Abstract
Background
Patients treated with anti-CD20 monoclonal antibodies could have a higher risk of adverse outcomes of coronavirus disease 2019 (COVID-19). The novel anti-CD20 monoclonal antibody obinutuzumab has shown greater B-cell depletion and superior in vitro efficacy than rituximab. We aimed to assess whether obinutuzumab would result in worse COVID-19 outcomes than rituximab.
Methods
We retrospectively reviewed 124 patients with B-cell lymphoma, 106 of whom received rituximab treatment and 18 of whom received obinutuzumab treatment. The adverse outcomes of COVID-19 were compared between patients in the two cohorts.
Results
The proportions of patients who were hospitalized (55.6% vs. 20.8%, p = 0.005), experienced prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (38.9% vs. 2.9%, p < 0.001), and developed severe COVID-19 (33.3% vs. 4.7%, p < 0.001) were higher in patients with obinutuzumab than in those with rituximab. Multivariate analyses showed that obinuzumab treatment was associated with higher incidences of prolonged SARS-CoV-2 infection (OR 27.05, 95% CI 3.75-195.22, p = 0.001) and severe COVID-19(OR 15.07, 95% CI 2.58–91.72, p = 0.003).
Conclusions
Our study suggested that patients treated with obinutuzumab had a higher risk of prolonged SARS-CoV-2 infection and severe COVID-19 than those treated with rituximab.
Similar content being viewed by others
Background
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in Wuhan, China, in December 2019 [1, 2], and today, it still has a great impact globally. Patients with hematological malignancies (HMs) usually have an immune deficiency and are at a higher risk for severe COVID-19 and death [3, 4]. Moreover, prolonged SARS-CoV-2 infection appears to be an emerging issue in HM, especially those treated with B-cell depleting immunotherapy [5,6,7].
Anti-CD20 monoclonal antibodies (mABs), as a single agent or in combination with chemotherapy, are widely used for B-cell lymphoma and significantly improve survival [8, 9]. However, these antibodies induce rapid B-cell depletion and suppress the humoral response to viruses [10]. Studies have reported that patients treated with anti-CD20 mAB (mainly type I anti-CD20 mAB rituximab) could have a higher risk of severe COVID-19, prolonged SARS-CoV-2 infection and death [6, 7, 11,12,13].
Obinutuzumab is a novel humanized type II anti-CD20 mAb approved for follicular lymphoma or chronic lymphocytic leukemia by U. S Food and Drug Administration in 2015 and was available in China in 2021. Obinutuzumab has shown greater B-cell depletion and superior in vitro efficacy than rituximab [14,15,16]. In clinical practice, obinutuzumab may result in a higher rate of grade ≥ 3 neutropenia than rituximab but without a significant increase in serious infections [17]. However, SARS-CoV-2 infections in B-cell lymphoma patients treated with obinutuzumab during the COVID-19 pandemic are not very clear.
In mid-December 2022, China lifted the strict COVID-19 control policy and experienced the pandemic of the SARS-CoV-2 Omicron variant. Within a short period of time, most people in China were infected with SARS-CoV-2. In this study, we reviewed the data of patents with B-cell lymphoma who received anti-CD20 mAB therapy in 2022 at a single center in China and aimed to determine whether patients treated with obinutuzumab were at a higher risk for adverse COVID-19 outcomes than those treated with rituximab during the COVID-19 pandemic.
Methods
Patients
We retrospectively reviewed all patients with B-cell lymphoma admitted to Ningbo Medical Center Li Huili Hospital between January 1,2022, and December 31,2022 and enrolled those who had received anti-CD20 mAb therapy as a single agent or in combination with other agents. None of the patients had been previously infected with SARS-CoV-2. The following patients were excluded: (a) Patients who died before China lifted the control of the COVID-19 epidemic in mid-December 2022. (b) Patients who underwent autologous stem cell transplantation during the study period. (c) Patients who were hospitalized due to infection and had typical characteristics of COVID-19 (symptoms, imaging findings, etc.) but SARS-CoV-2 was negative or not tested. (d) Patients had incomplete data. Enrolled patients were divided into two cohorts according to the type of anti-CD20 mAB they received: the rituximab cohort and the obinutuzumab cohort. The most commonly used combinations were CHOP-like chemotherapies. Other combinations included second-line chemotherapies (GeMOX, DICE, DHAP), bendamustine, methotrexate, lenalidomide and BTK inhibitors.
Baseline data collection
The baseline characteristics of patients before the COVID-19 wave in mid-December 2022 were collected, including sex, age, smoking, performance status (PS) according to Eastern Cooperative Oncology Group (ECOG) [18], comorbidities (hypertension, diabetes, pulmonary comorbidities, cardiac comorbidities), vaccination status, histological subtype (aggressive lymphoma or indolent lymphoma), newly diagnosed lymphoma or relapse/refractory disease, disease status, treatment strategies, the time from the last anti-CD20 mAb use to the COVID-19 wave, and the period between last antineoplastic therapy and COVID 19 wave, Inactivated COVID-19 vaccines were widely used in China before the COVID-19 wave, and “full vaccination” was defined as having received ≥ 2 doses of inactivated vaccine. Aggressive lymphomas included diffuse large B-cell lymphoma, Burkitt lymphoma, mantle cell lymphoma, and high-grade B-cell lymphoma. Indolent lymphomas included follicular lymphoma, marginal zone lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma.
SARS-CoV-2 infection
The diagnosis of COVID-19 was confirmed by a positive nucleic acid test by polymerase chain reaction (PCR) or next generation sequencing. At the follow-up assessment, we found that most patients experienced suspected SARS-CoV-2 infection based on their symptoms during the COVID-19 wave. However, most non-hospitalized patients did not undergo SARS-CoV-2 nucleic acid or antigen testing, resulting in an inaccurate number and characteristics of the overall population infected with SARS-CoV-2. Therefore, we calculated the proportion of hospitalization, prolonged SARS-CoV-2 infection, severe COVID-19 and COVID-19-related mortality in the overall population rather than in the infected patients. Blood cell characteristics and immune function-related indicators of hospitalized patients were collected and analyzed, as well as information on antiviral treatment, length of hospital stay and prognosis. Prolonged SARS-CoV-2 infection was defined as SARS-CoV-2 nucleic acid detection ≥ 30 days after initial positivity. Severe COVID-19 was defined as having an SpO2 < 94% on room air, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mmHg, a respiratory rate > 30 breaths/min, or lung infiltrates > 50% by the National Institutes of Health [19].
Statistical analysis
Absolute numbers and percentages were used for categorical variables, and differences between groups were analyzed by the chi-square test or Fisher’s exact test. Medians and ranges were used for continuous variables, and differences between groups were analyzed by the Mann‒Whitney test. Kaplan‒Meier curves were used to display the cumulative incidence of discharge from the hospital with improvement. Patients who were not discharged at the end of the follow-up or who died of COVID-19 were treated as censored data, and the log-rank test was used for comparison. Univariable and multivariate logistic regression analyses were conducted to assess the risk factors for prolonged SARS-CoV-2 infection and severe COVID-19. The 95% confidence intervals (CIs) were used to estimate odds ratios (ORs). Statistical tests were two-tailed, and p values ≤ 0.05 were considered statistically significant. SPSS V.25 was used for analyses, and GraphPad Prism was used for graphing.
Results
Patient characteristics
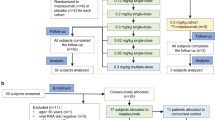
Between January 1, 2022, and December 31, 2022, 341 patients diagnosed with B-cell lymphoma were reviewed. Finally, 106 patients who had received rituximab-based treatments and 18 patients who had received obinutuzumab-based treatments were enrolled in the study (Fig. 1). The baseline characteristics of the two cohorts are shown in Table 1. In the rituximab cohort, 86.8% of the patients had aggressive lymphoma, whereas the majority of the patients in the obinutuzumab cohort had indolent lymphoma. A total of 57.5% of patients in the rituximab cohort and 88.9% of patients in the obinutuzumab cohort received anti-CD20 mAB treatment within 3 months before the COVID-19 wave, with a significant difference. More patients in obinutuzumab cohort received bendamustine(22.2% vs. 4.7%, p = 0.031). Other characteristics between the two cohorts were similar.
Outcomes of COVID-19
The proportions of patients who were hospitalized (55.6% vs. 20.8%, p = 0.005), experienced prolonged SARS-CoV-2 infection (38.9% vs. 2.9%, p < 0.001), and developed severe COVID-19 (33.3% vs. 4.7%, p < 0.001) were higher in patients treated with obinutuzumab than in those treated with rituximab (Fig. 2a). We did not find a significant difference in COVID-19-related mortality between the two cohorts (11.1% vs. 3.8%, p = 0.279).
Because there was a significant difference between rituximab and obinutuzumab groups of patients in whether bendamustine was used, and bendamustine may be a disadvantage factor for COVID-19 infection. Therefore, we compared the differences in COVID-19 outcomes between two groups of patients who had not received bendamustine in a subgroup analysis to exclude the interference of bendamustine(Fig. 2b). Subgroup analysis showed that patients who were hospitalized (50.0% vs. 19.8%, p = 0.031), experienced prolonged SARS-CoV-2 infection (28.6% vs. 2.0%, p = 0.002), and developed severe COVID-19 (21.4% vs. 4.0%, p = 0.038) were still higher in patients treated with obinutuzumab than in those treated with rituximab.
Among the 32 hospitalized patients, 25 patients received anti-viral therapies for COVID-19, and 9 patients received intravenous gamma globulin therapy. The median length of in-hospital stay was 10 days (range 4–47 days) in the rituximab cohort and 30 days (range 6–53 days) in the obinutuzumab cohort, with a significant difference. The Kaplan‒Meier curve of the cumulative incidence of discharge from the hospital with improvement is shown in Fig. 3.
Multivariate analyses showed that obinutuzumab treatment (OR 27.05, 95% CI 3.75-195.22, p = 0.001) was associated with a higher incidence of prolonged SARS-CoV-2 infection. Obinutuzumab treatment (OR 15.07, 95% CI 2.58–91.72, p = 0.003) and ECOG PS ≥ 2 (OR 8.03, 95% CI 1.34–47.91, p = 0.022) were associated with a higher incidence of severe COVID-19 (Table 2).
Laboratory data of hospitalized patients
We collected laboratory data from hospitalized patients at the time of COVID-19 diagnosis. The neutrophil count, lymphocyte count, CD4 + T-cell count and serum IgG level of patients with obinutuzumab were similar to those of patients with rituximab (neutrophil, median 2.30 vs. 2.60 G/L, p = 0.332; lymphocyte, median 0.65 vs. 0.50 G/L, p = 0.746; CD4 + T-cell, median 101 vs. 176/µL, p = 0.284; serum IgG, median 8.83 vs. 7.56 g/L, p = 0.476, Fig. 4). We also compared these laboratory data between patients with prolonged SARS-CoV-2 infection and those without prolonged SARS-CoV-2 infection and between patients with severe COVID-19 and those without severe COVID-19 (Fig. 4). There were no significant differences in neutrophil count, lymphocyte count, CD4 + T-cell count or serum IgG level between patients with and without prolonged SARS-CoV-2 infection. Patients with severe COVID-19 had lower neutrophil counts (median 1.89 vs. 3.95 G/L, p = 0.036) and CD4 + T-cell counts (median 75 vs. 233/µL, p = 0.007) than those without severe COVID-19.
Discussion
To the best of our knowledge, this is the first study to compare the outcomes of COVID-19 between patients with obinutuzumab and rituximab in an Asian population. Our study showed that during the COVID-19 epidemic, patients taking obinutuzumab were at a higher risk of prolonged SARS-CoV-2 infection and severe COVID-19 than those taking rituximab.
Studies in rituximab showed that complete B-cell depletion may occur within 72 h of anti-CD20 mAB infusion with recovery approximately 9–12 months after treatment [8]. Serum immunoglobulin levels mostly remain within the normal range or mildly hypogammaglobulinemia and return to normal within 1 year [8, 20,21,22]. Given the prolonged immunosuppression caused by anti-CD20 mAB, it significantly increases the risk of infection [23, 24]. In in vitro studies, obinutuzumab has shown greater B-cell depletion than rituximab [14,15,16], which could theoretically lead to a higher risk of infection, especially viral infection. Several studies have shown that patients treated with anti-CD20 mAbs have a higher risk of adverse outcomes of COVID-19 [7, 11, 13]. However, these studies did not distinguish between obinutuzumab and rituximab. Only two studies reported the outcomes of COVID-19 in patients treated with obinutuzumab, with a longer duration of infection(p = 0.012), a higher severe disease rate (35% vs. 7%) and a higher mortality rate (15% vs. 0%) than those in patients treated with rituximab [25, 26]. However, with no routine SARS-CoV-2 test, some infections may have been missed, resulting in patient selection bias, which is unavoidable in retrospective studies. In our study, the incidence of adverse outcomes was calculated in the whole population, including both SARS-CoV-2-infected and SARS-CoV-2-uninfected patients, suggesting that patients with obinutuzuma have a higher risk of adverse outcomes than those with rituximab during the COVID-19 epidemic. Maintenance therapy with obinutuzumab or rituximab increases the progression-free survival of follicular lymphoma [27, 28] and is now recommended by the NCCN guidelines. Under the COVID-19 pandemic, however, maintenance therapy may increase the risk of adverse outcomes of COVID-19, and the benefits and risks need to be weighed.
Prolonged SARS-CoV-2 infection now represents a new challenge for patients treated with anti-CD20 mAB. In a study of lymphoma patients treated with rituximab, the time from the first PCR positivity to final PCR negativity ranged from 33 to 77 days [29]. Another study reported a follicular lymphoma patient treated with obinutuzuma who experienced a prolonged SARS-CoV-2 infection of 187 days. In our study, the proportion of patients who experienced prolonged SARS-CoV-2 infection was 38.9% in the obinutuzumab cohort, which was much higher than that in the rituximab cohort. The median length of in-hospital stay in patients treated with obinuzumab was 30 days (range 6–53 days), which was significantly longer than that in patients treated with rituximab. These results indicate that the immune damage caused by obinutuzumab significantly prolongs the clearance of the virus. Long-term SARS-CoV-2 nucleic acid monitoring and appropriate delay in chemotherapy resumption should be considered in patients treated with obinutuzumab.
Previous studies have reported that some laboratory indicators are associated with the severity of COVID-19 [30, 31]. Several studies have shown that patients with severe COVID-19 have lower lymphocyte levels and higher white blood cells [32, 33]. Moreover, lower levels of CD4 + T cells are associated with severe COVID-19 [34, 35]. In our study, lower lymphocyte counts were observed in patients with severe COVID-19 than in those with mild COVID-19, which was consistent with previous studies. However, contrary to previous studies, patients with severe COVID-19 had lower levels of neutrophils. This may be related to the different characteristics between patients with hematological diseases receiving chemoimmunotherapy and other general patients. No significant differences in the levels of neutrophils, lymphocytes and CD4 + T-cells were observed between hospitalized patients with obinutuzumab and rituximab or between patients with prolonged SARS-CoV-2 infection and those without prolonged SARS-CoV-2 infection. Studies with larger sample sizes are needed to assess these differences more accurately.
There are several limitations of this study. First, most patients with suspected mild SARS-CoV-2 infection did not undergo SARS-CoV-2 nucleic acid testing, which resulted in an unclear COVID-19 infection rate and baseline characteristics of the total infected population. Second, because it was a retrospective study, the baseline characteristics between the obinutuzumab and rituximab cohorts were not completely compared. Finally, the sample size was not large enough to observe a difference in mortality between the obinutuzumab and rituximab cohorts.
Conclusions
Our study suggested that patients treated with obinutuzumab had a higher risk of prolonged SARS-CoV-2 infection and severe COVID-19 than those treated with rituximab. During the COVID-19 pandemic, rituximab might be considered an alternative to obinutuzuma in appropriate patients. The benefits and harms of maintenance therapy may need to be weighed.
Data availability
No datasets were generated or analysed during the current study.
References
Yu F, Du L, Ojcius DM, Pan C, Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020;22:74–9. https://doi.org/10.1016/j.micinf.2020.01.003.
Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–20. https://doi.org/10.1056/NEJMoa2002032.
Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737–45. https://doi.org/10.1016/S2352-3026(20)30251-9.
Pagano L, Salmanton-Garcia J, Marchesi F, et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA). J Hematol Oncol. 2021;14:168. https://doi.org/10.1186/s13045-021-01177-0.
Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for Cancer. N Engl J Med. 2020;383:2586–8. https://doi.org/10.1056/NEJMc2031670.
Lee CY, Shah MK, Hoyos D, et al. Prolonged SARS-CoV-2 infection in patients with lymphoid malignancies. Cancer Discov. 2022;12:62–73. https://doi.org/10.1158/2159-8290.CD-21-1033.
Dulery R, Lamure S, Delord M, et al. Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-hodgkin lymphoma treated with B-cell depleting immunotherapy. Am J Hematol. 2021;96:934–44. https://doi.org/10.1002/ajh.26209.
McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–33. https://doi.org/10.1200/JCO.1998.16.8.2825.
Maloney DG, Grillo-Lopez AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-hodgkin’s lymphoma. Blood. 1997;90:2188–95.
Tudesq JJ, Cartron G, Riviere S, et al. Clinical and microbiological characteristics of the infections in patients treated with rituximab for autoimmune and/or malignant hematological disorders. Autoimmun Rev. 2018;17:115–24. https://doi.org/10.1016/j.autrev.2017.11.015.
Loarce-Martos J, Garcia-Fernandez A, Lopez-Gutierrez F, et al. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int. 2020;40:2015–21. https://doi.org/10.1007/s00296-020-04699-x.
Hoffmann MS, Ganguly S, Delayed. COVID-19 respiratory failure in patients with lymphoma on Rituximab-based Chemoimmunotherapy. Clin Lymphoma Myeloma Leuk. 2021;21:e548–50. https://doi.org/10.1016/j.clml.2021.02.009.
Calderon-Parra J, Munez-Rubio E, Fernandez-Cruz A, et al. Incidence, clinical presentation, relapses and outcome of severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection in patients treated with Anti-CD20 monoclonal antibodies. Clin Infect Dis. 2022;74:1786–94. https://doi.org/10.1093/cid/ciab700.
Patz M, Isaeva P, Forcob N, et al. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br J Haematol. 2011;152:295–306. https://doi.org/10.1111/j.1365-2141.2010.08428.x.
Herter S, Herting F, Mundigl O, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013;12:2031–42. https://doi.org/10.1158/1535-7163.MCT-12-1182.
Mossner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115:4393–402. https://doi.org/10.1182/blood-2009-06-225979.
Freeman CL, Sehn LH. A tale of two antibodies: obinutuzumab versus rituximab. Br J Haematol. 2018;182:29–45. https://doi.org/10.1111/bjh.15232.
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
In Coronavirus Disease 2019 (COVID-19) treatment guidelines; Bethesda (MD), 2021.
Worch J, Makarova O, Burkhardt B. Immunreconstitution and infectious complications after rituximab treatment in children and adolescents: what do we know and what can we learn from adults? Cancers (Basel). 2015;7:305–28. https://doi.org/10.3390/cancers7010305.
Ghielmini M, Rufibach K, Salles G, et al. Single agent Rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK). Ann Oncol. 2005;16:1675–82. https://doi.org/10.1093/annonc/mdi320.
Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13:106–11. https://doi.org/10.1016/j.clml.2012.11.011.
Rafailidis PI, Kakisi OK, Vardakas K, Falagas ME. Infectious complications of monoclonal antibodies used in cancer therapy: a systematic review of the evidence from randomized controlled trials. Cancer. 2007;109:2182–9. https://doi.org/10.1002/cncr.22666.
Schulz H, Bohlius JF, Trelle S, et al. Immunochemotherapy with Rituximab and overall survival in patients with indolent or mantle cell lymphoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2007;99:706–14. https://doi.org/10.1093/jnci/djk152.
Shafat T, Grupel D, Porges T, Levi I, Yagel Y, Nesher L. Treatment with obinutuzumab leads to worse outcomes in haematological patients diagnosed with Omicron variant COVID-19. Br J Haematol. 2022;198:826–9. https://doi.org/10.1111/bjh.18315.
Aurer I, Jaksic O, Basic-Kinda S, et al. Treatment-related risk factors for adverse outcomes of COVID-19 in patients treated for lymphoid malignancies in the Pre-omicron Era-A study of KroHem, the Croatian Group for Hematologic Diseases. Biomedicines. 2024;12. https://doi.org/10.3390/biomedicines12020331.
Hill BT, Nastoupil L, Winter AM, et al. Maintenance rituximab or observation after frontline treatment with bendamustine-rituximab for follicular lymphoma. Br J Haematol. 2019;184:524–35. https://doi.org/10.1111/bjh.15720.
Marcus R, Davies A, Ando K, et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med. 2017;377:1331–44. https://doi.org/10.1056/NEJMoa1614598.
Furlan A, Forner G, Cipriani L, et al. Dramatic Response to Convalescent Hyperimmune Plasma in Association with an extended course of Remdesivir in 4 B cell-depleted Non-hodgkin Lymphoma patients with SARS-Cov-2 Pneumonia after Rituximab Therapy. Clin Lymphoma Myeloma Leuk. 2021;21:e731–5. https://doi.org/10.1016/j.clml.2021.05.013.
Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21:74. https://doi.org/10.1186/s12931-020-01338-8.
Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55. https://doi.org/10.1183/13993003.00524-2020.
Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. https://doi.org/10.1016/S2213-2600(20)30079-5.
Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. https://doi.org/10.1016/S0140-6736(20)30211-7.
Cossarizza A, De Biasi S, Guaraldi G, Girardis M, Mussini C. Modena Covid-19 Working, G. SARS-CoV-2, the Virus that causes COVID-19: cytometry and the New Challenge for Global Health. Cytometry A. 2020;97:340–3. https://doi.org/10.1002/cyto.a.24002.
Qin C, Zhou L, Hu Z, et al. Dysregulation of Immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8. https://doi.org/10.1093/cid/ciaa248.
Acknowledgements
We thank the patients for cooperating with our investigation and acknowledge all investigators who participated in this study, including physicians, nurses, and laboratory technicians.
Funding
This work is supported by Ningbo Medical Science and Technology Project (reference:2018A64).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization, Dian Jin; writing—original draft preparation, Wenxiu Shu and Qianqian Yang; methodology, Wenxiu Shu and Qianqian Yang; formal analysis, Bingrong Chen.; investigation, Dengbing Chen; resources, Hui Dai, Yanping Song, and Jiaqi Tong; data curation, Liufei Luo; writing—review and editing, Qianqian Cai and Jing Le. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Ningbo Medical Center Lihuili Hospital (protocol code YJZ2023SL2). Informed consent of patient was waived by Ningbo Medical Center Lihuili Hospital ethics committee due to the retrospective nature of the study.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shu, W., Yang, Q., Le, J. et al. Outcomes of COVID-19 in patients with obinutuzumab compared with patients with rituximab: a retrospective cohort study. Virol J 21, 212 (2024). https://doi.org/10.1186/s12985-024-02484-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02484-x