Abstract
Background
Several observational studies demonstrated that pregnant individuals with COVID-19 had a higher risk of preeclampsia and preterm birth. We aimed to determine whether women with COVID-19 diagnosis had adverse pregnancy outcomes.
Methods
A two-sample Mendelian randomization (MR) analysis in this study was used to evaluate the casual relationships between COVID-19 infection and obstetric-related diseases based on genome-wide association studies (GWAS) dataset. Inverse-variance weighted (IVW), MR-Egger and MR-PRESSO were used to infer the connection and estimate the pleiotropy respectively.
Results
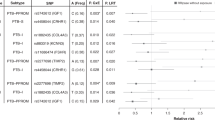
The significant connection was observed between COVID-19 and placental disorders with betaIVW of 1.57 and odds ratio (OR) of 4.81 (95% confidence interval [CI]: 1.05–22.05, p = 0.04). However, there were no associations between COVID-19 infection and gestational diabetes mellitus (GDM) (OR = 1.12; 95% CI: 0.85–1.45, p = 0.41), other disorders of amniotic fluid and membranes (OR = 0.90; 95% CI: 0.61–1.32, p = 0.59), Intrahepatic Cholestasis of Pregnancy (ICP) (OR = 1.42; 95% CI: 0.85–2.36, p = 0.18), birth weight (OR = 1.02; 95% CI: 0.99–1.05, p = 0.19), gestational hypertension (OR = 1.00; 95% CI: 1.00–1.00, p = 0.85), spontaneous miscarriages (OR = 1.00; 95% CI: 0.96–1.04, p = 0.90) and stillbirth (OR = 1.00; 95% CI: 0.98–1.01, p = 0.62).
Conclusion
There was no direct causal relationship between COVID-19 infection and maternal and neonatal poor outcomes. Our study could alleviate the anxiety of pregnant women under the COVID-19 pandemic conditions partly.
Highlights
1. COVID-19 was positively related to placental disorders.
2. COVID-19 didn’t cause stillbirths, spontaneous miscarriages, gestational hypertension/pre-eclampsia, low birth weight, intrahepatic cholestasis of pregnancy, gestational diabetes and other disorders of amniotic fluid and membranes.
Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) originated in Wuhan had killed millions of people and its pandemic led to panic worldwide and its pathogenic virus was severe acute respiratory disease coronavirus 2 (SARS-CoV-2) [1]. It was reported in 2020 that 60% newborns were born prematurely, 20% were small for gestational age (SGA) neonates and most infants had symptoms of shortness of breath in 10 newborns with negative nucleic acid born to mothers with COVID-19 [2]. The results from large multicenter cohort study showed that pregnant women with COVID-19 diagnosis had an increased risk of hypertension-related diseases, preterm birth, fetal distress, stillbirth, low birth weight and maternal deaths [3, 4]. Conversely, another retrospective study elucidated that COVID-19 couldn’t cause severe perinatal outcomes such as preterm birth nor can it be transmitted to the fetus through placenta [5], and some researchers also showed that COVID-19 pandemic had no effect on rates of spontaneous abortion through cross-sectional study [6]. In the meanwhile, to our knowledge, there were no studies to explore the specifical mechanism of COVID-19 and obstetric-related diseases. However, previous studies have pointed out that after pregnant women were infected with SARS-CoV-2, the transcription of placental syncytial trophoblast cells was changed, resulting in impaired cellular processes and reduced secretion of HCG hormone, resulting in impaired placental barrier [7]. The impaired placental barrier in pregnant women will not only cause damage to the pregnant women themselves, but also cause damage to the fetus through peroxide stress [8]. Thus, it is very essential to explore the related causes of obstetric-related diseases. And it was clear that there were conflicting results about the impact of COVID-19 on pregnancy, which might stem from economic instability and medical restrictions [9], therefore it was worthwhile for us to infer the causal relationships between COVID-19 infection and obstetric-related diseases.
Mendelian randomization (MR) was a data analysis technique to evaluate causal inference in epidemiological studies [10]. It used genetic variants as instrumental variables to assess the causal relationship between the exposure and the outcome of interest in non-experimental data [11]. “Exposure factor” referred to a putative causal risk factor, also known as an intermediate phenotype, which could be a biomarker, a physical measurement, or any risk factor that might affect outcomes [12]. Different from traditional randomized trials which were time-consuming, laborious, expensive, some not ethically supported and easy to cause bias because of behavioral, social, psychological and other factors [13], while MR analysis used single nucleotide polymorphisms as instrumental variables to make the results more reliable [14, 15]. In this study, we used MR analysis to evaluate the relationship between obstetric-related diseases and COVID-19, and inverse-variance weighted (IVW) MR analyses demonstrated that there was a statistically significant association between pregnant women diagnosed with COVID-19 infection and placental disorders, and the OR value was 4.81 (95%CI: 1.05–22.05, P = 0.04). Moreover, COVID-19 had null association with other traits. Furthermore, MR‐Egger regression revealed no statistically significant intercept for all traits and the p value in IVW test of heterogeneity analysis was greater than 0.05. In conclusion, our results showed that COVID-19 infection didn’t cause stillbirths, spontaneous miscarriages, gestational hypertension/pre-eclampsia, low birth weight, intrahepatic cholestasis of pregnancy, gestational diabetes and other disorders of amniotic fluid and membranes, but it led to placental disorders.
Materials and methods
We performed two-sample MR analysis with available summary-level data from the commonly available genome wide association studies (GWAS), The flow chart was shown in Fig. 1. Declaration of Helsinki statement and written informed consent had been obtained in the original publications. The summary-level data has been publicly published at https://gwas.mrcieu.ac.uk website for analysis.
COVID-19
Genetic instruments of COVID-19 (ID: ebi-a-GCST011073) were obtained from a large-scale study including 1,683,768 participants (1,644,784 controls vs 38,984 cases) from European and 8,660,177 SNPs [16].
Obstetric-related diseases
Obstetrician-related diseases refer to diseases with high incidence in obstetrics, including gestational diabetes, other disorders of amniotic fluid and membranes, Intrahepatic Cholestasis of Pregnancy (ICP), birth weight, gestational hypertension, spontaneous miscarriages, stillbirth and placental disorders [17]. The datasets we used were summary-level datasets and included populations from different European countries, therefore the diagnostic thresholds for the following common obstetrician-related diseases were not uniform. Gestational diabetes data (ID: finn-b-GEST_DIABETES) included 5687 cases and 117,892 controls, and 16,379,784 SNPs were obtained. Other disorders of amniotic fluid and membranes data (ID: finn-b-O15_AMNIOT_OTHER) included 1753 cases and 104,247 controls, and 16,379,393 SNPs were obtained. Intrahepatic Cholestasis of Pregnancy (ICP) (ID: finn-b-O15_ICP) included 940 cases and 122,639 controls, and 16,379,784 SNPs were obtained. Placental disorders (ID: finn-b-O15_PLAC_DISORD) included 102 cases and 104,247 controls, and 16,379,357 SNPs were obtained. Birth weight (ID: ukb-b-13378) included 261,932 participants, and 9,851,867 SNPs were obtained. Gestational hypertension/pre-eclampsia data (ID: ukb-b-13535) included 462,933 participants (1864 cases vs 461,069 controls), and 9,851,867 SNPs were obtained. Number of spontaneous miscarriages (ID: ukb-b-419) included 78,700 participants and 9,851,867 SNPs were obtained. Number of stillbirths (ID: ukb-b-6412) included 78,879 participants and 9,851,867 SNPs were obtained. All participants were of European descent.
Statistical analysis
R packages including TwoSampleMR (v 0.5.6), MendelianRandomization (v 0.7.0), and MRPRESSO (v 1.0) were used in this study. Instrumental variables (IVs) were obtained according to the three assumptions of MR. In the three assumption, we set the threshold of p-value as 1 × 10–5 and the threshold of r2 to include more IVs because some of the MR methods we used are less prone to weak instrument bias [18, 19]. Firstly, we selected SNPs that were closely associated with the COVID-19 at a significance level of p < 1 × 10–5, furthermore, SNPs with linkage disequilibrium (r2 = 0.05, kb = 10,000) and IVs with weak bias (F-statistics < 10) were removed. Secondly, we excluded SNPs that were associated with confounding factors (p < 1 × 10–5) that related to COVID-19 and obstetric-related diseases. Finally, SNPs that were directly related to the outcomes of interest (p < 1 × 10–5) were excluded to obtain the IVs. The formula for calculating R2 and F-statistics is in the form.

While MAF is minor allele frequency, SD = SE \(\times \sqrt{N}\), N and n are the sample size and R2 is a risk factor for the genotype the explanation the proportion of variability.
We used Cochran’s Q test in inverse-variance weighting (IVW) method to assess heterogeneity in the sensitivity analysis. Horizontal pleiotropy was estimated by the intercept of the MR-Egger regression and MR-pleiotropy residual sum and outlier (MR-PRESSO). We also assessed whether individual SNP had biases that independently affected the overall causal effect by leave-one-out methods. Odds ratios (OR) (p < 0.05) in this study was presented to evaluate the cause effects.
Results
SNP selection and validation
In summary, 9 IVs achieved genome-wide significance levels in gestational diabetes, stillbirths, intrahepatic cholestasis of pregnancy, placental disorders, other disorders of amniotic fluid and membranes, low birth weight and stillbirth, 7 IVs were obtained to be related to gestational hypertension/pre-eclampsia and COVID-19, 8 IVs were closely associated with spontaneous miscarriages and all F-statistics were greater than ten (Supplemental file 1).
Casual effects of COVID-19 on obstetric-related diseases
The IVW analysis revealed that COVID-19 infection was positively related to placental disorders with betaIVW of 1.57 and OR of 4.81 (95% CI: 1.05–22.05, p = 0.04). However, no associations were observed for gestational diabetes mellitus (GDM) (OR = 1.12; 95% CI: 0.85–1.45, p = 0.41), other disorders of amniotic fluid and membranes (OR = 0.90; 95% CI: 0.61–1.32, p = 0.59), Intrahepatic Cholestasis of Pregnancy (ICP) (OR = 1.42; 95% CI: 0.85–2.36, p = 0.18), birth weight (OR = 1.02; 95% CI: 0.99–1.05, p = 0.19), gestational hypertension (OR = 1.00; 95% CI: 1.00–1.00, p = 0.85), spontaneous miscarriages (OR = 1.00; 95% CI: 0.96–1.04, p = 0.90) and stillbirth (OR = 1.00; 95% CI: 0.98–1.01, p = 0.62) (Fig. 2). For all obstetric-related diseases, MR-Egger and MR-PRESSO revealed consistent results that no evidence of horizontal pleiotropy was detected. Furthermore, the p-value in heterogeneity analyses was greater than 0.05 (Table 1). Scatter plot and funnel plot of the association between COVID-19 and obstetric-related diseases displayed the similar results (Fig. 3). The forest plot revealed that no horizontal pleiotropy was observed and COVID-19 was positively related to placental disorders (Fig. 4). The leave-one-out plot indicated that individual SNP didn’t affect overall estimates (Fig. 5).
Discussion
In our study, we used two-sample MR analysis to analyze the association between COVID-19 and obstetric-related diseases, and comprehensively assessed the causal association. Our results indicated that COVID-19 was positively correlated with placental disorders, but not with stillbirths, spontaneous miscarriages, gestational hypertension/pre-eclampsia, birth weight, intrahepatic cholestasis of pregnancy, gestational diabetes and other disorders of amniotic fluid and membranes.
A recent study documented that SARS-CoV-2 colonized the placenta cells such as syncytiotrophoblasts, extravillous trophoblasts, immune cells and cytotrophoblasts by binding to angiotensin-converting enzyme 2 receptor and transmembrane serine protease 2 (TMPRSS2) resulting in placental inflammation and malperfusion [20]. Persistent inflammatory stimuli led to the perivillous deposition of massive fibrin which affected the gas exchange between mother and fetus eventually caused the stillbirth or fetal growth restriction [21]. Other possible mechanisms were that the long-term exposure of developing fetus to intrauterine inflammation and virus resulted in adverse outcomes of obstetrics and neonatology, and in severe cases, the diseases led to multisystemic defects and death in infants [22]. Wei et al. concluded that pregnant women with COVID-19 had 1.33- fold increased risk of preeclampsia, 1.82 times higher prevalence of preterm birth and 2.11-fold risk of stillbirth, moreover, the incidence of pregnancy-related adverse outcomes raised with the severity of infection [23]. However, our study showed that COVID-19 had null association with stillbirth etc. Placenta might be a barrier to mitigate adverse outcomes and some researchers found that no specific characteristics regardless of duration and severity of COVID-19 infection by collecting 138 placentas from 131 pregnant patients, but the limitation was that this study lacked a control group [24]. Andrea G Edlow etc. enrolled 127 pregnant women and found that no evidence to support definitive vertical transmission by virtue of detecting plasma SARS-CoV-2 viral load and antibodies in maternal, umbilical cord and neonates [25]. Based on the current evidence, it was difficult to decide whether COVID-19 was an independent risk factor for preterm birth, stillbirth, abortion and ICU admission. The reason for this limitation was that pregnant women, as a special group, had been excluded in some studies, and additionally, most retrospective studies were subject to bias caused by other confounding factors [26].
Mendelian randomization (MR) used genetic variants as instrumental variable to estimate the causal relationship, and it took advantage of allele randomization and excluded SNPs associated with confounding factors to successfully avoided bias [27, 28]. We investigated the association between COVID-19 and obstetric related diseases based on Mendelian randomization, which ensured the accuracy and authenticity of our results to a certain extent, and avoided the bias caused by other social factors. However, there were still some limitations. Firstly, we relaxed the p-value threshold to 1 × 10–5 and the value of r2 threshold to 0.05 to include more IVs [18, 19], while this may lead to an inaccurate description of the causal relationship between COVID-19 and obstetrical-related diseases. Secondly, the summary-level dataset on obstetrical-related diseases did not provide a detailed definition of each disease, which contributed to the extrapolation of the results of the present study.
Conclusion
COVID-19 had null association with stillbirths, spontaneous miscarriages, gestational hypertension/pre-eclampsia, low birth weight, intrahepatic cholestasis of pregnancy, gestational diabetes and other disorders of amniotic fluid and membranes. In the pandemic era, it was necessary to maintain regular prenatal care to stay away from panic, and our study helped to raise public awareness of COVID-19 and provided a theoretical basis for discovering the new triggers of other obstetric complications.
Availability of data and materials
The summary-level data on COVID-19 and Obstetric-related Diseases used in this study are publicly and freely available in the GWAS database (https://gwas.mrcieu.ac.uk), with ethical approval and informed consent of participants for each cohort in the GWAS.
References
SafiabadiTali SH, et al. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 detection. Clin Microbiol Rev. 2021;34(3):e00228-20.
Zhu H, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9(1):51–60.
Villar J, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–26.
Chmielewska B, et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9(6):e759–72.
Di Toro F, et al. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(1):36–46.
Travis-Lumer Y, et al. Rates of spontaneous abortion in Israel before and during the COVID-19 pandemic. JAMA Netw Open. 2023;6(2):e230233.
Chen J, et al. A placental model of SARS-CoV-2 infection reveals ACE2-dependent susceptibility and differentiation impairment in syncytiotrophoblasts. Nat Cell Biol. 2023;25(8):1223–34.
Cribiù FM, et al. Severe SARS-CoV-2 placenta infection can impact neonatal outcome in the absence of vertical transmission. J Clin Invest. 2021;131(6):e145427.
Carr MJ, et al. Effects of the COVID-19 pandemic on primary care-recorded mental illness and self-harm episodes in the UK: a population-based cohort study. Lancet Public Health. 2021;6(2):e124–35.
Skrivankova VW, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. 2021;326(16):1614–21.
Ren Z, et al. Relationship between NAFLD and coronary artery disease: a Mendelian randomization study. Hepatology. 2023;77(1):230–8.
Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. 2017;14(10):577–90.
Greene SJ, et al. Pragmatic design of randomized clinical trials for heart failure: rationale and design of the TRANSFORM-HF trial. JACC Heart Fail. 2021;9(5):325–35.
Coffee, et al. Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol Psychiatry. 2015;20(5):647–56.
Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: a review. Res Synth Methods. 2019;10(4):486–96.
Initiative C-HG. The COVID-19 Host Genetics Initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur J Hum Genet. 2020;28(6):715–8.
Dumitriu D, Gyamfi-Bannerman C. Understanding risk for newborns born to SARS-CoV-2-positive mothers. JAMA. 2021;325(20):2051–2.
Julian TH, et al. A review of Mendelian randomization in amyotrophic lateral sclerosis. Brain. 2022;145(3):832–42.
Zhang J, et al. Mediators of the association between educational attainment and type 2 diabetes mellitus: a two-step multivariable Mendelian randomisation study. Diabetologia. 2022;65(8):1364–74.
Kumar D, Verma S, Mysorekar IU. COVID-19 and pregnancy: clinical outcomes; mechanisms, and vaccine efficacy. Transl Res. 2023;251:84–95.
Cornish EF, McDonnell T, Williams DJ. Chronic inflammatory placental disorders associated with recurrent adverse pregnancy outcome. Front Immunol. 2022;13:825075.
Prochaska E, Jang M, Burd I. COVID-19 in pregnancy: placental and neonatal involvement. Am J Reprod Immunol. 2020;84(5):e13306.
Wei SQ, et al. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193(16):E540–8.
Corbetta-Rastelli CM, et al. Analysis of placental pathology after COVID-19 by timing and severity of infection. Am J Obstet Gynecol MFM. 2023;5(7):100981.
Edlow AG, et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open. 2020;3(12):e2030455.
Wastnedge EAN, et al. Pregnancy and COVID-19. Physiol Rev. 2021;101(1):303–18.
Sanderson E. Multivariable Mendelian randomization and mediation. Cold Spring Harb Perspect Med. 2021;11(2):a038984.
Gul A, Harford A, Zager P. Mendelian randomization to establish the causality of uric acid with diabetic nephropathy in type 1 diabetics. Kidney Int. 2017;91(5):1005–7.
Acknowledgements
This study acknowledged that Guangzhou Women and Children’ Medical Center, Guangzhou Medical University that funded my research.
Funding
1. Research foundation of Guangzhou Women and Children’s Medical Center for Clinical Doctor; 2. Funding by Science and Technology Projects in Guangzhou (grant number:SL2022A03J00794); 3. Funding by Science and Technology Projects in Guangzhou (grant number: SL2022A04J00864).
Author information
Authors and Affiliations
Contributions
Conception and design: YF and DF; Provision of materials: YF and DF; Manuscript writing: YF; Manuscript revision: DF; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Guangzhou Women and Children’ Medical Center, Guangzhou Medical University.
Consent for publication
No applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fang, Y., Fang, D. Mendelian randomization analysis reveals causal relationship between obstetric-related diseases and COVID-19. Virol J 21, 73 (2024). https://doi.org/10.1186/s12985-024-02348-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02348-4