Abstract
Background
Epstein-Barr virus (EBV) can be reactivated and proliferated with fatal outcome in immuno-compromised people, but the clinical consequences of EBV infection in patients with severe fever with thrombocytopenia syndrome (SFTS) remain uncertain. In this study, we investigated the infection rate, the influence and the early predictors of EBV infection in SFTS patients.
Methods
In this retrospective study, SFTS patients who were treated in the First Affiliated Hospital of Nanjing Medical University from May 2011 to August 2021 were enrolled and divided into infected and non-infected groups. We compared the demographic characteristics, clinical manifestations and signs, laboratory tests and prognosis, and explored the risk factors of EBV infection by receiver operating characteristic (ROC) curve and logistic regression.
Results
A total of 120 hospitalized SFTS patients with EBV-DNA testing were enrolled in this study. Patients with EBV infection had statistically significant higher mortality rate (32.0% vs. 11.43%, P = 0.005). Compared with the non-infected group, the EBV-infected group had higher levels of C-reactive protein (CRP), creatine-kinase (CK), fasting blood glucose (FBG), blood urea nitrogen (BUN), D-dimer, and CD56+ cell counts, lower levels of immunoglobulin G (IgG), IgM, complement 3 (C3), and C4. The proportion of patients with age ≥ 60 years and ferritin > 1500.0 ng/ml in the EBV-infected group was significantly higher than that in the non-infected group. The results of ROC analysis showed that the cut-off values of CRP, IgG, C3, C4, and CD56+ cell counts to predict EBV infection were 13.2 mg/l, 12.5 g/l, 1.1 g/l, 0.6 g/l, 0.3 g/l, and 94.0 cells/µl. Multivariable logistic analysis showed that age ≥ 60 years old, CRP > 13.2 mg/l, BUN > 5.4 mmol/l, ferritin > 1500.0 ng/ml, IgG < 12.5 g/l, IgM < 1.1 g/l, C4 < 0.3 g/l, and CD56+ cell counts > 94.0 cells/µl were the independent risk factors of EBV infection in SFTS patients.
Conclusions
SFTS combined with EBV infection is associated with high morbidity and mortality. It is necessary to strengthen screening for EBV infection and its early predictive markers after admission in SFTS patients.
Similar content being viewed by others
Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is a severe tick-borne disease caused by Dabie bandavirus (DBV), which is a negative-strand RNA virus and belongs to the genus Bandavirus in family Phenuiviridae [1]. This viral hemorrhagic fever disease was first identified in 2009 in the rural areas of China [2]. After DBV infection, the patients were characterized by fever, headache, thrombocytopenia, leukopenia, gastrointestinal symptoms, diffuse intravascular coagulation, multiple organ dysfunction syndrome (MODS) and shock [3]. The emergence of SFTS has had a huge impact on population health mainly in East and Southeast Asia. Despite the fact that the case fatality rate (CFR) of SFTS ranges between 10.5% and 35%, there are currently no effective treatments available for SFTS patients [4,5,6]. Previous studies have proved that ribavirin can suppress the replication of DBV in vivo and in vitro [7, 8]. However, there is no specific clinical evidence to prove if the antivirus effect of ribavirin can be useful to increase the survival rates of SFTS patients [3]. Controlling the progression and reducing the mortality of SFTS is still challenging.
The Epstein-Barr virus (EBV), which is a ubiquitous gamma herpesvirus infects more than 90% of the adulthood worldwide [9]. Most immunocompetent people have no obvious manifestations after infection, but in immunocompromised individuals, EBV may be reactivated and proliferated with fatal outcome. Previous studies have presented that EBV infection can affect the progression and outcome of several diseases, including coronavirus disease 2019 (COVID-19) and multiple sclerosis (MS) [10, 11]. Cytokine storm, which is characterized by imbalanced immune responses with an exaggerated systemic inflammation, played a crucial role in the deterioration of SFTS [12, 13]. However, there is no specific evidence to support the relationship between EBV infection and the prognosis of SFTS up to date. Moreover, the risk factors associated with EBV infection in SFTS patients have never been investigated. This study systematically assessed the impact that different potential factors have on the risk of EBV infection in patients with SFTS, and explore the early predictors.
Materials and methods
Study design and participants
From May 2011 to August 2021, the clinical records of SFTS patients who were hospitalized in the First Affiliated Hospital of Nanjing Medical University were collected in this retrospective case-control study. All these patients should undergo a complete clinical examination at the time of admission, including the plasma EBV DNA levels. Patients were divided into two groups according to EBV-DNA levels. The peripheral whole blood samples of SFTS patients were collected in tubes containing Ethylenediaminetetraacetic acid (EDTA). EBV PCR Fluorescence Quantitative Diagnostic Kit (Daan Gene Co., Guangzhou, China) was used to extract DNA from whole blood and a real-time quantitative PCR with ABI PRISM 7500 (Applied Biosystems, Foster City, CA, USA) was used to quantify EBV-specific sequences. It was performed at the department of Laboratory Medicine of the hospital. EBV DNA load > 500 copies/ml was defined as positive and ≤ 500 copies/ml was defined as a negative level. This research was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.
Data collection
The retrospective analysis evaluated information from the electronic medical record system, including epidemiological, demographic characteristics (age, gender, basic diseases, smoking and drinking history), clinical data (clinical manifestations and signs), laboratory tests (routine blood tests, renal and liver function tests, and inflammatory biomarkers), treatment, and prognosis. All patients were followed up for 30 days post-discharge.
Diagnostic criteria
Based on the confirmed definition published by the Chinese Ministry of Health in 2010 [14], SFTS was diagnosed according to the following criteria: (1) patients having a history of bite from a tick in the two weeks prior to onset of illness, or having been to hilly terrain, forest region or mountainous area in epidemic seasons. (2) patients presented with fever, fatigue, anorexia and so on with acute-onset. (3) laboratory tests indicated low white blood cell and platelet counts. (4) patients who were tested to meet one of the following four criteria: positive for DBV RNA in peripheral blood using polymerase chain reaction; seroconversion in immunoglobulin G (IgG) antibody specific for DBV; compared with the samples during acute stage, the specific antibody was detected more than four times during convalescent stage; the isolation of DBV from the samples. Central nervous system (CNS) manifestation was identified as at least one of the following symptoms: muscle and limb tremor, cognitive dysfunction, lethargy, confusion, stupor, delirium, coma, dysphoria, convulsion and seizures [15, 16].
Statistical analysis
We used IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 9.0 (GraphPad Software, San Diego, USA) for all statistical analysis. Normal and non-normal continuous variables were presented as means with standard deviations (SDs) and medians with inter-quartile range (IQR) and analyzed by Mann-Whitney U test. Categorical variables were presented as number with percentages and analyzed by chi-square test or Fisher’s test. Variables were considered statistically significant with a P-value of < 0.05. Receiver operating characteristic (ROC) curve analyses were used to evaluate the best cut-off values and the prognostic value of the clinical indicators. The risk factors for EBV infection were performed by multivariate logistic regression analysis and the results were described as the odds rations (ORs) and 95% confidence intervals (CIs). Survival analysis comparisons between the two groups were analyzed by the log rank (Mantel-Cox) test with the 30-day follow-up period.
Results
Overview of clinical information
Figure 1 showed the flow chart for inclusion and exclusion of SFTS patients. Among 142 SFTS patients with EBV-DNA testing from May 2011 to August 2021, 120 patients were included in the final analysis according to the inclusion and exclusion criteria. There were 50 cases (41.7%) in EBV-infected group and 70 cases (58.3%) in non-infected group. Comparisons of demographics, clinical symptoms and signs, laboratory findings, and other clinical information were presented in Table 1 (at the end of the article). The proportion of SFTS patients who were at least 60 years old was 70.0% in EBV-infected group, and that in the non-EBV infection group was 50.0%, the difference was statistically significant (P < 0.05). Twenty-two (44.0%) were males in EBV-infected group, while 36 (51.4%) were males in non-infected group. In terms of underlying diseases there was no significant difference between the two groups in our study. The patients had symptoms and signs as fever, cough, myalgia, gastrointestinal symptom, and CNS manifestation with no significant difference.
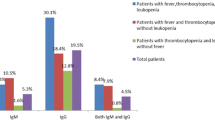
As shown in Table 1, the median levels of inflammatory and immunological markers including C-reactive protein (CRP), IgG, IgM, complement 3 (C3), C4, and CD56+ cell counts in EBV-infected patients were 12.7 (IQR 5.0-18.9) mg/l, 10.9 (IQR 7.3–13.3) g/l, 0.8 (IQR 0.6–1.1) g/l, 0.6 (IQR 0.4–0.7) g/l, 0.3 (IQR 0.2–0.3) g/l, and 146.6 (IQR 97.5–246.0) cells/µl. The levels of CRP and CD56+ cell counts in EBV-infected group were significantly higher than those in non-infected group and the levels of IgG, IgM, C3, and C4 were lower (P < 0.05). The proportion of patients whose ferritin level was more than 1500.0 ng/ml was 92.0% in EBV-infected group and 74.3% in non-infected group (P < 0.05). With respect to coagulative indicators, the level of D-dimer was higher in EBV-infected group than in non-infected group (P < 0.05). In addition, there was significant difference of creatine-kinase (CK), fasting blood glucose (FBG), and blood urea nitrogen (BUN) between the two groups, while other biochemical indexes were no significant difference.
Outcomes of the EBV-infected group and the non-infected group
The proportion of patients admitted to intensive care unit (ICU) was higher in EBV-infected group, but the difference was not statistically significant (P > 0.05). In addition, the median duration of hospital stay was 8.0 (IQR 6.0–13.0) days in EBV-infected group and 7.0 (IQR 4.0–11.0) days in non-infected group with significant difference (P < 0.05). For these SFTS patients, the mortality rate was 32.0% for the EBV-infected group and 11.4% for the non-infected group with significant difference (P < 0.05). As shown in Fig. 2, the infected group always had a higher mortality than the non-infected group during the one-month follow-up period. In addition, the difference of mortality rate between the two groups increased significantly after the first two weeks.
Risk factors for EBV infection in SFTS patients
Table 2 showed the results of ROC analysis of immune and inflammatory biomarkers based on the comparable findings in Table 1. The cut-off values of CRP, IgG, IgM, C3, C4, and CD56+ cell counts to predict EBV infection were 13.2 mg/l, 12.5 g/l, 1.1 g/l, 0.6 g/l, 0.3 g/l and 94.0 cells/µl, with sensitivities of 50.0%, 72.0%, 74.0%, 60.0%, 58.0%, and 78.0% and specificities of 77.1%, 51.4%, 51.4%, 64.3%, 64.3%, and 47.1%, respectively. As shown in Table 3, cut-off values of CK, FBG, BUN, and D-dimer to predict the incidence of EBV infection in SFTS patients were 299.5 U/l, 7.7 mmol/l, 5.4 mmol/l, and 2.2 mg/l, with sensitivities of 82.6%, 61.7%, 70.0%, and 69.4%, and specificities of 50.0%, 68.1%, 55.7%, and 59.4%, respectively.
Multivariate logistic regression analysis was adopted to identify if the significant indexes in Table 1 correlated with EBV infection in SFTS patients. The results were summarized in Table 4, which showed that the following several significant risk factors remained: age ≥ 60 years old (OR 3.196, 95% CI 1.046–9.763, P = 0.041), CRP > 13.2 mg/l (OR 3.097, 95% CI 1.077–8.908, P = 0.036), BUN > 5.4 mmol/l (OR 4.670, 95% CI 1.424–15.311, P = 0.011), ferritin > 1500.0 ng/ml (OR 10.638, 95% CI 1.990-56.868, P = 0.006), IgG < 12.5 g/l (OR 3.589, 95% CI 1.218–10.580, P = 0.020), IgM < 1.1 g/l (OR 8.141, 95% CI 2.469–26.844, P = 0.001), C4 < 0.3 g/l (OR 3.296, 95% CI 1.132–9.598, P = 0.029), and CD56+ cell counts > 94.0 cells/µl (OR 5.710, 95% CI 1.839–17.732, P = 0.003).
Discussion
DBV has been identified that it can affect the cellular and humoral immune responses of patients, which may contribute to the high possibility of EBV infection in SFTS patients [17]. And given the increasing popularity and mortality of SFTS, we performed the first study to date to reveal the strong evidence that EBV infection is strongly associated with the worse outcome in SFTS patients. Meanwhile, our multivariate analysis clearly showed the risk factors for EBV infection in SFTS patients.
Referred to as one of the most common viruses, EBV can persist for the remainder of the life in individuals following the acute phase of infection and can be reactivated by primary or secondary immunodeficiency [18]. Immunosuppressive agents, high-dose corticosteroid treatment, acute virus coinfection have been identified as the most common risk factors for EBV infection in several studies, which were based on immunodeficient or immunosuppressed patients [19,20,21]. In the present study, we focused on patients with SFTS. DBV has been confirmed to have the ability to attenuate cellular and humoral immune responses. Aberrant production of pro- and anti-inflammatory cytokines are produced in severe cases, resulting in systemic inflammatory response syndrome (SIRS) and mixed/compensatory anti-inflammatory response syndrome (MARS/CARS) [22]. SIRS coupled with MARS/CARS can increase the occurrence of secondary infection, multi-organ failure and fatal outcomes [23]. The immune damage including cellular immunosuppression and cytokine storms provided the probability that EBV infection has a strong association with SFTS. Our study evaluated a cohort of 120 hospitalized patients with SFTS, of which 41.7% patients were EBV-DNA positive.
In this study, we found that EBV infection contributed to prolonged length of hospital stay, higher rates of ICU admission, and increased mortality of SFTS patients. Prior studies have also shown that SFTS patients with microbial infections exhibit a higher mortality rate compared to those without co-infections [24]. Several studies have highlighted that EBV infection substantially elevates mortality rates when juxtaposed with uninfected patients across various conditions such as COVID-19, multiple myeloma, and acute-on-chronic liver failure (ACLF) [25,26,27]. On the one hand, increasing evidence proved that in critically ill patients with SFTS, the mortality might be associated with an excessive upregulation of proinflammatory mediators, which may further be triggered by EBV infection [28]. On the other hand, studies have indicated that an older age and elevated levels of BUN serve as valuable prognostic indicators of mortality in SFTS patients [29]. Our study has identified BUN levels > 5.4 mmol/l and an age of ≥ 60 years as predictors of EBV infection in SFTS patients. EBV infection likely serves as a marker of illness severity in SFTS patients, aligning well with our understanding of herpes virus co-infections.
In this research, several immune and inflammatory biomarkers were confirmed to be useful to the prediction of EBV infection in SFTS patients. CRP and ferritin, biochemical markers of inflammation, have been reported to be associated with poor prognosis in SFTS patients and can be significantly increased when tissues are extensively damaged by DBV [30,31,32,33]. Our study confirmed the link between CRP and ferritin levels and EBV infection in SFTS patients. Hyperinflammatory response impaired immunocompetence were leaded by DBV and then EBV infection, which results in a worse outcome. This may have a strong association with the outbreak of inflammatory cytokines after EBV infection, which still needs further mechanism studies to confirm.
From the perspective of other laboratory indicators, we observed that the levels of complement and immunoglobulins can serve as biomarkers for predicting DBV-associated immune dysfunction and EBV infection in SFTS patients. The production of immunoglobulins plays a strong part in the adaptive immune system by binding to pathogens and facilitating their elimination [34]. Previous studies have suggested deficiency in immunoglobulins may lead to immunodeficiency and severe infections [35]. The abnormality of immunoglobulins in relation to EBV infection risk in SFTS patients has not been investigated. In our cohort of SFTS, we discovered that low IgM and IgG levels were risk factors for EBV infection. The complement system can also label pathogens through the alternative, lectin and classical pathways for promoting an inflammatory immune response [36]. C4 is a key component of the complement system and can be decreased through consumption after DBV infection [37]. Our result showed that lower serum concentrations of C4, which indicates excessive complement activation and product consumption were associated with the risk of EBV infection in SFTS.
In addition, there was no significant difference in lymphocyte subsets between the two groups except for CD56+ cells (NK cells), which might due to the small sample size and individual difference. NK cells have been identified as an important immunomodulatory part in both innate immune and adaptive immune response. Individuals with NK cell deficiency can be more susceptible to herpes virus [38]. In contrast, in our multivariable model, higher level of NK cells had an increased risk of EBV infection. Various studies have proved that the proportion and absolute count of NK cells in patients with acute phase and severe SFTS was higher than in convalescent cases and mild SFTS [39]. It was supposed that increased numbers of NK cells may contribute to the extensive tissue damage in SFTS patients, which has been proved to be correlated with EBV infection [40].
To our knowledge, this study is the first large population-based study to prove that SFTS patients with EBV infection are at increased risk for worse prognosis. There was a significant dysregulation of several essential components of the immune and inflammatory system in SFTS patients with EBV infection. Acute-phase proteins, complement factors, immunoglobulins and lymphocyte subsets are dysregulated in SFTS patients, which indicate disruptions of innate and adaptive immunity and contribute to the high risk of EBV infection. Results of our study also provide the insight that further research is needed to determine if anti-herpesvirus drugs can improve the prognosis in SFTS patients with EBV infection. In addition, due to the limited data of cases in this study, it was not possible to further explore the prognosis in SFTS patients with different levels of EBV infection. Finally, efforts need to be maintained to elucidate the impact of SFTS in the reactivation of latent EBV.
Conclusions
In conclusion, EBV infection may be frequent in SFTS patients and increase the mortality. The levels of acute-phase proteins, complement factors, immunoglobulins and lymphocyte subsets have crucial predictive values for the early identification of EBV infection in SFTS patients. It is necessary to strengthen screening for EBV infection and its early predictive markers after admission in these patients. Attention should be paid to SFTS patients with dysregulation of several essential components of the immune and inflammatory system.
Data availability
No datasets were generated or analysed during the current study.
References
Casel MA, Park SJ, Choi YK. Severe fever with thrombocytopenia syndrome virus: emerging novel phlebovirus and their control strategy. Exp Mol Med. 2021;53(5):713–22.
Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, RenJ, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364(16):1523–32.
Zhang Y, Miao W, Xu Y, Huang Y. Severe fever with thrombocytopenia syndrome in Hefei: clinical features, risk factors, and Ribavirin therapeutic efficacy. J Med Virol. 2021;93(6):3516–23.
Miao D, Liu MJ, Wang YX, Ren X, Lu QB, Zhao GP, Dai K, Li XL, Li H, Zhang XA, Shi WQ, Wang LP, Yang Y, Fang LQ, Liu W. Epidemiology and Ecology of severe fever with Thrombocytopenia Syndrome in China, 2010–2018. Clin Infect Dis. 2021;73(11):e3851–8.
Chen Q, Yang D, Zhang Y, Zhu M, Chen N, Yushan Z. Transmission and mortality risk assessment of severe fever with thrombocytopenia syndrome in China: results from 11-years’ study. Infect Dis Poverty. 2022;11(1):93.
Yokomizo K, Tomozane M, Sano C, Ohta R. Clinical presentation and mortality of severe fever with Thrombocytopenia Syndrome in Japan: a systematic review of Case Reports. Int J Environ Res Public Health. 2022;19(4).
Tani H, Fukuma A, Fukushi S, Taniguchi S, Yoshikawa T, Iwata-Yoshikawa N, Sato Y, Suzuki T, Nagata N, Hasegawa H, Kawai Y, Uda A, Morikawa S, Shimojima M, Watanabe H, Saijo M. Efficacy of T-705 (Favipiravir) in the treatment of infections with Lethal severe fever with Thrombocytopenia Syndrome Virus. mSphere. 2016;1(1).
Lee MJ, Kim KH, Yi J, Choi SJ, Choe PG, Park WB, Kim NJ, Oh MD. In vitro antiviral activity of Ribavirin against severe fever with thrombocytopenia syndrome virus. Korean J Intern Med. 2017;32(4):731–7.
Damania B, Kenney SC, Raab-Traub N. Epstein-Barr virus: Biology and clinical disease. Cell. 2022;185(20):3652–70.
Simonnet A, Engelmann I, Moreau AS, Garcia B, Six S, El Kalioubie A, Robriquet L, Hober D, Jourdain M. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect Dis Now. 2021;51(3):296–9.
Olmez O, Baba C, Abasiyanik Z, Ozakbas S. Epstein-Barr virus antibody in newly diagnosed multiple sclerosis patients and its association with relapse severity and lesion location. Mult Scler Relat Disord. 2022;68:104149.
Jin K, Dai Y, Ouyang K, Huang H, Jiang Z, Yang Z, Zhou T, Lin H, Wang C, Wang C, Sun X, Lu D, Liu X, Hu N, Zhu C, Zhu J, Li J. TRIM3 attenuates cytokine storm caused by Dabie Bandavirus via promoting toll-like receptor 3 degradation. Front Microbiol. 2023;14:1209870.
Khalil J, Yamada S, Tsukamoto Y, Abe H, Shimojima M, Kato H, Fujita T. The nonstructural protein NSs of severe fever with Thrombocytopenia Syndrome Virus causes a cytokine storm through the hyperactivation of NF-κB. Mol Cell Biol. 2021;41(3):e0054220.
Ministry of Health P. Guideline for prevention and treatment of sever fever with thrombocytopenia syndrome (2010 vesrion). Chin J Clin Infect Dis. 2011;04(4):193–4.
Fei X, Feng B, Fang K, Ren W. Risk factors for mortality in severe fever with Thrombocytopenia Syndrome patients with Central Nervous System complications. Med Sci Monit. 2023;29:e938427.
Wang M, Huang P, Liu W, Tan W, Chen T, Zeng T, Zhu C, Shao J, Xue H, Li J, Yue M. Risk factors of severe fever with thrombocytopenia syndrome combined with central neurological complications: a five-year retrospective case-control study. Front Microbiol. 2022;13:1033946.
Yang T, Huang H, Jiang L, Li J. Overview of the immunological mechanism underlying severe fever with thrombocytopenia syndrome (review). Int J Mol Med. 2022;50(3).
Sánchez-Ponce Y, Fuentes-Pananá EM. The role of coinfections in the EBV-Host broken equilibrium. Viruses. 2021;13(7).
Fujimoto K, Hatanaka KC, Hatanaka Y, Kasahara I, Yamamoto S, Tsuji T, Nakata M, Takakuwa Y, Haseyama Y, Oyamada Y, Yonezumi M, Suzuki H, Sakai H, Noguchi H, Mori A, Nishihara H, Teshima T, Matsuno Y. Association of Epstein-Barr virus with regression after withdrawal of immunosuppressive drugs and subsequent progression of iatrogenic immunodeficiency-associated lymphoproliferative disorders in patients with autoimmune diseases. Hematol Oncol. 2020;38(5):799–807.
Naendrup JH, Garcia Borrega J, Eichenauer DA, Shimabukuro-Vornhagen A, Kochanek M, Böll B. Reactivation of EBV and CMV in severe COVID-19-Epiphenomena or trigger of hyperinflammation in need of treatment? A large Case Series of critically ill patients. J Intensive Care Med. 2022;37(9):1152–8.
Zhou JR, Shi DY, Wei R, Wang Y, Yan CH, Zhang XH, Xu LP, Liu KY, Huang XJ, Sun YQ. Co-reactivation of Cytomegalovirus and Epstein-Barr Virus was Associated with Poor Prognosis after allogeneic stem cell transplantation. Front Immunol. 2020;11:620891.
Mendoza CA, Ebihara H, Yamaoka S. Immune Modulation and Immune-mediated pathogenesis of emerging Tickborne Banyangviruses. Vaccines (Basel). 2019;7(4).
Hu L, Kong Q, Yue C, Xu X, Xia L, Bian T, Liu Y, Zhang H, Ma X, Yin H, Sun Q, Gao Y, Ye Y, Li J. Early-warning Immune predictors for Invasive Pulmonary aspergillosis in severe patients with severe fever with Thrombocytopenia Syndrome. Front Immunol. 2021;12:576640.
Ge HH, Wang G, Guo PJ, Zhao J, Zhang S, Xu YL, Liu YN, Ye XL, Wu YX, Li S, Yue M, Ji WJ, Geng SY, LiH, Zhang XA, Yang ZD, Cui N, Li W, Lin L, Liu W. Coinfections in hospitalized patients with severe fever with thrombocytopenia syndrome: a retrospective study. J Med Virol. 2022;94(12):5933–42.
Xia B, Wang X, Yang R, Mengzhen L, Yang K, Ren L, Li S, Wang S, Zhang Y. Epstein-Barr virus infection is associated with clinical characteristics and poor prognosis of multiple myeloma. Biosci Rep. 2019;39(10).
Hu J, Zhao H, Lou D, Gao H, Yang M, Zhang X, Jia H, Li L. Human cytomegalovirus and Epstein-Barr virus infections, risk factors, and their influence on the liver function of patients with acute-on-chronic liver failure. BMC Infect Dis. 2018;18(1):577.
Meng M, Zhang S, Dong X, Sun W, Deng Y, Li W, Li R, Annane D, Wu Z, Chen D. COVID-19 associated EBV reactivation and effects of ganciclovir treatment. Immun Inflamm Dis. 2022;10(4):e597.
Kang SY, Yoo JR, Park Y, Kim SH, Heo ST, Park SH, Kim M, Kim TJ, Oh S, Lee MS, Kim JM, Cho NH, Lee KM, Lee KH. Fatal outcome of severe fever with thrombocytopenia syndrome (SFTS) and severe and critical COVID-19 is associated with the hyperproduction of IL-10 and IL-6 and the low production of TGF-β. J Med Virol. 2023;95(7):e28894.
Gong L, Zhang L, Wu J, Lu S, Lyu Y, Zhu M, Liu B, Zhu Y, Song D, Su B, Liu Z. Clinical progress and risk factors for death from severe fever with Thrombocytopenia Syndrome: a multihospital Retrospective Investigation in Anhui, China. Am J Trop Med Hyg. 2021;104(4):1425–31.
Xiong L, Xu L, Lv X, Zheng X. Effects of corticosteroid treatment in patients with severe fever with thrombocytopenia syndrome: a single-center retrospective cohort study. Int J Infect Dis. 2022;122:1026–33.
Gao H, Wang B, Yao H, Zhang W, Teng H. Application of blood purification technology in severe fever with thrombocytopenia syndrome. Biotechnol Genet Eng Rev. 2023:1–10.
Chen K, Sun H, Geng Y, Yang C, Shan C, Chen Y. Ferritin and procalcitonin serve as discriminative inflammatory biomarkers and can predict the prognosis of severe fever with thrombocytopenia syndrome in its early stages. Front Microbiol. 2023;14:1168381.
Xie J, Su M, Dang Y, Zhao L. Prognostic Value of Serum Ferritin for patients with severe fever with Thrombocytopenia Syndrome: a single-Center Retrospective Cohort Study. Infect Dis Ther. 2023;12(3):979–88.
Sun Y, Huang T, Hammarström L, Zhao Y. The immunoglobulins: New insights, implications, and applications. Annu Rev Anim Biosci. 2020;8:145–69.
Labrosse R, Barmettler S, Derfalvi B, Blincoe A, Cros G, Lacombe-Barrios J, Barsalou J, Yang N, Alrumayyan N, Sinclair J, Ong MS, Camargo CA Jr., Walter J, Haddad E. Rituximab-induced hypogammaglobulinemia and infection risk in pediatric patients. J Allergy Clin Immunol. 2021;148(2):523–e532528.
Garred P, Tenner AJ, Mollnes TE. Therapeutic targeting of the complement system: from Rare diseases to Pandemics. Pharmacol Rev. 2021;73(2):792–827.
Lee SY, Yun SH, Lee H, Lee YG, Seo G, Kim NH, Park EC, Lee CS, Kim SI. Serum proteomics of severe fever with thrombocytopenia syndrome patients. Clin Proteom. 2022;19(1):32.
Mujal AM, Delconte RB, Sun JC. Natural killer cells: from innate to adaptive features. Annu Rev Immunol. 2021;39:417–47.
Zhao J, Lu QB, Li H, Yuan Y, Cui N, Yuan C, Zhang XA, Yang ZD, Ruan SM, Liu LZ, Du J, Fang LQ, Liu W. Sex differences in Case Fatality rate of patients with severe fever with Thrombocytopenia Syndrome. Front Microbiol. 2021;12:738808.
Cantan B, Luyt CE, Martin-Loeches I. Influenza Infections and emergent viral infections in Intensive Care Unit. Semin Respir Crit Care Med. 2019;40(4):488–97.
Funding
Supported by the National Natural Science Foundation of China (No. 81871242).
Author information
Authors and Affiliations
Contributions
QP and YD participated in the research design, writing of the paper, performance of the research, and data analysis; NH and ZT participated in the research design, revision of the paper, and data analysis; PS, NJ, LS, ZF, RW, XH and KJ participated in the research design and data analysis. JL was responsible for the conceptualization and reviewed and edited the manuscript. All authors approved the final version for publication and agree to be held accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University (protocol no. 2023-SR-336). However, no informed consent was signed. Due to the retrospective nature of the study, except for personal information, only the clinical data of anonymous patients were collected without infringing on the rights and interests of the patients.
Consent for publication
Written informed consent for publication was obtained from all participants and their parents.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pu, Q., Dai, Y., Hu, N. et al. Early predictors of Epstein-Barr virus infection in patients with severe fever with thrombocytopenia syndrome. Virol J 21, 179 (2024). https://doi.org/10.1186/s12985-024-02452-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02452-5