Abstract
Background
In the aftermath of the COVID-19 pandemic, there has been a surge in human metapneumovirus (HMPV) transmission, surpassing pre-epidemic levels. We aim to elucidate the clinical and epidemiological characteristics of HMPV infections in the post-COVID-19 pandemic era.
Methods
In this retrospective single-center study, participants diagnosed with laboratory confirmed HMPV infection through Targeted Next Generation Sequencing were included. The study encompassed individuals admitted to Henan Children's Hospital between April 29 and June 5, 2023. Demographic information, clinical records, and laboratory indicators were analyzed.
Results
Between April 29 and June 5, 2023, 96 pediatric patients were identified as infected with HMPV with a median age of 33.5 months (interquartile range, 12 ~ 48 months). The majority (87.5%) of infected children were under 5 years old. Notably, severe cases were statistically younger. Predominant symptoms included fever (81.3%) and cough (92.7%), with wheezing more prevalent in the severe group (56% vs 21.1%). Coinfection with other viruses was observed in 43 patients, with Epstein–Barr virus (EBV) (15.6%) or human rhinovirus A (HRV type A) (12.5%) being the most common. Human respiratory syncytial virus (HRSV) coinfection rate was significantly higher in the severe group (20% vs 1.4%). Bacterial coinfection occurred in 74 patients, with Haemophilus influenzae (Hin) and Streptococcus pneumoniae (SNP) being the most prevalent (52.1% and 41.7%, respectively). Severe patients demonstrated evidence of multi-organ damage. Noteworthy alterations included lower concentration of IL-12p70, decreased lymphocytes percentages, and elevated B lymphocyte percentages in severe cases, with statistical significance. Moreover, most laboratory indicators exhibited significant changes approximately 4 to 5 days after onset.
Conclusions
Our data systemically elucidated the clinical and epidemiological characteristics of pediatric patients with HMPV infection, which might be instructive to policy development for the prevention and control of HMPV infection and might provide important clues for future HMPV research endeavors.
Similar content being viewed by others
Introduction
Human metapneumovirus (HMPV), a member of paramyxovirus family, was first identified in 2001 [1]. It has since been commonly implicated in acute respiratory tract infections (ARTI) affecting both pediatric and adult populations worldwide. Despite efforts, a live-attenuated recombinant HMPV vaccine has demonstrated inadequate immunogenicity in children aged 6–59 months [2], and to date, no licensed vaccines or specific drugs are available for HMPV infections. Primary infections typically occur before the age of 5 years, with HMPV prevalence among this age group ranging from 1.1% to 86% globally [3]. In 2018, HMPV-associated hospital admissions among children under 5 years old globally amounted to 643,000, with 16,100 (hospital and community) HMPV-associated ARTI deaths [4]. These figures underscore the substantial socio-economic impact and disease burden associated with HMPV infection.
Over the past 3 years, the unprecedented implementation of non-pharmaceutical interventions during the COVID-19 pandemic has significantly impacted the epidemiology of various pediatric infectious diseases [5,6,7]. The return of respiratory virus circulation to pre-pandemic levels is anticipated as COVID-19 mitigation measures gradually ease [8]. The concept of “immunity debt” has been proposed to characterize the paucity of protective immunity resulting from prolonged decreased exposure to various pathogens [9]. In Western Australia, the incidence of HMPV infection surged threefold in 2021 compared to the period of 2017 ~ 2019. Moreover, the proportion of respiratory-coded admissions undergoing HMPV testing doubled in 2021 [10, 11]. Similarly, in the United States, the number of HMPV infections suddenly spiked to record levels in the spring of 2023 (https://www.cdc.gov/surveillance/nrevss/hmpv/region.html). In China, several studies conducted before the COVID-19 pandemic have examined the prevalence and genotypic diversity of HPMV, as well as the epidemiological and clinical characteristics of hospitalized patients with HPMV infection [12,13,14,15,16]. A study conducted in the Netherlands demonstrated that the clinical impact of HMPV infection remained consistent between the non-COVID-19 period and the examined COVID-19 period, with no significant changes observed in terms of incidence and/or disease severity [17]. Nevertheless, the epidemiological and clinical characteristics of HMPV infections have shown disparities following the COVID-19 pandemic. Consequently, there is an urgent need for clinical investigations, particularly focusing on children hospitalized with HMPV infection, to provide a reference for clinical diagnosis and management. In this study, we present a detailed analysis of the clinical and epidemiological features of 96 pediatric patients in central China from April 29 to June 5, 2023. Our findings contribute to a comprehensive understanding of the characteristics of HMPV infections in the post-COVID-19 pandemic era.
Materials and methods
Study design and participants
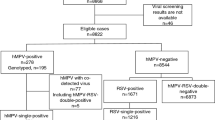
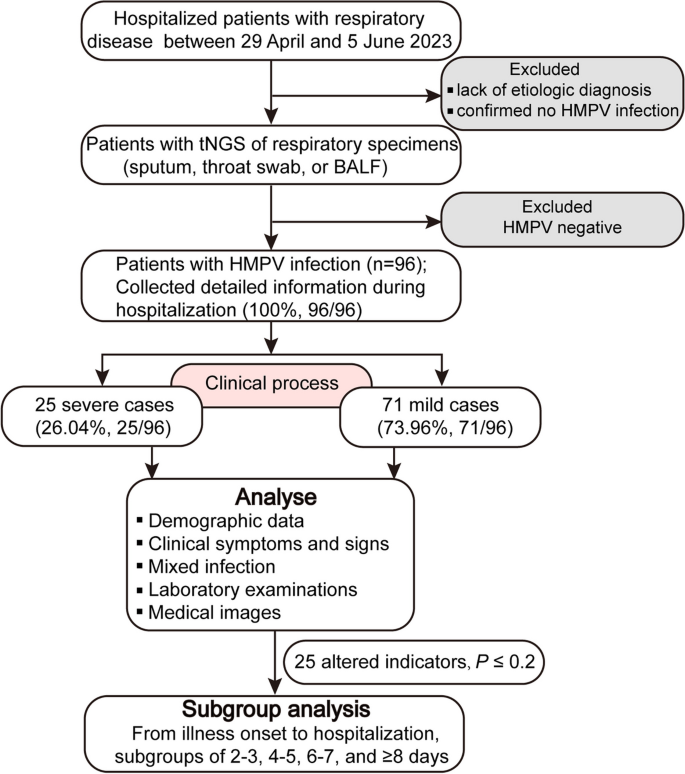
For this retrospective study, we included a total of 96 hospitalized pediatric patients diagnosed with HMPV infection who were admitted to Children’s Hospital Affiliated to Zhengzhou University between 29 April and 5 June 2023 (Fig. 1). Children’s Hospital Affiliated to Zhengzhou University (Henan Children’s Hospital) is the largest tertiary pediatric referral hospital in central China with 2,200 beds, which is located in Zhengzhou of Henan province. Respiratory specimens (including sputum, throat swab, or bronchoalveolar lavage fluid (BALF)) from the majority of hospitalized patients with respiratory illness underwent testing for respiratory pathogens using Targeted Next Generation Sequencing (tNGS) conducted by Guangzhou Kingmed Ctr for Clin Lab Co ltd. (https://www.kingmed.com.cn/) [18]. All enrolled patients were categorized as either mild or severe based on the guidelines for the management of community-acquired pneumonia in children of the People’s Republic of China (2013 Edition) [19, 20]. This observational study was approved by the Committee for Ethical Review of Zhengzhou University (ethical approval No: ZZUIRB2023-180), and written informed consent was obtained from the parents.
Data collection
The electronic medical records data of the included patients underwent independently review and retrospective collection by trained researchers. To ensure quality control, two additional researchers cross-checked the data collection forms. Detailed information was extracted, encompassing sequencing data, demographic data, clinical symptoms and signs, laboratory examinations, medical images, and outcomes of treatment. Laboratory examinations comprised routine testing, coagulation function tests, lymphocyte subsets, inflammatory or infection-related biomarkers, analysis of immunological responses, and measurement of biomarkers for monitoring liver, myocardial, and renal functions.
Statistical analysis
The extracted data were initially entered into Microsoft Excel software (2016), and subsequently imported into SPSS 25.0 or GraphPad Prism 8.3 software for statistical analysis. Binomial or categorical variables were expressed as percentages, while clinical characteristics and laboratory findings (continuous variables) were presented as median with interquartile range (IQR). To compare variables across groups, the Mann–Whitney test was used for continuous variables, and Chi-squared test or Fisher exact test was employed for categorical variables. All statistical tests were two-sided, and a P-value < 0.05 was considered statistically significant.
Results
Epidemiology and demographic characteristics of HPMV infections
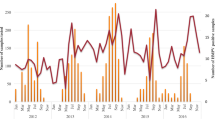
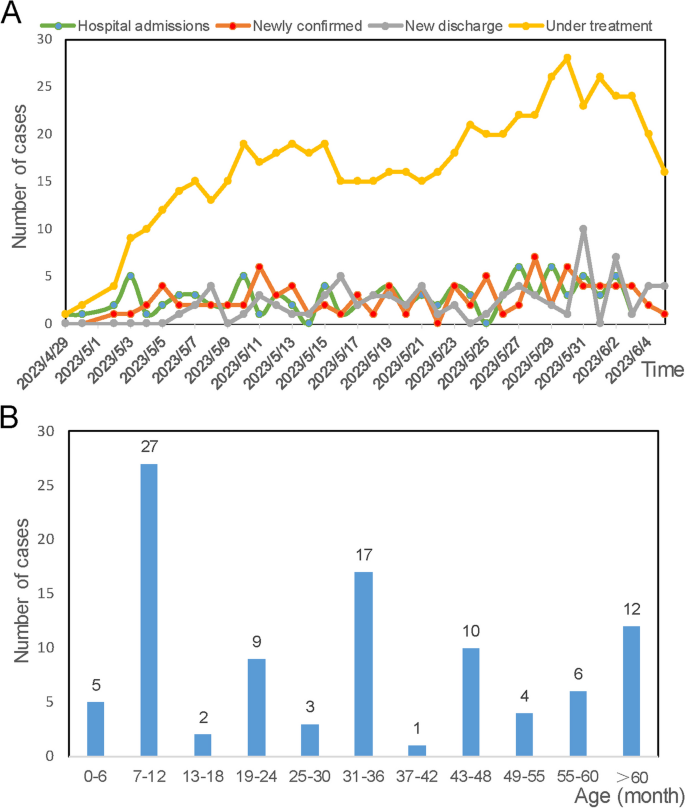
HMPV infections were almost detected and hospitalized every day between April 29 and June 5, 2023. The number of infected patients under treatment is highest at May 30 (28 patients), and 16 infected patients remained hospitalized at June 5 (Fig. 2A). In total, 96 pediatric patients (25 severe cases and 71 mild cases), were included in this study, with a median age of 33.5 months (IQR: 12–48 months). Sequencing data (partial M and N gene) from 9 patients were randomly selected and subjected to analysis. Detailed sequencing data are provided in Supplementary Fig. S1. Through multiple alignments with the nearest homologies from the NCBI databases, it was determined that all the selected viruses belong to subtype A2b. The majority (87.5%, 84/96) of infected children were under 5 years old, and more than half (65.63%, 63/96) were under 3 years old (Fig. 2B). As shown in Table 1, children in the severe group tended to be younger, with a median age of 1 year, compared to a median age of 3 years in the mild group. The age distribution significantly differed between the severe and mild groups (P = 0.005). The male to female ratio was 1.53 (58/38). Although the difference was not significant (P = 0.06, 77.5% vs 48%), a higher proportion of patients in the mild group resided in urban areas.
In Table 1, we present a summary of the characteristics observed in HMPV infections. Upon admission, the majority of patients presented with fever (81.3%, 78/96) or cough (92.7%, 89/96), and almost one-third of patients (30.2%, 29/96) exhibited wheezing. The other 8 patients (8.3%, 9/96) also have exhibited fever, although this symptom was not recorded as the main complaint. Compared to mild cases, a significantly higher proportion of patients in the severe group exhibited wheezing symptoms (P = 0.001, 21.1% vs 56%). Twelve patients presented with dyspnea on admission, all of whom were categorized into the severe group. Furthermore, 11 severe cases were admitted to the Pediatric Intensive Care Unit during their hospitalization. The majority of patients exhibited two or more respiratory symptoms. A higher proportion of patients in the mild group presented with symptoms of both cough and fever (P = 0.003, 66.2% vs 32%). Patients with both cough and wheezing (P = 0.018, 5.6% vs 24.0%), as well as those exhibiting concomitant fever, cough, wheezing (P = 0.063, 12.7% vs 32.0%), were more severely ill. The respiratory rate and heart rate upon admission were significantly higher in severe patients compared to mild cases. Notably, severe patients experienced longer hospital stays, with a median duration of 8 days, in contrast to 6 days for mild patients (P = 0.000321). Clinical respiratory symptoms resolved or disappeared by the time of discharge in almost all patients, except for one critically ill patient with a high suspicion of hemophagocytic syndrome who ultimately dropped out of treatment.
Coinfections with other causative agents based on tNGS
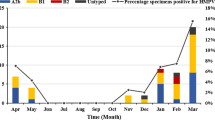
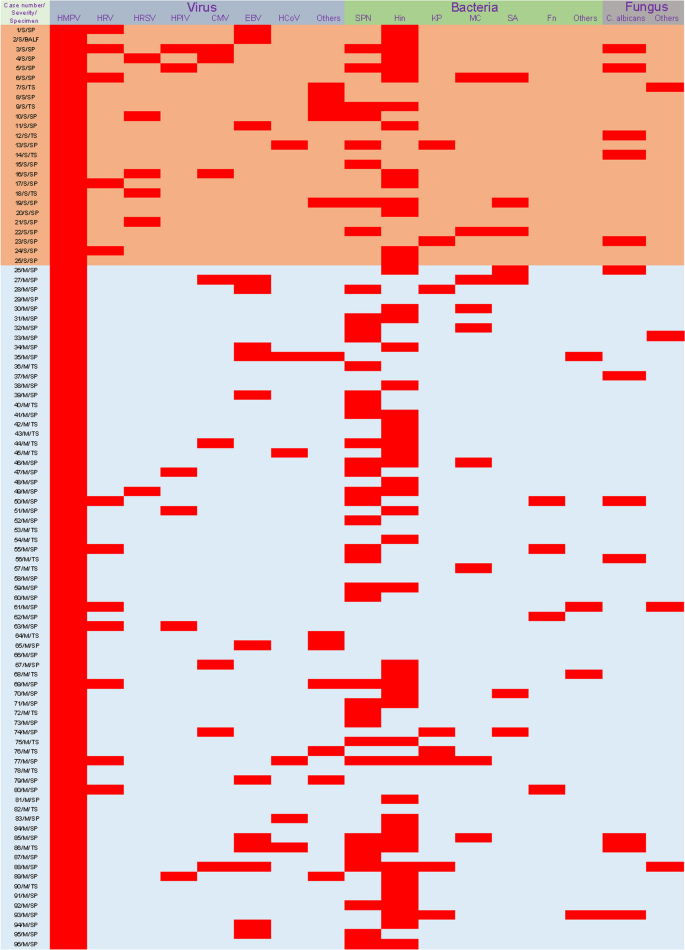
Among the 96 HMPV-infected patients, 91 (94.8%) were coinfected with other causative agents (Table 2). Correspondingly, 5 patients were solely infected with HMPV, presenting symptoms of fever and coughing. Additionally, forty-three patients were infected with another virus. Coinfections of HMPV and EBV (15.6%, 15/96) or HRV type A (12.5%, 12/96) were the most common. The rate of HRSV coinfections (20%) was significantly higher in the severe group compared to the mild group (1.4%). Bacterial coinfections were identified in 74 patients, with Hin detected in 50 children (52.1%), SNP in 40 children (41.7%), MC in 9 children (9.4%), KP in 8 children (8.3%), and SA in 7 children (7.3%). Regarding fungal coinfections, C. albicans infection in the upper respiratory tract was the most prevalent. Further details about the pathogens infecting all patients are shown in Fig. 3.
Coinfections in HMPV-infected patients. Red squares represent infection. Samples from case 44, 55 and 92 were subjected to multiple targeted sequencing of upper respiratory tract pathogens, including 105 pathogens. The others were subjected to targeted sequencing of multiple respiratory pathogens contained 198 pathogens. See Supplementary file 1 for more details of the targeted sequencing project. SP, sputum; TS, throat swab
Laboratory test findings
As shown in Table 3, severe patients exhibited a significantly lower count of EOS (P = 0.001), ESR count (P = 0.013), percentage of BASO (P = 0.013), percentage of EOS (P = 0.000273), and percentage of LYMPH (P = 0.000007), compared to mild group. Conversely, severe patients exhibited an increased percentage of NEUT (P = 0.000022), and a higher count of NEUT (P = 0.001), MONO (P = 0.04). Additionally, severe patients exhibited more evidence of multiple-organ damage compared to mild cases, as indicated by higher levels of unconjugated bilirubin (P = 0.038), alanine aminotransferase (P = 0.004), aspartate aminotransferase (P = 0.014), gamma-glutamyl transferase (P = 0.000004), lactate dehydrogenase (P = 0.004), and creatine kinase-MB (P = 0.035). In contrast, levels of conjugated bilirubin (P = 0.032), creatinine (P = 0.014) and uric acid (P = 0.04) decreased. Furthermore, several coagulation-related indices showed statistical significance between the two groups, including prolonged thrombin time, elevated prothrombin activity, decreased fibrinogen concentration, shortened prothrombin time and reduced international normalized ratio in severe group. Besides, decreased concentrations of Immunoglobulin G and Immunoglobulin A were observed in the severe group compared to the mild group. Other laboratory indices or organ damage biomarkers in the two groups were presented in Table 3.
Inflammatory responses in HMPV-infected patients
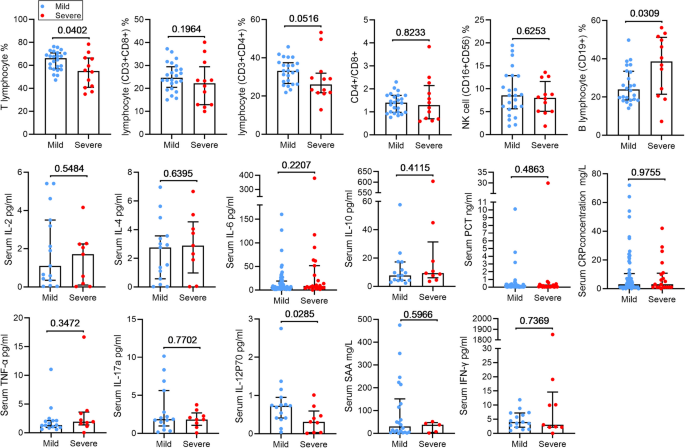
To further elucidate the immune response, we analyzed the changes in lymphocyte subpopulations, serum cytokines, and chemokines levels were also analyzed (Fig. 4). We observed higher levels of cytokines, including interleukin (IL)-2, IL-4, IL-6, IL-10, tumor necrosis factor (TNF) -α, and SAA in severe patients compared to mild patients, although these differences did not reach statistical significance. Conversely, the levels of interferon (IFN)-γ, IL-17a, IL-12p70, were decreased in the severe patients, but only the lower levels of IL-12p70 exhibited statistical significance between the two groups (P = 0.029). Regarding immune cells, the decline in lymphocytes percentage was more pronounced in severe patients (P = 0.0402), while the percentage of B lymphocyte was significantly elevated (P = 0.0309). There was no significant difference in the percentage of CD4 + T cells, CD8 + T cell, and NK cell, as well as the ratio of CD4 + T cells to CD8 + T cells between the two groups. Notably, severe patients exhibited more pronounced aggravated inflammatory responses, lymphopenia, and multiple-organ damage compared to those in mild cases.
Dynamic change of several altered indicators
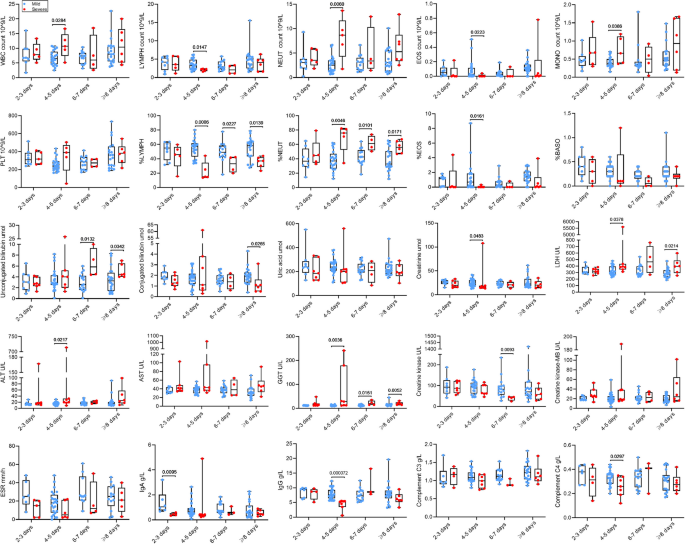
All subjects were stratified into different subsets based on the duration from illness onset to hospitalization, including subsets of 2–3, 4–5, 6–7, and ≥ 8 days. The altered indicators (P ≤ 0.2) were analyzed within these subgroups. As shown in Fig. 5, there was no statistically significant difference in PLT, percentage of BASO, uric acid, AST, CK-MB, ESR and C3 between the two groups. Obviously, most indicators exhibited significant changes after 4–5 days of illness onset. Compared to mild patients, severe cases exhibited significantly increased WBC count, NEUT count, MONO count, percentage of NEUT, and LDH, ALT, GGT in the peripheral blood at 4–5 days after illness onset. Conversely, LYMPH count, EOS count, percentage of LYMPH, percentage of LYMPH, and creatinine, IgG, and C4 in the serum were significantly reduced during the same period. The changes in percentage of LYMPH and NEUT remained consistent after 4–5 days after illness onset. Figure 5 showed specific changes in other indicators.
Subgroup analyses of laboratory indicators. The box plots show the medians (middle line) and first and third quartiles (boxes), and the whiskers show range of the measured values.
Abbreviations: WBC white blood cell, PLT platelet count, NEUT neutrophil, LYMPH lymphocyte, MONO monocyte, EOS eosinophilic granulocyte, BASO basophilic granulocyte, LDH lactate dehydrogenase, ALT alanine aminotransferase, AST aspartate aminotransferase, GGT gamma-glutamyl transferase, ESR erythrocyte sedimentation rate, Ig immunoglobulin
Radiologic findings
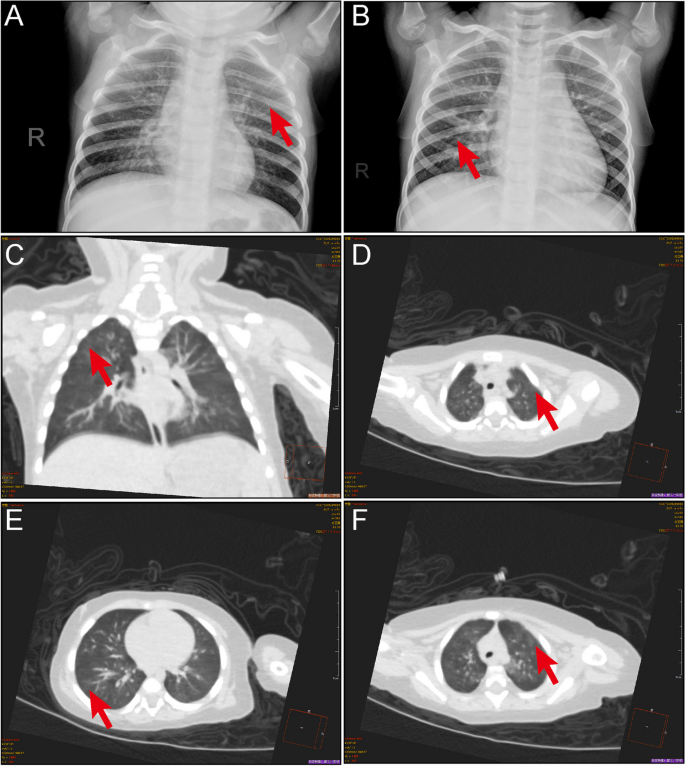
As mentioned previously, nearly all patients were coinfected with other causative agents, while only 5 patients were solely infected with HMPV. Among these, imaging examinations were conducted for three of the five patients. Despite presenting with mild clinical symptoms such as fever and cough, all three patients exhibited abnormal radiological findings, including focal ground-glass opacities and/or stripe shadows (Fig. 6).
Pulmonary manifestations of HMPV infection. A CT scan of 1-year-old girl showing multiple ground-glass opacities in both lungs (arrow). B Chest radiograph of a 4-year-old girl displaying multiple ground-glass opacities (arrow). C-F Chest CT scans from a 7-month-old girl showing focal ground-glass opacities and stripes coexisting in the lung (arrows)
Discussion
The etiological significance of HMPV in ARTI has been the focus of increasing attention worldwide [3]. HMPV infection among hospitalized children with ARTIs experienced a significant decrease during COVID-19 pandemic [14]. However, with the relaxation of COVID-19 mitigation measures, HMPV transmission has surpassed pre-epidemic levels [10]. The immunity debt [9] and the alterations in prevalent genetic subtypes [14] following the COVID-19 pandemic have raised our concerns. Hence, this study aimed to evaluate the epidemiological and clinical characteristics of HMPV infections in hospitalized children in Henan, China from April 29 to June 5, 2023.
In this study, we observed that the majority (87.5%) of infected children were under 5 years, with more than half (65.63%) being under 3 years old. Previous study has indicated that the hospital admission rate for HMPV-associated ARTI is notably higher among infants compared to older children, with approximately 58% of hospital admissions in children under 5 years occurring within the first year of life [4, 21]. The elevated burden on infants may be attributed to the immaturity of their immune systems and the decline of maternal antibodies during the first months of life [22].
Upon admission, we observed that patients presenting with concurrent cough and wheezing, as well as those with simultaneous fever, cough, and wheezing, tended to be more severely ill. Generally, HMPV-associated respiratory diseases manifest along a spectrum from mild fever and cough to severe bronchiolitis and pneumonia [13, 15]. A study involving hospitalized children in Beijing similarly reported that the majority of patients presented with cough and fever [14]. Wheezing is a common manifestation in children with HMPV infection, often progressing to life-threatening bronchiolitis and pneumonia [23]. A study has indicated that 13.0% ~ 60.7% of children infected with HMPV experience recurrent wheezing or receive a diagnosis of asthma [24]. However, distinguishing HMPV-associated pneumonia from infection caused by other pathogens based solely on clinical features remains challenging [25,26,27]. Several respiratory pathogens, including HMPV, exhibit similar incidence rates and clinical characteristics, thereby posing a diagnostic challenge for attending physicians.
A previous study revealed that HMPV was the third most common virus associated with coinfection [28]. Viral cocirculation can lead to competition among viruses, whereby the stronger virus may survive and/or mutate, potentially resulting in higher mortality and morbidity rates [29]. In our study, we identified 43 patients who were coinfected with another virus, with coinfections of HMPV with EBV (15.6%) or HRV type A (12.5%) being common. EBV infects over 95% of the global population and is often asymptomatic, typically occurring at a young age [30]. Unlike EBV, HRV type A is responsible for more than 50% of upper respiratory tract infections globally and it is the most common virus associated with wheezing in children aged between one and two years [31]. Previous studies have identified influenza, HRSV, adenoviral, and human bocavirus as the most common respiratory viruses involved in coinfections [14, 15, 23, 32]. Our results findings diverged from previously published data, which could be attributed to the seasonality of different viruses [33], variations in sample types, and differences in sample sizes among studies. In our study, the rate of HRSV coinfections was significantly higher in the severe group compared to the mild group. Previous research has indicated that dual infections with HMPV and RSV are associated with severe bronchiolitis and an increased risk of Intensive Care Unit admission for mechanical ventilation [34]. Additionally, coinfections with bacterial pathogens often lead to a more severe course and increased mortality. While HMPV-associated pneumonia is generally less severe than bacterial pneumonia, it can be as severe as or even more severe than infections caused by other common pathogens [27]. In our study, Hin and SNP were the two most common bacterial coinfections, accounting for 52.1% and 41.7%, respectively. Therefore, further data are necessary to elucidate the cumulative clinical effects on disease severity resulting from coinfecting with other respiratory pathogens.
Significant alterations in several indicators among severe patients were notably observed at 4–5 days after the onset of illness, marking a critical time point for disease progression, and necessitating timely decisions regarding treatment interventions. Therefore, we should pay more attention to the diagnosis and intervention in the early stage of the disease. In our study, we observed a lower level of IL-12p70 in severe patients. However, previous research suggested that HMPV failed to induce production of IL-12p70 in BALF in a mouse model [35]. In terms of immune cells, we observed a more pronounced decline in the lymphocyte percentage among severe patients, while the percentage of B lymphocyte was significantly elevated. In other words, the function of the specific cellular immunity was decreased, while the function of humoral immune was increased. Nevertheless, the decreased concentrations of Immunoglobulin G and Immunoglobulin A in the severe group contradicted these results. In fact, interpreting these results is often challenging due to the complexities of the disease and the involvement of mixed infections. In clinical practice, patients with single HMPV infections are rare, emphasizing the necessity for further research on patients with mixed infections.
This study also had several limitations. Firstly, our focus was primarily on the epidemiological and clinical features of HMPV positive cases. Thus, other common respiratory pathogens were not excluded. Subsequently, our results more reflect the cumulative clinical effects of coinfection. Secondly, children with more severe symptoms are more likely to receive hospital care, which may affect our understanding of the clinical features of HMPV-positive patients. Despite these shortcomings, our results offer a valuable insight into the epidemiological and clinical characteristics of hospitalized patients with HMPV infection in Henan, China.
Conclusion
Our study elucidated important epidemiological and clinical characteristics of HMPV infection in Henan, China. These findings hold potential significance for informing policy development aimed at the prevention and control of HMPV infection. Additionally, our results may provide important insights for guiding HMPV research efforts in the post- COVID-19 pandemic era.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- ARTI:
-
Acute respiratory tract infections
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- α-HBBDH:
-
α-hydroxybutyrate dehydrogenase
- BALF:
-
Bronchoalveolar lavage fluid
- BASO:
-
Basophilic granulocyte
- CK:
-
Creatine kinase
- CK-MB:
-
Creatine kinase-MB
- CRP:
-
C-reactive protein
- CMV:
-
Cytomegalovirus
- C. albicans:
-
Canidia albicans
- EOS:
-
Eosinophilic granulocyte
- ESR:
-
Erythrocyte sedimentation rate
- EBV:
-
Epstein–Barr virus
- GGT:
-
Gamma-glutamyl transferase
- HRV-A:
-
Human rhinovirus A
- HRSV:
-
Human respiratory syncytial virus
- HPIV type3:
-
Human parainfluenza virus type 3
- HCoV:
-
Human coronavirus
- Hin:
-
Haemophilus influenza
- HMPV:
-
Human metapneumovirus
- IQR:
-
Interquartile range
- Ig:
-
Immunoglobulin
- IFN:
-
Interferon
- IL:
-
Interleukin
- KP:
-
Klebsiella pneumoniae
- LYMPH:
-
Lymphocyte
- LDH:
-
Lactate dehydrogenase
- MONO:
-
Monocyte
- MC:
-
Moraxella catarrhalis
- NEUT:
-
Neutrophil
- PLT:
-
Platelet count
- PCT:
-
Procalcitonin
- RBC:
-
Red blood cell
- SPN:
-
Streptococcus pneumoniae
- SAA:
-
Serum amyloid A
- SA:
-
Staphylococcus aureus
- TNF:
-
Tumor necrosis factor
- tNGS:
-
Targeted Next Generation Sequencing
- WBC:
-
White blood cell
References
van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–24.
Karron RA, San Mateo J, Wanionek K, Collins PL, Buchholz UJ. Evaluation of a Live Attenuated Human Metapneumovirus Vaccine in Adults and Children. J Pediatric Infect Dis Soc. 2018;7(1):86–9.
Divarathna MVM, Rafeek RAM, Noordeen F. A review on epidemiology and impact of human metapneumovirus infections in children using TIAB search strategy on PubMed and PubMed Central articles. Rev Med Virol. 2020;30(1): e2090.
Wang X, Li Y, Deloria-Knoll M, Madhi SA, Cohen C, Ali A, et al. Global burden of acute lower respiratory infection associated with human metapneumovirus in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob Health. 2021;9(1):e33–43.
Belingheri M, Paladino ME, Piacenti S, Riva MA. Effects of COVID-19 lockdown on epidemic diseases of childhood. J Med Virol. 2021;93(1):153–4.
Izu A, Nunes MC, Solomon F, Baillie V, Serafin N, Verwey C, et al. All-cause and pathogen-specific lower respiratory tract infection hospital admissions in children younger than 5 years during the COVID-19 pandemic (2020–22) compared with the pre-pandemic period (2015–19) in South Africa: an observational study. Lancet Infect Dis. 2023.
Perez A, Lively JY, Curns A, Weinberg GA, Halasa NB, Staat MA, et al. Respiratory Virus Surveillance Among Children with Acute Respiratory Illnesses - New Vaccine Surveillance Network, United States, 2016–2021. MMWR Morb Mortal Wkly Rep. 2022;71(40):1253–9.
Hatter L, Eathorne A, Hills T, Bruce P, Beasley R. Respiratory syncytial virus: paying the immunity debt with interest. Lancet Child Adolesc Health. 2021;5(12):e44–5.
Cohen R, Ashman M, Taha MK, Varon E, Angoulvant F, Levy C, et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now. 2021;51(5):418–23.
Foley DA, Yeoh DK, Minney-Smith CA, Shin C, Hazelton B, Hoeppner T, et al. A surge in human metapneumovirus paediatric respiratory admissions in Western Australia following the reduction of SARS-CoV-2 non-pharmaceutical interventions. J Paediatr Child Health. 2023;59(8):987–91.
Foley DA, Sikazwe CT, Minney-Smith CA, Ernst T, Moore HC, Nicol MP, et al. An Unusual Resurgence of Human Metapneumovirus in Western Australia Following the Reduction of Non-Pharmaceutical Interventions to Prevent SARS-CoV-2 Transmission. Viruses. 2022;14(10).
Zhao H, Feng Q, Feng Z, Zhu Y, Ai J, Xu B, et al. Clinical characteristics and molecular epidemiology of human metapneumovirus in children with acute lower respiratory tract infections in China, 2017 to 2019: A multicentre prospective observational study. Virol Sin. 2022;37(6):874–82.
Wang C, Wei T, Ma F, Wang H, Guo J, Chen A, et al. Epidemiology and genotypic diversity of human metapneumovirus in paediatric patients with acute respiratory infection in Beijing, China. Virol J. 2021;18(1):40.
Cong S, Wang C, Wei T, Xie Z, Huang Y, Tan J, et al. Human metapneumovirus in hospitalized children with acute respiratory tract infections in Beijing. China Infect Genet Evol. 2022;106: 105386.
Zeng SZ, Xiao NG, Zhong LL, Yu T, Zhang B, Duan ZJ. Clinical features of human metapneumovirus genotypes in children with acute lower respiratory tract infection in Changsha. China J Med Virol. 2015;87(11):1839–45.
Zhang C, Du LN, Zhang ZY, Qin X, Yang X, Liu P, et al. Detection and genetic diversity of human metapneumovirus in hospitalized children with acute respiratory infections in Southwest China. J Clin Microbiol. 2012;50(8):2714–9.
Jongbloed M, Leijte WT, Linssen CFM, van den Hoogen BG, van Gorp ECM, de Kruif MD. Clinical impact of human metapneumovirus infections before and during the COVID-19 pandemic. Infect Dis (Lond). 2021;53(7):488–97.
Li SY, Tong J, Liu Y, Shen W, Hu P. Targeted next generation sequencing is comparable with metagenomic next generation sequencing in adults with pneumonia for pathogenic microorganism detection. J Infection. 2022;85(5):E127–9.
Subspecialty Group of Respiratory Diseases TSoP, Chinese Medical Association The Editorial Board CJoP. [Guidelines for management of community acquired pneumonia in children(the revised edition of 2013) (II)]. Zhonghua Er Ke Za Zhi. 2013;51(11):856-62.
Subspecialty Group of Respiratory Diseases TSoPCMA, Editorial Board CJoP. [Guidelines for management of community acquired pneumonia in children (the revised edition of 2013) (I)]. Zhonghua Er Ke Za Zhi. 2013;51(10):745-52.
Wang X, Li Y, O’Brien KL, Madhi SA, Widdowson MA, Byass P, et al. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob Health. 2020;8(4):e497–510.
Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 1821;2015(282):20143085.
Schildgen V, van den Hoogen B, Fouchier R, Tripp RA, Alvarez R, Manoha C, et al. Human Metapneumovirus: lessons learned over the first decade. Clin Microbiol Rev. 2011;24(4):734–54.
Coverstone AM, Wang L, Sumino K. Beyond Respiratory Syncytial Virus and Rhinovirus in the Pathogenesis and Exacerbation of Asthma: The Role of Metapneumovirus, Bocavirus and Influenza Virus. Immunol Allergy Clin North Am. 2019;39(3):391–401.
Wilkesmann A, Schildgen O, Eis-Hubinger AM, Geikowski T, Glatzel T, Lentze MJ, et al. Human metapneumovirus infections cause similar symptoms and clinical severity as respiratory syncytial virus infections. Eur J Pediatr. 2006;165(7):467–75.
Cui A, Xie Z, Xu J, Hu K, Zhu R, Li Z, et al. Comparative analysis of the clinical and epidemiological characteristics of human influenza virus versus human respiratory syncytial virus versus human metapneumovirus infection in nine provinces of China during 2009–2021. J Med Virol. 2022;94(12):5894–903.
Howard LM, Edwards KM, Zhu Y, Grijalva CG, Self WH, Jain S, et al. Clinical Features of Human Metapneumovirus-Associated Community-acquired Pneumonia Hospitalizations. Clin Infect Dis. 2021;72(1):108–17.
Zhu Y, Xu B, Li C, Chen Z, Cao L, Fu Z, et al. A Multicenter Study of Viral Aetiology of Community-Acquired Pneumonia in Hospitalized Children in Chinese Mainland. Virol Sin. 2021;36(6):1543–53.
Nickbakhsh S, Mair C, Matthews L, Reeve R, Johnson PCD, Thorburn F, et al. Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc Natl Acad Sci U S A. 2019;116(52):27142–50.
Damania B, Kenney SC, Raab-Traub N. Epstein-Barr virus: Biology and clinical disease. Cell. 2022;185(20):3652–70.
Vandini S, Biagi C, Fischer M, Lanari M. Impact of Rhinovirus Infections in Children. Viruses. 2019;11(6).
Du Y, Li W, Guo Y, Li L, Chen Q, He L, et al. Epidemiology and genetic characterization of human metapneumovirus in pediatric patients from Hangzhou China. J Med Virol. 2022;94(11):5401–8.
Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of Respiratory Viral Infections. Annu Rev Virol. 2020;7(1):83–101.
Esposito S, Daleno C, Prunotto G, Scala A, Tagliabue C, Borzani I, et al. Impact of viral infections in children with community-acquired pneumonia: results of a study of 17 respiratory viruses. Influenza Other Respir Viruses. 2013;7(1):18–26.
Guerrero-Plata A, Casola A, Garofalo RP. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. J Virol. 2005;79(23):14992–7.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (YJ., NO. 82002147 and NO. 82372229), China Postdoctoral Science Foundation (YJ., NO. 2019M662543), and by Open Research Fund of National Health Commission Key Laboratory of Birth Defects Prevention & Henan Key Laboratory of Population Defects Prevention (YJ., NO. ZD202301).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design. Conceptualization: Wangquan Ji, Fang Wang, and Yuefei Jin; Methodology: Qingmei Wang, Jiaying Zheng, Chenyu Wang, Qiujing Liang, and Shujuan Han; Formal analysis and investigation: Yu Chen, Shujie Han, Bowen Dai, Kang Li, Shuang Li, Zijie Li, Qingmei Wang, Jiaying Zheng, Chenyu Wang, Qiujing Liang, Xiaolong Li, and Shujuan Han; Writing - original draft preparation: Wangquan Ji and Yuefei Jin; Writing - review and editing: Yuefei Jin, and Fang Wang; Funding acquisition: Yuefei Jin; Resources: Shouhang Chen, Yaodong Zhang, Xiaolong Zhang, and Ruyu Zhang; Supervision: Yuefei Jin, and Fang Wang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study received ethical approval from the Committee for Ethical Review of Zhengzhou University (ethical approval No: ZZUIRB2023-180) and written informed consent was obtained from the parents.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 6.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ji, W., Chen, Y., Han, S. et al. Clinical and epidemiological characteristics of 96 pediatric human metapneumovirus infections in Henan, China after COVID-19 pandemic: a retrospective analysis. Virol J 21, 100 (2024). https://doi.org/10.1186/s12985-024-02376-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02376-0