Abstract
Objective
This systematic review and meta-analysis of randomized controlled trials (RCTs) was conducted to determine the effect of alpha-lipoic acid (ALA) supplementation on the inflammatory markers among patients with metabolic syndrome (MetS) and related disorders.
Methods
We searched the following databases until November 2017: PubMed, MEDLINE, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials. Three reviewers independently assessed study eligibility, extracted data, and evaluated risk of bias of included primary studies. Statistical heterogeneity was assessed using Cochran’s Q test and I-square (I2) statistic. Data were pooled by using the random-effect model and standardized mean difference (SMD) was considered as the summary effect size.
Results
Eighteen trials out of 912 potential citations were found to be eligible for our meta-analysis. The findings indicated that ALA supplementation significantly decreased C-reactive protein (CRP) (SMD = − 1.52; 95% CI, − 2.25, − 0.80; P < 0.001), interlokin-6 (IL-6) (SMD = − 1.96; 95% CI, − 2.60, − 1.32; P < 0.001), and tumor necrosis factor alpha levels (TNF-α) (SMD = − 2.62; 95% CI, − 3.70, − 1.55; P < 0.001) in patients diagnosed with metabolic diseases.
Conclusion
In summary, the current meta-analysis demonstrated the promising impact of ALA administration on decreasing inflammatory markers such as CRP, IL-6 and TNF-α among patients with MetS and related disorders.
Similar content being viewed by others
Introduction
Increased pro-inflammatory markers and oxidative stress occurs in adipose tissues are the two factors that may play a key role in the incidence of metabolic-related comorbidities among patients with metabolic disorders [1]. Increased chronic inflammation is associated with increased risk of metabolic disorders, including type 2 diabetes mellitus (T2DM) [2] and arteriosclerosis, endothelial dysfunction, vascular calcification, increased activity of metalloproteinases, oxidative damage, and degradation of collagen [3,4,5]. It was reported that metabolic syndrome (MetS) is associated with a 2-fold increased risk of cardiovascular disease (CVD) over the next 5 to 10 years [6]. Inflammatory cytokines including interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are usually produced by different cells including endothelial, immune and arterial smooth muscle cells inducing the migration of additional immune cells into the atherosclerotic lesion and the activation of the acute phase C-reactive protein (CRP) in the liver [7, 8]. Increased levels of CRP are associated with increased risk of CVD and diabetes [9, 10]. In addition to CRP, other inflammatory biomarkers such as IL-6 and TNF-α may be correlated with the development of CVD in diabetic patients [11].
Complementary therapies such as antioxidants supplementation are recommended in patients with metabolic abnormalities to improve their nutritional status and boost their immune system [12]. Existing evidence has proved the beneficial effects of several antioxidants supplements including pentoxifylline [13] and lycopene [14] on reducing inflammation. Available data regarding the effects of alpha-lipoic acid (ALA) supplementation on inflammatory markers are controversial. In a study by Carbonelli et al. [15], obese Caucasian people showed significant reduction in CRP and TNF-α concentrations following ALA supplementation (800 mg/day) for 4 months. ALA supplementation (600–1000 mg/day) during a period ranging from 2 wk. to 1 year in patients with impaired glucose tolerance showed contradictory results. Zhang et al. [16] demonstrated that ALA supplementation decreased TNF-α and IL-6 while increased adiponectin levels, however others did not observe any beneficial effects of ALA on inflammatory markers [17, 18]. Discrepancies in these findings may be due to differences in study design, characteristics of study populations, dosage of ALA used and duration of the intervention.
We are aware of no systematic review or meta-analyses of randomized controlled trials (RCTs) evaluating the effect of ALA supplementation on inflammatory markers among patients with MetS and related disorders. Thus, the current meta-analysis was performed to summarize the available evidence regarding the effect of ALA supplementation on inflammatory markers among patients with MetS and related disorders.
Materials and methods
Search strategy and selection studies
We searched the following databases until November 2017: PubMed, MEDLINE, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials. Additionally, a manual search was conducted among the references lists of all eligible articles and review studies to identify potential articles that were not captured by the electronic searches. Three authors (VO, MM and MA) independently performed the literature search to retrieve RCTs that have examined the association between ALA supplementation and the inflammatory markers by using the following MeSH and text keywords: patients [“Mets” OR “disorders related to MetS” OR “diabetes” OR “T1DM” OR “T2DM” OR “overweight” OR “obese” OR “chronic kidney disease (CKD)” OR “hypertension” OR “high blood pressure” OR “dyslipidemia” OR “CVD”], intervention (“alpha-lipoic acid” OR “ALA” OR “α-lipoic acid” AND “supplementation” OR “intake”), and outcomes [“CRP” OR “IL-6” OR “TNF-α”]. Eligible studies were restricted to those RCTs published in English language.
Inclusion and exclusion criteria
RCTs were selected using the following inclusion criteria: being a placebo-controlled randomized trial (either parallel or cross-over designs), human studies conducted in adults, the target population was patients diagnosed with metabolic diseases, and studies reported mean changes between pre- and post-intervention CRP and/or IL-6 and/or TNF-α following ALA supplementation for the intervention and placebos groups. Other types of human studies (cross-sectional, cohort studies), animal, in vitro studies, and review papers were excluded. Case reports or cases series, and the studies did not achieve the minimum quality assessment score, those receiving any non-steroidal anti-inflammatory drug or ALA supplements within the last month were also excluded from the study.
Data extraction and quality assessment
Three authors (VO, MM, and MA) reviewed each trial and extracted all related data, independently. The disagreement among them was resolved by discussion with a fourth author (ZA). The quality of the included RCTs was assessed using the Cochrane Collaboration risk of bias tool based on the following information: randomization generation, allocation concealment, blinding of participants and outcome assessment, incomplete outcome data, and selective outcome reporting, as well as the other sources of bias. The extracted data included: first author, publication year, demographical variables, study design, sample size, dose of intervention, duration of study, type of intervention, type of disease, the mean and standard deviation (SD) for CRP, IL-6, and TNF-α.
Data synthesis and statistical analysis
We preformed a comprehensive electronic and manual search to avoid publication bias. Additionally, Egger’s regression test was used to assess publication bias statistically [19]. Statistical heterogeneity was assessed using Cochran’s Q and I-square (I2) tests [20]. I2 greater than 50% or P < 0.05 was considered as significant heterogeneity. We estimated the difference between intervention (ALA supplementation) and placebo group by calculating the standardized mean difference (SMD) with 95% confidence interval (CI) using STATA software version 12.0 (Stata Corp., College Station, TX) and RevMan V.5.3 software (Cochrane Collaboration, Oxford, UK). Since the indications could effect on pooled SMD were different between included studies, we used random-effects models to perform meta-analyses. Subgroup and sensitivity analyses were conducted to assess the source of heterogeneity and to explore the contribution of each study to the reliability of the pooled mean difference, respectively. P-values < 0.05 were considered as statistically significant.
Results
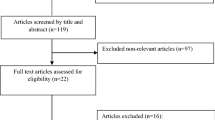
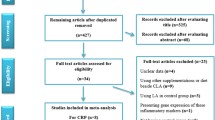
The process of the step by step study selection has shown in Additional file 1. Overall, 18 trials out of 912 potential citations were found to be eligible for this meta-analysis. Seven studies were RCTs design, and eleven were randomized, double-blind, placebo-controlled trials. Eleven trials have assessed the effects of ALA supplementation on CRP [21,22,23,24,25,26,27,28,29,30,31], eleven on IL-6 [16, 22,23,24,25, 30,31,32,33,34,35], and nine on TNF-α levels [16, 22, 24, 25, 28, 32, 33, 36, 37]. Intervention duration among included studies varied from 2 weeks to 12 months. The dosage of ALA supplements ranged from 300 to 600 (mg/day). Location of studies included; four studies in Italy [25, 28, 29, 33], three in Iran [23, 27, 37], three in China [16, 21, 22], two in Egypt [35, 36], one in Spain [30], one in Romania [32], two in United States [31, 34], one in Korea [26], and one in New Zealand [24]. Details of the included studies are summarized in Table 1. The quality of included trials is presented in Additional file 2.
Main outcomes
The results of current meta-analysis showed that ALA supplementation significantly decreased CRP (SMD = − 1.52; 95% CI, − 2.25, − 0.80; P < 0.001; I2: 93.7), IL-6 (SMD = − 1.96; 95% CI, − 2.60, − 1.32; P < 0.001; I2: 90.6), and TNF-α levels (SMD = − 2.62; 95% CI, − 3.70, − 1.55; P < 0.001; I2: 94.3) in patients with MetS and related disorders (Table 2 and Fig. 1).
We also performed subgroup analyses according to geographic area (Asia, European, USA, Oceania, and Africa), dosage of ALA supplements (> 600 vs. ≤600 mg/day), co-administration with other nutrients (ALA vs. ALA plus other nutrients), duration of the intervention (≥8 vs. < 8 weeks), and type of diseases (diabetic, ESRD vs. other diseases). We found that heterogeneity may decrease by duration of the intervention (< 8 weeks = I2: 89.9 and ≥ 8 weeks = I2:85.1 vs. overall I2:90.6%) and type of diseases (diabetic = I2: 75.1 and other = I2:89.5 vs. overall I2:90.6%) for IL-6 and type of diseases (diabetic = I2: 92.8 and other = I2:94.0 vs. overall I2:94.3%) for TNF-α levels (< 8 weeks = I2: 88.2 and ≥ 8 weeks = I2:92.1 vs. overall I2:94.5%). The detailed of subgroup analysis are presented in Table 3.
In sensitivity analysis, we found no significant difference between the pre- and post-sensitivity analysis for all inflammatory markers. The smallest and greatest pooled SMDs in the sensitivity analyses for the level of inflammatory markers are shown in Additional file 3. Egger’s regression tests showed no significant publication bias for the effects of ALA on CRP (B = − 11.35, P = 0.01). We found publication bias for IL-6 (B = − 6.88, P = 0.00) and TNF-α (B = − 7.28, P = 0.01), so we non parametric method was applied (Duval and Tweedie) to estimate the findings of censored studies. Findings showed that the summary of effect size for IL-6 and TNF-α did not significantly changed between before and after inclusion of censored studies for CRP (SMD = − 1.69; 95% CI, − 2.48, − 0.90), IL-6 (SMD = − 1.96; 95% CI, − 2.60, − 1.32), and TNF-α (SMD = − 2.62; 95% CI, − 3.70, − 1.55).
Discussion
This systematic review and meta-analysis assessed the effect of ALA supplementation on inflammatory markers in patients with MetS and related disorders. Our findings supported the beneficial impact of ALA administration on lowering inflammatory markers in patients suffering from metabolic syndrome and related disorders.
Few studies have reported the beneficial effects of antioxidant supplementation on inflammatory cytokines. In a meta-analysis conducted by Ju et al. [38], selenium supplementation significantly decreased serum CRP levels in patients with coronary heart disease, suggesting its potential impact on reducing inflammation in chronic conditions. In addition, supplementation with vitamin E in the form of either α-tocopherol or γ-tocopherol resulted in a significant reduction in CRP concentrations [39]. Available information regarding the effects of ALA supplementation on inflammatory cytokines is inconclusive. ALA supplementation for 12 months significantly decreased serum levels of common markers of inflammation in ablated patients [25]. Furthermore, dietary supplementation with ALA for 10 weeks significantly improved systemic inflammation and cardiovascular disease-related risk factors in healthy overweight women [30]. However, no benefits of resveratrol supplementation were reported on cardiovascular risk factors in the meta-analysis conducted by Sahebkar et al. [40]. In another study, taking ALA supplements for 8 weeks did not affect IL-8 and TNF-α levels in hemodialysis patients [37]. Increased inflammatory markers, especially TNF-α, might promote insulin resistance, and alter expression of cytokines in adipose tissues which is considered an important link between MetS and insulin resistance [41]. In addition, high levels of inflammatory markers in diabetic patients and those suffering from diabetic nephropathy are positively correlated with the severity of albuminuria [42]. Local inflammation plays also an important role in the development of diabetic retinopathy [43].
ALA intake may reduce inflammatory markers through scavenging free radicals, down-regulating pro-inflammatory redox-sensitive signal transduction processes including nuclear factor kappa B translocation, leading to lower release of other free radicals and cytotoxic cytokines [44, 45]. Moreover, ALA administration improves cellular antioxidant capacity and phases 2 enzymes such as catalase, reduced glutathione, glutathione reductase, and glutathione-S-transferase [46]. ALA can also inhibit the activation of serine kinases including IKKβ to suppress inflammatory cytokines [47]. Zhang et al. [48] mentioned to ALA potential to inhibit TNF-α-induced I kappa B kinase activation. It is speculated that the ALA treatment effects might be influenced by its baseline values and improved blood levels over time. In the current meta-analysis it was not possible to consider the effect of baseline ALA values in determining the impact of it on inflammatory markers. Furthermore, different geographical latitudes where study conducted might further complicate the effect of baseline ALA values. Overall, on top of those explained above, different study designs, sample size, different dosages of ALA used along with characteristics of study participants might explain the discrepancies among different studies.
There are several strengths for this study. Higher numbers of studies included in this analysis and longer period of supplementation in included trials have added to the value of this meta-analysis. All included studies were placebo-controlled randomized trials with acceptable methodological quality and the least probable chance of bias. Further, we relied on independent judgment in which different reviewers independently performed the systematic review process.
The current meta-analysis had a few limitations. There were few eligible RCTs, and most of them had a modest number of participants. Various doses of ALA were administered for intervention in the included studies. We were unable to evaluate the dose response association between supplementation dose and inflammatory markers due to the low number of studies included. In addition, we did not evaluate the residual confounding and bias of each study that could not be addressed through pooling. Considerable heterogeneity across studies made our findings complicated to interpret the main outcomes. Thus, evaluation of heterogeneity is a crucial part of any meta-analysis.
Conclusions
Overall, the current meta-analysis supported the beneficial impacts of ALA administration on decreasing inflammatory markers such as CRP, IL-6 and TNF-α among patients with MetS and related disorders.
Abbreviations
- ALA:
-
Alpha-lipoic acid
- CRF:
-
Chronic renal failure
- CRP:
-
C-reactive protein
- ESRD:
-
End-stage renal disease
- IL-6:
-
Interlokin-6
- IV:
-
Intravascular
- MetS:
-
Metabolic syndrome
- NIDDM:
-
Non-insulin-dependent diabetes mellitus
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- TNF-α:
-
Tumor necrosis factor alpha
References
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61.
Liu C, Feng X, Li Q, Wang Y, Hua M. Adiponectin, TNF-alpha and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine. 2016;86:100–9.
Mozos I, Malainer C, Horbanczuk J, Gug C, Stoian D, Luca CT, et al. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front Immunol. 2017;8:1058.
Dinh QN, Chrissobolis S, Diep H, Chan CT, Ferens D, Drummond GR, et al. Advanced atherosclerosis is associated with inflammation, vascular dysfunction and oxidative stress, but not hypertension. Pharmacol Res. 2017;116:70–6.
Elcioglu OC, Afsar B, Bakan A, Takir M, Ozkok A, Oral A, et al. Chronic rhinosinusitis, endothelial dysfunction, and atherosclerosis. Am J Rhinol Allergy. 2016;30:58–61.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; world heart federation; international atherosclerosis society; and International Association for the Study of obesity. Circulation. 2009;120:1640–5.
Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38.
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation. 2003;108:1664–72.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34.
Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97.
Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97:3a–11a.
Tabrizi R, Akbari M, Moosazadeh M, Lankarani KB, Heydari ST, Kolahdooz F, et al. The effects of selenium supplementation on glucose metabolism and lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res. 2017;49:826–30.
Brie D, Sahebkar A, Penson PE, Dinca M, Ursoniu S, Serban MC, et al. Effects of pentoxifylline on inflammatory markers and blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2016;34:2318–29.
Cheng HM, Koutsidis G, Lodge JK, Ashor A, Siervo M, Lara J. Tomato and lycopene supplementation and cardiovascular risk factors: a systematic review and meta-analysis. Atherosclerosis. 2017;257:100–8.
Carbonelli MG, Di Renzo L, Bigioni M, Di Daniele N, De Lorenzo A, Fusco MA. Alpha-lipoic acid supplementation: a tool for obesity therapy? Curr Pharm Des. 2010;16:840–6.
Zhang Y, Han P, Wu N, He B, Lu Y, Li S, et al. Amelioration of lipid abnormalities by alpha-lipoic acid through antioxidative and anti-inflammatory effects. Obesity (Silver Spring). 2011;19:1647–53.
McNeilly AM, Davison GW, Murphy MH, Nadeem N, Trinick T, Duly E, et al. Effect of alpha-lipoic acid and exercise training on cardiovascular disease risk in obesity with impaired glucose tolerance. Lipids Health Dis. 2011;10:217.
Mollo R, Zaccardi F, Scalone G, Scavone G, Rizzo P, Navarese EP, et al. Effect of alpha-lipoic acid on platelet reactivity in type 1 diabetic patients. Diabetes Care. 2012;35:196–7.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Xiang G, Pu J, Yue L, Hou J, Sun H. Alpha-lipoic acid can improve endothelial dysfunction in subjects with impaired fasting glucose. Metabolism. 2011;60:480–5.
Hong Y, Peng J, Cai X, Zhang X, Liao Y, Lan L. Clinical efficacy of Alprostadil combined with alpha-lipoic acid in the treatment of elderly patients with diabetic nephropathy. Open medicine (Warsaw, Poland). 2017;12:323–7.
Ahmadi A, Mazooji N, Roozbeh J, Mazloom Z, Hasanzade J. Effect of alpha-lipoic acid and vitamin E supplementation on oxidative stress, inflammation, and malnutrition in hemodialysis patients. Iran j kidney dis. 2013;7:461–7.
Manning PJ, Sutherland WH, Williams SM, Walker RJ, Berry EA, De Jong SA, et al. The effect of lipoic acid and vitamin E therapies in individuals with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2013;23:543–9.
Sardu C, Santulli G, Santamaria M, Barbieri M, Sacra C, Paolisso P, et al. Effects of alpha lipoic acid on multiple cytokines and biomarkers and recurrence of atrial fibrillation within 1 year of catheter ablation. Am J Cardiol. 2017;119:1382–6.
Chang JW, Lee EK, Kim TH, Min WK, Chun S, Lee KU, et al. Effects of alpha-lipoic acid on the plasma levels of asymmetric dimethylarginine in diabetic end-stage renal disease patients on hemodialysis: a pilot study. Am J Nephrol. 2007;27:70–4.
Khabbazi T, Mahdavi R, Safa J, Pour-Abdollahi P. Effects of alpha-lipoic acid supplementation on inflammation, oxidative stress, and serum lipid profile levels in patients with end-stage renal disease on hemodialysis. J Ren Nutr. 2012;22:244–50.
Marfella R, Barbieri M, Sardu C, Rizzo MR, Siniscalchi M, Paolisso P, et al. Effects of α-lipoic acid therapy on sympathetic heart innervation in patients with previous experience of transient takotsubo cardiomyopathy. J Cardiol. 2016;67:153–61.
Gianturco V, Bellomo A, D'Ottavio E, Formosa V, Iori A, Mancinella M, et al. Impact of therapy with alpha-lipoic acid (ALA) on the oxidative stress in the controlled NIDDM: a possible preventive way against the organ dysfunction? Arch Gerontol Geriatr. 2009;49(Suppl 1):129–33.
Huerta AE, Prieto-Hontoria PL, Sainz N, Martinez JA, Moreno-Aliaga MJ. Supplementation with alpha-lipoic acid alone or in combination with eicosapentaenoic acid modulates the inflammatory status of healthy overweight or obese women consuming an energy-restricted diet. J Nutr. 2016; [Epub ahead of print]
Ramos LF, Kane J, McMonagle E, Le P, Wu P, Shintani A, et al. Effects of combination tocopherols and alpha lipoic acid therapy on oxidative stress and inflammatory biomarkers in chronic kidney disease. J Ren Nutr. 2011;21:211–8.
Cinteza D, Berteanu M, Vladoiu S, Manolescu BN, Dinu H. The consumption of alanerv(R) nutritional supplement and the dynamic of some inflammatory markers in post-acute stroke patients undergoing rehabilitation. Maedica. 2013;8:137–42.
Nasole E, Nicoletti C, Yang ZJ, Girelli A, Rubini A, Giuffreda F, et al. Effects of alpha lipoic acid and its R+ enantiomer supplemented to hyperbaric oxygen therapy on interleukin-6, TNF-alpha and EGF production in chronic leg wound healing. J Enzyme Inhib Med Chem. 2014;29:297–302.
Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, et al. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and lipoic acid in endothelial dysfunction (ISLAND) study. Circulation. 2005;111:343–8.
El-Nakib GA, Mostafa TM, Abbas TM, El-Shishtawy MM, Mabrouk MM, Sobh MA. Role of alpha-lipoic acid in the management of anemia in patients with chronic renal failure undergoing hemodialysis. Int J Nephrol Renovasc Dis. 2013;6:161–8.
Hegazy SK, Tolba OA, Mostafa TM, Eid MA, El-Afify DR. Alpha-lipoic acid improves subclinical left ventricular dysfunction in asymptomatic patients with type 1 diabetes. Rev Diabet Stud. 2013;10:58–67.
Safa J, Ardalan MR, Rezazadehsaatlou M, Mesgari M, Mahdavi R, Jadid MP. Effects of alpha lipoic acid supplementation on serum levels of IL-8 and TNF-alpha in patient with ESRD undergoing hemodialysis. Int Urol Nephrol. 2014;46:1633–8.
Ju W, Li X, Li Z, Wu GR, Fu XF, Yang XM, et al. The effect of selenium supplementation on coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. J Trace Elem Med Biol. 2017;44:8–16.
Saboori S, Shab-Bidar S, Speakman JR, Yousefi Rad E, Djafarian K. Effect of vitamin E supplementation on serum C-reactive protein level: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2015;69:867–73.
Sahebkar A, Serban C, Ursoniu S, Wong ND, Muntner P, Graham IM, et al. Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors--results from a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2015;189:47–55.
Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801.
Fornoni A, Ijaz A, Tejada T, Lenz O. Role of inflammation in diabetic nephropathy. Curr Diabetes Rev. 2008;4:10–7.
Gologorsky D, Thanos A, Vavvas D. Therapeutic interventions against inflammatory and angiogenic mediators in proliferative diabetic retinopathy. Mediat Inflamm. 2012;2012:629452.
Wong A, Dukic-Stefanovic S, Gasic-Milenkovic J, Schinzel R, Wiesinger H, Riederer P, et al. Anti-inflammatory antioxidants attenuate the expression of inducible nitric oxide synthase mediated by advanced glycation endproducts in murine microglia. Eur J Neurosci. 2001;14:1961–7.
Bierhaus A, Chevion S, Chevion M, Hofmann M, Quehenberger P, Illmer T, et al. Advanced glycation end product-induced activation of NF-kappaB is suppressed by alpha-lipoic acid in cultured endothelial cells. Diabetes. 1997;46:1481–90.
Cao Z, Tsang M, Zhao H, Li Y. Induction of endogenous antioxidants and phase 2 enzymes by alpha-lipoic acid in rat cardiac H9C2 cells: protection against oxidative injury. Biochem Biophys Res Commun. 2003;310:979–85.
Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxid Redox Signal. 2005;7:1040–52.
Zhang WJ, Frei B. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001;15:2423–32.
Acknowledgements
The present study was supported by a grant from the Vice-chancellor for Research, SUMS, Shiraz, and Iran.
Funding
The research grant provided by Research Deputy of Shiraz University of Medical Sciences (SUMS).
Availability of data and materials
The primary data for this study is available from the authors on direct request.
Author information
Authors and Affiliations
Contributions
ZA, MA and RT contributed in conception, design, statistical analysis and drafting of the manuscript. VO, KB-L, RT, MM, S-TH and FK. contributed in conception, data collection and manuscript drafting. MM and MC contributed in revised version. The final version was confirmed by all authors for submission.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Literature search and review flowchart for selection of studies. (DOC 44 kb)
Additional file 2:
The methodological quality of included studies (risk of bias). (DOC 44 kb)
Additional file 3:
The effects of alpha-lipoic acid supplementation on inflammatory markers based on sensitivity analysis. (DOC 33 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Akbari, M., Ostadmohammadi, V., Tabrizi, R. et al. The effects of alpha-lipoic acid supplementation on inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab (Lond) 15, 39 (2018). https://doi.org/10.1186/s12986-018-0274-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-018-0274-y