacterials, family Coriobacteriaceae, genus Collinsella) decreased significantly at the end of study compared to baseline in each group. The abundance of Parabacteroids distasonis in L_K56 group, Clostridium scindens in placebo group and Veillonella atypica in VH_K56 group were significantly increased than baseline.
Linear discriminant analysis (LDA) effect size (LEfSe) was used to calculate the taxa that best discriminated between the baseline and end of study in each group. Taxa that reached a linear discriminant analysis score (log10) > 2.0 are highlighted and labelled at taxonomic levels from phylum to species
Discussion
This study presents a comprehensive analysis of the effects of supplementation with a novel probiotic strain on obesity, metabolic parameters and gut microbiota in free-living adults with obesity. After 60 days of intervention period, we found that supplementation with Lacticaseibacillus paracasei K56 at a daily dose of 109 CFU determined beneficial effects on obesity and glucose metabolism by reducing body fat mass, body fat percentage, trunk fat mass and visceral fat area, waist circumferences and glycosylated hemoglobin. Since central fat has a greater negative impact on the metabolic risk associated with obesity, the reduction of fat compartment may represent a beneficial effect of probiotics, even though there was no significant reduction in body weight. However, the effects of K56 were no longer significant when the dose of supplementation was higher (1010 CFU/day) or lower (107 CFU/day), and at very high doses(1011 CFU/day), there was a trend toward opposite effects. Such dose–response effects of probiotics have been observed in a previous clinical study in which, after obese adults were randomized to receive low-dose lactobacillus gasseri BNR17 (BNR-L, 109 CFU/day), or high-dose BNR17 (BNR-H, 1010 CFU/day) for 12 weeks, reduced visceral adipose tissue was only observed in high dose of L. gasseri BNR17 group [21]. In a randomized clinical trial, obese subjects received 200 g/d fermented milk contains 108 CFU/g Lactobacillus gasseri SBT2055 for 12 weeks, the abdominal visceral and subcutaneous fat area reduced from baseline significantly by an average of 4.6% and 3.3% respectively [16]. However, when the concentrations of SBT2055 were 106 or 107 CFU/g, significant reductions were didn’t present, suggesting a possible diminution of effectiveness at lower doses [17]. However, in a recent study, researchers observed that there were no unequivocal relationships between the effect of probiotics and the dose [25]. In a previously reported animal study, high fat diet induced obese mice were treated by gavage five times a week with freshly prepared K56 (106 CFU/day or 108 CFU/day) alone or in combination with α- galactooligosaccharides for 12 weeks. After treatment, all probiotic groups significantly decreased body weight gain and visceral fat than high fat diet (HFD) group, especially at the dosage of 108CUF/day alone or combined with α-GOS had lower body weight and fat gain than 106 CFU/day group [23]. In another animal study, HFD-fed mice were administered K56 suspension of 107 CFU/day, 109 CFU/day, and 1011 CFU/day. After 10 weeks of intervention, the three K56 groups did lowered the weight gain and abdominal fat than HFD group, and there were no significant differences between the three k56 groups. However, the abdominal fat by MRI scanning in 107group was significantly higher than normal diet (ND) group, whereas no significant increases or comparable to ND group in 109 and 1011 groups. Moreover, regarding the impact to glucose metabolism, the AUC of oral glucose tolerance test was significantly reduced in 109 and 1011 groups than HFD group, especially in the 109group [24]. Lactobacillus johnsonni 3121 and Lactobacillus. rhamnosus 86 were also evaluated for their anti-obesity effects using a high-fat diet-induced obese mouse model. Daily oral administration of L. johnsonni 3121 and L. rhamnosus 86 for 12 weeks (1010 CFU/day) significantly improved serum lipid profile and downregulated the expression of genes related to adipogenesis and lipogenesis in epididymal white adipose tissue of high-fat diet fed obese mice (p < 0.05) [26]. Fat mass expansion of ketonic diet induced diabetic mice was ameliorated by treatment with Bifidobacterium animalis ssp. Lactis 420 at a dose of 1010 CFU/day (p = 0.020), and there was a marked trend of fat mass reduction by 109 CFU/day (p = 0.066) [27]. These findings suggest that, the dose probiotics need varies greatly depending on the strain. Although the recommended intake of probiotics is mainly between 107 and 1011 CFU/day, some strains have been shown to be efficacious at lower levels, while some requires substantially more [28]. In this study, obesity parameters such as PBF, VFA, BFM, WC were reduced significantly after treatment with K56 in L_K56 group, and trend to improvements were observed in VL_K56 and H_K56 groups. Although the results were not as robust as animal experiments, but generally consistent with the results of above-mentioned animal experiments in which the anti-obesity effects of K56 were evaluated.
An accumulating body of evidence has suggested that the gut microbiota of obese individuals is characterized by a decrease of α diversity, an alteration of β diversity, an increased abundance of phylum Firmicutes and Firmicutes-to-Bacteroidetes ratio, while some other studies have suggest that no significant difference existed in obese and lean individuals concerning Firmicutes/Bacteroidetes ratio and the abundance of Bacteroidetes [29]. Numerous mechanisms of action for probiotic-mediated weight loss have been proposed. These include the modification of the gut microbiota, reduction of intestinal permeability, and modulation of the immune system [10, 30]. In our study, concerning the changes in abundance of bacterial phyla after intervention period, there were no statistically significant changes in each group. This is in accordance with a previously reported clinical trial in which multi-species probiotic includes nine strains of Bifidobacterium and Lactobacillus altered the influence of microbiota on biochemical, physiological and immunological parameters, but it didn’t affect overall composition of gut microbiota after 12-weeks administration to obese, postmenopausal women. It is noteworthy that, low-dose K56 supplementation increased the abundance of genus Parabacteroides and species Parabacteroides distasonis significantly. According to previous papers, the gut microbial community of obese patients exhibited a significant decrease in the relative abundance of several Bacteroidetes taxa including Parabacteroides spp., Bacteroides spp. when compared to normal weight subjects and negatively correlated with body fat and waist circumferences [31, 32]. Besides, researchers recently have found that Parabacteroides distasonis could affect the proportion of secondary non-12α-hydroxylated bile acids and metabolism of glucose and lipid, ameliorate weight regain via increased thermogenesis [33]. Although bile acids were not analyzed in this study, it is possible that treatment with K56 induces weight loss in subjects with obesity by increasing the abundance of Parabacteroides distasonis species, followed by increased secondary non-12α-hydroxylated bile acids and increased thermogenesis. In addition, genus Bacteroides in L_K56 and VH_K56 groups, Alistipes and Parasutterella in L_K56 group each trended towards increased abundance in the gut after intervention. In an animal study, it has been reported that Bacteroides has protective effects against weight gain [34]. Alistipes, a genus belongs to Bacteroidetes phylum, has been reported to inversely correlated to adiposity, lipid, and glucose homeostasis parameters [35], and may have protective effects against some diseases, including liver fibrosis, colitis, cancer immunotherapy, and cardiovascular disease [36]. Parasutterella was reported to have potential role in bile acid maintenance and cholesterol metabolism [37]. After administration of K56, we also noted a trend of reduction in the abundance of Prevotella in L_K56 group. In a previous clinical trial, it has been reported that high abundance of Prevotellaceae and Veillonellaceae associated with obesity and impaired glucose metabolism [38]. Recently, researchers have proposed that high abundance of Prevotella, especially P. copri in the gut may be associated with excessive energy uptake and increase fat accumulation [39]. In addition, Individuals with reduced microbial gene richness present more pronounced dys-metabolism and low-grade inflammation that were the main characteristics of obesity, suggesting that reduced gut microbial diversity accompanied changes in key species is the decisive factor in obesity [40]. According to the ACE, Shannon, Simpson and Chao1 indices, there were significant changes in alpha diversity of the intestinal microbial community in L_k56 and H_K56 groups from baseline to end of study and the changes in alpha diversity in L_K56 group differed significantly from other groups. This in agreement with a previous RCT that also reported significant differences in alpha diversity after supplementation with probiotic Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 [41]. However, the PCoA scatter plot for baseline and after intervention didn’t differed in each group except for a slight trend to separation in L_K56 group. Taken together, the results indicate that K56 administration is expected to enrich the microbial community, modulate the gut microbiota associated with obesity. This is in consistent with previous preclinical study in which K56 supplementation restored the gut microbiota of HFD fed mouse and ameliorated HFD induced obesity and associated metabolic parameters such as blood glucose and lipid profile [24]. But we didn’t observe significant changes in plasma lipid profile and fasting blood glucose in present study, except for a statistically significant reduction in HbA1c in L_K56 group. However, the average levels of plasma lipid and glucose were within normal range at baseline, and after 60 days of K56 supplementation in all groups. This result warrants further investigations in patients with hyperlipidemia and prediabetes to evaluate a metabolic benefit of K56. Also, this exploratory study enrolled a small number of individuals, which affects statistical power, especially when the effects of an intervention on clinical features were investigated. As a result, the study was not powered to deliver definitive conclusions on the end points related to energy balance. However, all the groups were randomized and investigated blindly. We may argue that any confounding factors were probably equally distributed between different groups. And we didn’t observe any improvements in Placebo group over the intervention period. Based on this exploratory study and preclinical animal studies, we could suggest that administration of K56 in adequate amount, may help improve obesity and related metabolic parameters, and the dosage as high as 1011 CFU/day is safe. If we take efficiency and economy into account, the dose of 109 CFU/day could probably be a better option. Meanwhile, this study was a promising start for future clinical trials with propriate design to confirm and extend our study results.
Conclusion
This was the first randomized single-blind placebo controlled exploratory study to investigate the effects of supplementation with a novel probiotic strain K56 in obese free-living adults. The results suggest that, under the condition of maintaining original dietary intake and physical activity, supplementation with different doses of Lacticaseibacillus paracasei K56 has certain effect on reducing body fat, improving glucose metabolism and modulating the gut microbiota to favor anti-obesity characteristics, especially at a dose of 109 CFU/day.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author Ying Feng, upon reasonable request.
Abbreviations
- BMI:
-
Body mass index
- BFM:
-
Body fat mass
- PBF:
-
Percent body fat
- VFA:
-
Visceral fat area
- HbA1:
-
Glycosylated hemoglobin
- GA:
-
Glycated albumin
- FBG:
-
Fasting blood glucose
- TCH:
-
Total cholesterol
- TG:
-
Triglycerides
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- DBP:
-
Diastolic blood pressure
- SBP:
-
Systolic blood pressure
- ANOVA:
-
Analysis of variance
References
World Health Organization. Obesity. https://www.who.int/health-topics/obesity. Accessed 20 Nov 2022.
Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6(12):944–53.
Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101(44):15718–23.
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102(31):11070–5.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31.
Aazmi S, Teh LK, Ramasamy K, Rahman T, Salleh MZ. Comparison of the anti-obesity and hypocholesterolaemic effects of single Lactobacillus casei strain Shirota and probiotic cocktail. Int J Food Sci Technol. 2015;50(7):1589–97.
Cani PD, Delzenne NM. Gut microflora as a target for energy and metabolic homeostasis. Curr Opin Clin Nutr Metab Care. 2007;10(6):729–34.
Gomes AC, Hoffmann C, Mota JF. Gut microbiota is associated with adiposity markers and probiotics may impact specific genera. Eur J Nutr. 2020;59(4):1751–62.
Cerdó T, García-Santos JA, Bermúdez M, Campoy C. The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients. 2019;11(3):635.
Abenavoli L, Scarpellini E, Colica C, Boccuto L, Salehi B, Sharifi-Rad J, Aiello V, Romano B, De Lorenzo A, Izzo AA, Capasso R. Gut microbiota and obesity: a role for probiotics. Nutrients. 2019;11(11):2690.
Kadooka Y, Ogawa A, Ikuyama K, Sato M. The probiotic Lactobacillus gasseri SBT2055 inhibits enlargement of visceral adipocytes and upregulation of serum soluble adhesion molecule (sICAM-1) in rats. Int Dairy J. 2011;21(9):623–7.
Shirouchi B, Nagao K, Umegatani M, Shiraishi A, Morita Y, Kai S, Yanagita T, Ogawa A, Kadooka Y, Sato M. Probiotic Lactobacillus gasseri SBT2055 improves glucose tolerance and reduces body weight gain in rats by stimulating energy expenditure. Br J Nutr. 2016;116(3):451–8.
Soundharrajan I, Kuppusamy P, Srisesharam S, Lee JC, Sivanesan R, Kim D, Choi KC. Positive metabolic effects of selected probiotic bacteria on diet-induced obesity in mice are associated with improvement of dysbiotic gut microbiota. FASEB J. 2020;34(9):12289–307.
Karimi G, Jamaluddin R, Mohtarrudin N, Ahmad Z, Khazaai H, Parvaneh M. Single-species versus dual-species probiotic supplementation as an emerging therapeutic strategy for obesity. Nutr Metab Cardiovasc Dis. 2017;27(10):910–8.
Rahman MS, Kang I, Lee Y, Habib MA, Choi BJ, Kang JS, Park DS, Kim YS. Bifidobacterium longum subsp. infantis YB0411 inhibits adipogenesis in 3T3-L1 pre-adipocytes and reduces high-fat-diet-induced obesity in mice. J Agric Food Chem. 2021;69(21):6032–42.
Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64(6):636–43.
Kadooka Y, Sato M, Ogawa A, Miyoshi M, Uenishi H, Ogawa H, Ikuyama K, Kagoshima M, Tsuchida T. Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal adiposity in adults in a randomised controlled trial. Br J Nutr. 2013;110(9):1696–703.
Gomes AC, Machado de Sousa RG, Botelho PB, Nogueira Gomes TL, Prada PO, Mota JF. The additional effects of a probiotic mix on abdominal adiposity and antioxidant status: a double-blind, randomized trial. Obesity. 2017;25(1):30–8.
Szulinska M, Loniewski I, van Hemert S, Sobieska M, Bogdanski P. Dose-dependent effects of multispecies probiotic supplementation on the lipopolysaccharide (LPS) level and cardiometabolic profile in obese postmenopausal women: a 12-week randomized clinical trial. Nutrients. 2018;10(6):773.
Perna S, Ilyas Z, Giacosa A, Gasparri C, Peroni G, Faliva MA, Rigon C, Naso M, Riva A, Petrangolini G, Redha AA, Rondanelli M. Is probiotic supplementation useful for the management of body weight and other anthropometric measures in adults affected by overweight and obesity with metabolic related diseases? A systematic review and meta-analysis. Nutrients. 2021;13(2):666.
Kim J, Yun JM, Kim MK, Kwon O, Cho B. Lactobacillus gasseri BNR17 supplementation reduces the visceral fat accumulation and waist circumference in obese adults: a randomized, double-blind, placebo-controlled trial. J Med Food. 2018;21(5):454–61.
Stenman LK, Lehtinen MJ, Meland N, Christensen JE, Yeung N, Saarinen MT, Courtney M, Burcelin R, Lahdeaho M-L, Linros J, Apter D, Scheinin M, Smerud HK, Rissanen A, Lahtinen S. Probiotic with or without fiber controls body fat mass, associated with serum zonulin, in overweight and obese adults-randomized controlled trial. EBioMedicine. 2016;13:190–200.
Min QQ, Sun T, Xu JY, Chen YZ, Liu WH, Zhao W, Hao JY, Zhao ZF, Hung WL, Qin LQ. Differential modulation of the metabolic effects of diet-induced obesity by probiotic Lactobacillus paracasei K56 and prebiotic alpha-galactooligosaccharides. Curr Top Nutraceutical Res. 2021;19(1):21–8.
Miao Z, Zheng H, Liu WH, Cheng R, Lan H, Sun T, Zhao W, Li J, Shen X, Li H, Feng H, Hung WL, He F. Lacticaseibacillus paracasei K56 attenuates high-fat diet-induced obesity by modulating the gut microbiota in mice. Probiotics Antimicrob Proteins 2022;35067837.
Kaczmarczyk M, Szulińska M, Łoniewski I, Kręgielska-Narożna M, Skonieczna-Żydecka K, Kosciolek T, Bezshapkin V, Bogdański P. Treatment with multi-species probiotics changes the functions, not the composition of gut microbiota in postmenopausal women with obesity: a randomized, double-blind, placebo-controlled study. Front Cell Infect Microbiol. 2022;12: 815798.
Lee CS, Park MH, Kim BK, Kim SH. Antiobesity effect of novel probiotic strains in a mouse model of high-fat diet-induced obesity. Probiotics Antimicrob Proteins. 2021;13(4):1054–67.
Stenman LK, Waget A, Garret C, Klopp P, Burcelin R, Lahtinen S. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef Microbes. 2014;5(4):437–45.
World Gastroenterology Organisation. https://www.worldgastroenterology.org/guidelines/probiotics-and-prebiotics. Accessed 1 Dec 2022.
Cheng Z, Zhang L, Yang L, Chu H. The critical role of gut microbiota in obesity. Front Endocrinol (Lausanne). 2022;13:1025706.
Mazloom K, Siddiqi I, Covasa M. Probiotics: how effective are they in the fight against obesity? Nutrients. 2019;11(2):258.
Palmas V, Pisanu S, Madau V, Casula E, Deledda A, Cusano R, Uva P, Vascellari S, Loviselli A, Manzin A, Velluzzi F. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci Rep. 2021;11(1):5532.
Gallardo-Becerra L, Cornejo-Granados F, García-López R, Valdez-Lara A, Bikel S, Canizales-Quinteros S, López-Contreras BE, Mendoza-Vargas A, Nielsen H, Ochoa-Leyva A. Metatranscriptomic analysis to define the Secrebiome, and 16S rRNA profiling of the gut microbiome in obesity and metabolic syndrome of Mexican children. Microb Cell Fact. 2020;19(1):61.
Li M, Wang S, Li Y, Zhao M, Kuang J, Liang D, Wang J, Wei M, Rajani C, Ma X, Tang Y, Ren Z, Chen T, Zhao A, Hu C, Shen C, Jia W, Liu P, Zheng X, Jia W. Gut microbiota-bile acid crosstalk contributes to the rebound weight gain after calorie restriction in mice. Nat Commun. 2022;13(1):2060.
Yoshida N, Yamashita T, Osone T, Hosooka T, Shinohara M, Kitahama S, Sasaki K, Sasaki D, Yoneshiro T, Suzuki T, Emoto T, Saito Y, Ozawa G, Hirota Y, Kitaura Y, Shimomura Y, Okamatsu-Ogura Y, Saito M, Kondo A, Kajimura S, Inagaki T, Ogawa W, Yamada T, Hirata KI. Bacteroides spp promotes branched-chain amino acid catabolism in brown fat and inhibits obesity. iScience. 2021;24(11): 103342.
Garcia-Ribera S, Amat-Bou M, Climent E, Llobet M, Chenoll E, Corripio R, Ibáñez L, Ramon-Krauel M, Lerin C. Specific dietary components and gut microbiota composition are associated with obesity in children and adolescents with Prader–Willi syndrome. Nutrients. 2020;12(4):1063.
Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A. The genus alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front Immunol. 2020;11:906.
Ju T, Kong JY, Stothard P, Willing BP. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. 2019;13(6):1520–34.
Serena C, Ceperuelo-Mallafré V, Keiran N, Queipo-Ortuño MI, Bernal R, Gomez-Huelgas R, Urpi-Sarda M, Sabater M, Pérez-Brocal V, Andrés-Lacueva C, Moya A, Tinahones FJ, Fernández-Real JM, Vendrell J, Fernández-Veledo S. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018;12(7):1642–57.
Chen C, Fang S, Wei H, He M, Fu H, Xiong X, Zhou Y, Wu J, Gao J, Yang H, Huang L. Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome. 2021;9(1):175.
Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto JM, Renault P, Doré J, Zucker JD, Clément K, Ehrlich SD. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–8.
Mo SJ, Lee K, Hong HJ, Hong DK, Jung SH, Park SD, Shim JJ, Lee JL. Effects of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 on overweight and the gut microbiota in humans: randomized, double-blinded, placebo-controlled clinical trial. Nutrients. 2022;14(12):2484.
Acknowledgements
The test materials were kindly provided by YILI industrial company Ltd. (China).
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
YF, GK, WLH, HF conceptualized and designed the study. GK and YF analyzed and interpreted the data; GK, WF, YH and YF performed the experiments; GK drafted the initial manuscript; YF and WHL reviewed and edited the writing; YF and WLH supervised the project; YF, WLH, WLH and WZ resources. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Board Committee of Huadong Hospital (protocol code 2020K203, 2020.12.28). Written informed consent was obtained from all participants prior to their inclusion in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kadeer, G., Fu, W., He, Y. et al. Effect of different doses of Lacticaseibacillus paracasei K56 on body fat and metabolic parameters in adult individuals with obesity: a pilot study. Nutr Metab (Lond) 20, 16 (2023). https://doi.org/10.1186/s12986-023-00739-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-023-00739-y