Abstract
Objective
Routine viral load and drug resistance testing are well supported in most resource-rich settings and provide valuable benefits in the clinical care of PLWH in these communities. Undoubtedly, there exist financial and political constraints for the scale-up of viral load and drug resistance testing in Sub-Saharan Africa. To achieve the global UNAIDS 95/95/95 targets, there is the need to bridge this inequity in patient care and allow for a universal approach that leaves no community behind.
Methods
Venous blood from 96 PLWH on second-line ART from Korle-Bu Teaching Hospital were collected and processed into plasma for CD4+ T- cell and viral load assessments. Ribonucleic acid (RNA) was extracted from stored plasma and the protease gene amplified, sequenced and analyzed for subtype and drug resistance mutations using the Stanford HIV drug resistance database.
Results
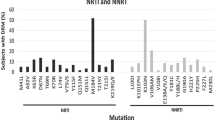
Out of the 96 PLWH, 37 experienced virological failure with 8 patients’ samples successfully sequenced. The predominant HIV-1 subtype identified was CRF02_AG (6/8, 75.0%) with 12.5% (1/8) each of CFR06_cpx infection and one case unable to subtype. The major PI resistance mutations identified were; M46I, I54V, V82A, I47V, I84V and L90M.
Conclusions
Persons living with HIV who had experienced virologic failure in this study harboured drug resistance mutations to PI, thus compromise the effectiveness of the drugs in the second line. Resistance testing is strongly recommended prior to switching to a new regimen. This will help to inform the choice of drug and to achieve optimum therapeutic outcome among PLWH in Ghana.
Highlights
Protease inhibitors (PI) are prescribed for patients failing on reverse transcriptase-based regimen without adequate monitoring for viral load and drug resistance testing.
The study provides evidence of PI resistance mutations and their implications for the relatively fewer individuals on PI-based regimen in the era of dolutegravir (DTG) use.
Close monitoring on all HIV infected patients on PI is needed.
Similar content being viewed by others
Introduction
At the end of 2022, an estimated number of 39 million people were living with HIV (PLWH) globally with 25.6 million in Africa [1]. Within the same year, 1.3 million new infections and 630,000 deaths from AIDS-related illnesses were reported [1]. In Ghana, 354,927 people were living with HIV with prevalence rate of 1.7% at the end of 2021 [2, 3]. Since the scale up of anti-retroviral therapy (ART), gradual increase in resistance to the drugs in Sub-Saharan Africa has been reported in several studies which has warranted current recommendations on ARTs for treatment and prevention [4, 5]. Protease inhibitors (PI) generally have high genetic barrier to the selection of ART resistant variants, which means after failure with first line drugs, suppression with PI-based regimen could be achieved [6]. Even though there is increased ART coverage, the price of PI such as ritonavir-boosted lopinavir (LPV/r) and ritonavir-boosted atazanavir (ATV/r) are still about two-fold higher than the first-line drugs in low resource countries [7].
Genotyping to confirm drug resistance mutations is key to inform therapy especially in cases where viremia is detected [8, 9]. However, this is not the case for most resource-limited settings including Ghana where genotypic antiretroviral testing (GART) presents financial and technical burden. Present data from the Ghana AIDS Commission (GAC) on the UNAIDS 95-95-95 target reveals a deficiency in optimizing GART to guide therapy and achieve the UNAIDS targets [2]. Better therapeutic outcome from second-line regimen amongst persons failing first-line therapy in Ghana is of great concern due to the extensive use of PI as the ART of choice in second and third-line regimens [10]. Even though, recently, there are integrase inhibitors such as dolutegravir (DTG) rolled-out in the ART guidelines in Ghana and other low-and middle-income countries [10,11,12], their cost and accessibility has been factors for their relatively slower use in patients’ regimen [13]. Currently, there is still a minority of patients still on PI, but with emphasis on DTG therapy it will be important to understand the dynamics of PI resistance to enable proper monitoring of PLWH on PIs. This study therefore sought to determine drug resistance mutations to PI containing regimen among PLWH virologically failing second-line antiretroviral therapy in Ghana.
Methods
Study design and population
A total of 96 PLWH on antiretroviral regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) and one PI consented and enrolled onto the study between July 2016 and June 2017.
Laboratory analysis
The CD4+ T-cell count and plasma HIV-1 viral load were determined at the Fever’s unit laboratory immediately after sample collection. All viral RNA extraction, complementary DNA synthesis, nested-polymerase chain reaction and sequencing were done as previously published [14].
Phylogenetic analysis
Nucleotide sequences for each sample were assembled to form a contig using SeqManPro 13 (DNASTAR Incorporation, U.S.A). Consensus sequence obtained was aligned with an HIV reference sequence (B-HXB2-PRT_2253–3700) in BioEdit (http://www.mbio.ncsu.edu/Bioedit/bioedit.html). Sequences were submitted to the Stanford University HIV Drug Resistance Database (https://hivdb.stanford.edu/hivdb/by-sequences/) to assign subtypes and analysed for HIV drug resistance mutations. The subtypes were confirmed with the Los Alamos National Laboratory HIV Database (http://www.hiv.lanl.gov). Neighbor-joining tree with the Kimura’s 2-parameter distances was employed in Molecular Evolutionary Genetic Analysis version 6.0 (MEGA 6).
Statistical analysis
The mean CD4 count and mean viral load were calculated across patient demographics and statistical significance determined at 95% confidence level via non-parametric tests- Mann-Whitney U test for two patient categories (sex, adherence at 1 week and adherence at 1 month) and Kruskal-Wallis H test for four patient categories (age group & education). All analyses were done with Microsoft Excel and Statistical Package for Social Science (SPSS version 22).
Results
Study population
Ninety-six (96) PLWH were enrolled in this study. Most of the respondents 66% (n = 63) were above 40 years and were females, 70% (n = 67). A high non-adherence level of 96% (n = 92) was observed in the week of medication, which increased to 97% (n = 93) after a month where one patient was reported to be non-adherent after initial adherence at 1 week (Table 1). Importantly, there was significant reduction in viral load copies within 1 month of adherence but not with CD4+ T cell count. Also, significance was observed for viral load copies with educational level where patients with tertiary level education had the lowest relative mean viral load count of 4542.09 copies/ml and those without any formal education reported to have the highest mean viral load of 143697.40 copies/ml (Table 1).
ART prescriptions for enrolled patients
Records from patient folders indicated that they were on second-line ART regimen which were primarily made of two of NRTIs; Lamivudine (3TC), Emtricitabine (FTC), Tenofovir (TDF), Zidovudine (ZDV), and either LPV/r or ATV/r as PI in the following combinations; TDF/FTC/LPV/r- 10 (9.8%), 3TC/TDF/LPV/r- 43 (42.2%), 3TC/TDF/ATV/r- 15 (14.7%), 3TC/ZDV/LPV/r- 20 (19.6%), 3TC/ZDV/ATV/r- 13 (12.7%) and TDF/FTC/ATV/r- 1 (0.9%) (Table 2).
Phylogenetic analysis of PR gene sequences
Phylogenetic analysis of PR gene sequences. A phylogenetic analysis using the eight successfully sequenced samples was done. Except for one sequence, PI0011_PRTS_GHA that clustered with the Burkinabe HIV-isolate B202 C1_BFA, study sequences clustered well with one Nigerian sequence (HQ843555.1 HIV-1 isolate_07NG.SN330_NIG). The red squares represent the sequences under consideration
Discussion
This study sought to investigate the PI drug resistance mutations among PLWH virologically failing second line antiretroviral therapy. Most of the participants were females, and aged above 40 years, this agrees with other studies in Sub-Saharan Africa and the nationally reported incidence of HIV in Ghana where females happen to be more affected than males [2, 15].
In most Sub-Saharan African countries including Ghana, where there exist technical and financial challenges in routinely assessing viral load and drug resistance testing in patient care, assessing CD4+ T-cell counts and clinical symptoms are necessary to monitoring the success of ART, hence their continuous and consistent investigation is important [16]. In this study, a significant reduction in viral load was observed after 1 month of adherence but relatively stable CD4+ T cell count. The importance of monitoring viral load for patient management has been well communicated by the World Health Organisation (WHO) [17, 18]. In the recent 2023 International AIDS Society (IAS) conference in Australia, the WHO reported evidence of achieving better treatment outcomes and programmatic success such as in U = U (Undetectable = Untransmissible) when viral load is below < 1000 copies/ml [19]. Even though, monitoring CD4 + T cell count is accepted, it is not recommended and done occasionally when countries can afford the cost of routine viral load. Thus, most developed settings do not routinely use it in patient care as several clinical factors can affect the results [20].
Overall, the trends suggested that the second-line drugs used over the period had been effective, and only when used appropriately. Notwithstanding their effectiveness, only about a limited number of HIV patients in Ghana are on PI partly due to financial constraints and unavailability [10]. These findings confirmed the self-reported adherent behaviour from patients reporting low adherence rate of 97% for one month. This agrees with other studies that showed that patients with 100% adherence had reduced risk of developing resistance to PI boosted regimens [21]. Again, tertiary educational level significantly correlated with reduced viral load copies. Even though this would need a more prospective study to arrive at concrete conclusions, patients who fairly appreciate their clinical situation and the importance of taking their medication and assessing their viral load can benefit the patient and healthcare providers [22, 23].
The 463 bp fragments of the protease coding genes of the pol gene of HIV-1 were amplified and sequenced. All mutations were non-polymorphic and have been associated with LPV/r and ATV/r. It is worth noting that a significant number of PLWH in Ghana are on LPV/r and ATZ/r regimens [10]. Positions 46, 54 and 82 of the protease gene are the main sites for developing a high-level resistance to LPV/r [24], whereas positions 84 and 90 are known to cause high-level resistance to ATV/r [25]. The failure to continuously assess patients for drug resistance variants impact treatment success. The findings corroborates with studies in South Africa and Tanzania where a third of South African patients had PI-drug resistance mutations impacting treatment with darunavir-containing regimen [26] and those in Tanzania with treatment failure after 6–12 months of PI-containing therapy [27]. We recommend here the use a drug safety monitory protocol among clinicians as part of treatment guidelines. Even though the study measured viral load at 1 week and 1 month timepoints and studied fewer patients who consented to make their clinical folders accessible, the clinical picture of the patients in the study emphasizes the need for political and financial willingness to implement HIV viral load monitoring, and couple it with genotypic antiretroviral testing for PLWH failing therapy as a standard of care in Ghana, especially in the era of DTG roll-out.
Conclusion
The results indicate that majority of patients who have previously failed first line therapy and currently receiving PI-based second-line ART at the Korle-Bu Teaching Hospital were still not virologically unsuppressed due to reported cases of non-adherence. This presents with programmatic concerns and thus, routine HIV-1 viral load and drug resistance testing is necessary to early detect virologic failure and guide treatment.
Data availability
The data sets analysed during this study are available with the corresponding author on request.
References
WHO. World Health Organization. HIV and AIDS- Key facts. 2023.
GAC, Ghana AIDS. Commission: People Living with HIV in Ghana. 2023.
WB. The World Bank. Prevalence of HIV, total (% of population ages 15–49.)– Ghana 2021.
Hammer SM, Saag MS, Schechter M, Montaner JS, Schooley RT, Jacobsen DM, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society–USA panel. JAMA. 2006;296(7):827–43.
Gandhi RT, Bedimo R, Hoy JF, Landovitz RJ, Smith DM, Eaton EF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society–USA panel. JAMA. 2023;329(1):63–84.
Garone D, Conradie K, Patten G, Cornell M, Goemaere E, Kunene J, et al. High rate of virological re-suppression among patients failing second-line antiretroviral therapy following enhanced adherence support: a model of care in Khayelitsha, South Africa. South Afr J HIV Med. 2013;14(4):170–5.
Nichols BE, Sigaloff KC, Kityo C, Hamers RL, Baltussen R, Bertagnolio S, et al. Increasing the use of second-line therapy is a cost‐effective approach to prevent the spread of drug‐resistant HIV: a mathematical modelling study. J Int AIDS Soc. 2014;17(1):19164.
Hosseinipour MC, Gupta RK, Van Zyl G, Eron JJ, Nachega JB. Emergence of HIV drug resistance during first-and second-line antiretroviral therapy in resource-limited settings. J Infect Dis. 2013;207(suppl2):S49–56.
Swenson LC, Min JE, Woods CK, Cai E, Li JZ, Montaner JS, et al. HIV drug resistance detected during low-level viremia is associated with subsequent virologic failure. AIDS. 2014;28(8):1125.
NACP-Ghana. National AIDS Control Programme: 2019 Annual Report-June 2020. 2019:38.
NACP-Tanzania. National AIDS Control Program United Republic of Tanzania: National Guidelines for the Management of HIV and AIDS. 2019.
Jenkins SY, Resar D, Panos Z, Staple A, Watkins M, Ripin D, et al. Securing accelerated access to long-acting injectable cabotegravir for HIV prevention in low‐and middle‐income countries. J Int AIDS Soc. 2023;26:e26101.
Meyer-Rath G, Jamieson L, Pillay Y. What will it take for an injectable ARV to change the face of the HIV epidemic in high‐prevalence countries? Considerations regarding drug costs and operations. J Int AIDS Soc. 2023;26:e26106.
Fujisaki S, Fujisaki S, Ibe S, Asagi T, Itoh T, Yoshida S, et al. Performance and quality assurance of genotypic drug-resistance testing for human immunodeficiency virus type 1 in Japan. Jpn J Infect Dis. 2007;60(2/3):113.
Sia D, Onadja Y, Hajizadeh M, Heymann SJ, Brewer TF, Nandi A. What explains gender inequalities in HIV/AIDS prevalence in sub-saharan Africa? Evidence from the demographic and health surveys. BMC Public Health. 2016;16(1):1–18.
Badri M, Lawn SD, Wood R. Utility of CD4 cell counts for early prediction of virological failure during antiretroviral therapy in a resource-limited setting. BMC Infect Dis. 2008;8(1):1–8.
Bennett DE. The requirement for surveillance of HIV drug resistance within antiretroviral rollout in the developing world. Curr Opin Infect Dis. 2006;19(6):607–14.
Organization WH. Guidelines: updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring. 2021. https://iris.who.int/bitstream/handle/10665/340190/9789240022232-eng.pdf; [Accessed March 14, 2024].
Broyles LN, Luo R, Boeras D, Vojnov L. The risk of sexual transmission of HIV in individuals with low-level HIV viraemia: a systematic review. Lancet. 2023.
Montarroyos UR, Miranda-Filho DB, César CC, Souza WV, Lacerda HR, Militão Albuquerque MFP, et al. Factors related to changes in CD4 + T-cell counts over time in patients living with HIV/AIDS: a multilevel analysis. PLoS ONE. 2014;9(2):e84276.
Gathe JC Jr, Ive P, Wood R, Schürmann D, Bellos NC, DeJesus E, et al. SOLO: 48-week efficacy and safety comparison of once-daily fosamprenavir/ritonavir versus twice-daily nelfinavir in naive HIV-1-infected patients. AIDS. 2004;18(11):1529–37.
Aggleton P, Yankah E, Crewe M. Education and HIV/AIDS—30 years on. AIDS Educ Prev. 2011;23(6):495–507.
Govathson C, Ndlovu N, Rambally-Greener L, Schmucker L, Chetty-Makkan CM, Miot J et al. Increasing HIV treatment literacy among people living with HIV using a novel health communication aid: evidence from KwaZulu Natal province, South Africa. medRxiv. 2023:2023.06. 15.23291430.
Mo H, King MS, King K, Molla A, Brun S, Kempf DJ. Selection of resistance in protease inhibitor-experienced, human immunodeficiency virus type 1-infected subjects failing lopinavir-and ritonavir-based therapy: mutation patterns and baseline correlates. J Virol. 2005;79(6):3329–38.
Colonno R, Rose R, McLaren C, Thiry A, Parkin N, Friborg J. Identification of I50L as the signature atazanavir (ATV)-resistance mutation in treatment-naive HIV-1-infected patients receiving ATV-containing regimens. J Infect Dis. 2004;189(10):1802–10.
Chimukangara B, Lessells RJ, Sartorius B, Gounder L, Manyana S, Pillay M, et al. HIV-1 drug resistance in adults and adolescents on protease inhibitor-based antiretroviral therapy in KwaZulu-Natal Province, South Africa. J Global Antimicrob Resist. 2022;29:468–75.
Bircher RE, Ntamatungiro AJ, Glass TR, Mnzava D, Nyuri A, Mapesi H, et al. High failure rates of protease inhibitor-based antiretroviral treatment in rural Tanzania–A prospective cohort study. PLoS ONE. 2020;15(1):e0227600.
Acknowledgements
We thank the Clinical and Laboratory staff of the Fevers’ Unit of Korle-Bu Teaching hospital, for assisting us to recruit the patients on the study. Technical support by the HIV genotyping team of Noguchi Memorial Institute for medical research is acknowledged.
Funding
No external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: MS, BMO, SYG, VEB, MB, EYB and KWCS; Data curation: MS and BMO; Formal analysis: MS, BMO, SYG, VEB, MB, AKA, EYB and KWCS. Methodology: MS and EYB. Writing review and editing: MS, BMO, SYG, VEB, MB, EYB and KWCS. Supervision: EYB,, KWCS, SYG. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Human ethical approval and consent to participate
Ethical clearance for this study was obtained from the Korle-Bu Scientific and Technical Committee with approval number for the study as KBTH-STC/IRB0005. Prior to enrolment, informed consent to participate in the study was obtained from all 96 PLWH.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Seshie, M., Obeng, B.M., Boamah, V.E. et al. Resistance to protease inhibitors among persons living with HIV in Ghana: a case for viral load and drug resistance monitoring. Virol J 21, 159 (2024). https://doi.org/10.1186/s12985-024-02354-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02354-6