Abstract
Background
Azvudine has been approved for the treatment of coronavirus disease 2019 (COVID-19) patients in China, and this meta-analysis aims to illustrate the safety of azvudine and its effectiveness in reducing mortality.
Methods
PubMed, Embase, Web of science, Cochrane Library and the Epistemonikos COVID-19 Living Overview of Evidence database (L.OVE) were searched to aggregate currently published studies. Cochrane risk of bias tool and ROBINS-I tool were used to assess the risk of bias of randomized controlled study and cohort study respectively. Odds radios (ORs) with 95% confidence interval (CIs) were combined for dichotomous variables. Publication bias was assessed by Egger’s test and funnel plots.
Results
A total of 184 articles were retrieved from the included databases and 17 studies were included into the final analysis. Pooled analysis showed that azvudine significantly reduced mortality risk in COVID-19 patients compared with controls (OR: 0.41, 95%CI 0.31–0.54, p < 0.001). Besides, either mild to moderate or severe COVID-19 patients could benefit from azvudine administration. There was no significant difference in the incidence of ICU admission (OR: 0.90, 95%CI 0.47–1.72, p = 0.74) and invasive ventilation (OR: 0.94, 95%CI 0.54–1.62, p = 0.82) between azvudine and control group. The incidence of adverse events was similar between azvudine and control (OR: 1.26, 95%CI 0.59–2.70, p = 0.56).
Conclusions
This meta-analysis suggests that azvudine could reduce the mortality risk of COVID-19 patients, and the safety of administration is acceptable.
Trial registration
PROSPERO; No.: CRD42023462988; URL: https://www.crd.york.ac.uk/prospero/.
Similar content being viewed by others
Background
Since the outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at the end of 2019, more than 700 million people have been infected, and nearly 7 million people have died due to the viral infection as of September 9, 2023, which has caused a serious burden on the public and the economy [1, 2]. During the Omicron variant of SARS-CoV-2 epidemic in China at the end of 2022, a large number of patients emerged in less than 2 months, which called for an urgent need for effective, safe and economical antiviral drugs [3, 4]. Besides Nirmatrelvir/Ritonavir (Paxlovid), Molnupiravir and Remdesivir, Azvudine, a nucleotide analogue, has gained emergency approval for treatment of coronavirus disease 2019 (COVID-19) patients in China since September, 2022 [5, 6].
Azvudine can block the RNA replication of the virus through inhibiting RNA-dependent RNA polymerase, and has been shown to be effective against SARS-CoV-2 [7, 8]. Several studies exploring azvudine treatment for COVID-19 patients had reported different clinical outcomes and adverse events, without conclusive results [9,10,11,12,13]. Besides, there have been retrospective studies comparing the efficacy of azvudine with Paxlovid in the treatment of patients with COVID-19, and the results remain controversial [14, 15]. There was a published meta-analysis illustrating the efficacy of azvudine on shortening time to nucleic-acid negative conversion and its safety [16]. However, quantitative pooled analyses were lack, and other clinical outcomes were not discussed. We thus conducted this meta-analysis, to comprehensively clarify its antiviral efficacy, and safety of azvudine for SARS-CoV-2 in details.
Methods
We conducted and reported this meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [17]. The protocol was registered with PROSPERO (CRD42023462988).
Search strategy and study identification
We performed a systematic literature search using the online databases: PubMed, Embase, Web of sciences, Cochrane Library and the Epistemonikos COVID-19 Living Overview of Evidence (L.OVE) database. All publications before August 23, 2022, were identified using the following keywords: “COVID-19”, “SARS-CoV-2”, “Azvudine” and “FNC”. We also manually reviewed the reference lists of relevant articles to identify additional studies.
Studies eligible for this meta-analysis met the following selection criteria: (1) randomized controlled studies (RCTs) or cohort studies investigating patients confirmed coronavirus-19 disease (COVID-19); (2) Azvudine as the treatment intervention with or without control; (3) efficacy and safety outcomes of interest; (4) studies written in English. Reviews, case reports, case series were excluded. Conference abstracts reporting similar results and conducted by the same research group were superseded by publications.
Study selection, data extraction, and risk of bias assessment
Two reviewers (Yaqi Wang and Huaiya Xie) independently performed eligibility evaluation, data extraction, and risk of bias assessment. Disagreements between the two reviewers were resolved through discussion until a consensus was reached. We extracted data on general information (first author, publication year, country, study design), participants (sample size, age, and sex), antiviral therapies specific to COVID-19, efficacy and safety outcomes (mortality risks showed with Hazards ratios (HRs) or odds ratios (ORs) with 95% confidence intervals (95% CIs), time to first nucleic-acid negative conversion, intensive care unit (ICU) admission, rate of progression to invasive mechanical ventilation (IMV) or extracorporeal membrane oxygenation (ECMO), and incidence of any AEs. We preferred results using propensity score matching for cohort studies; otherwise, data from total sample were extracted. Moreover, we also did further analyses for patients over 65 years old, with different severity, and with comorbidities (hypertension, diabetes mellitus, and cardiovascular diseases).
We employed Cochrane risk of bias tool to evaluate methodological quality of RCTs [18], and assessed risk of bias in nonrandomized studies using ROBINS-I tool [19].
Statistics
We presented combined results as ORs with 95% CIs for dichotomous variables. We measured heterogeneity between studies using Higgins’s test. A random-effects model for pooled quantitative analysis was applied when high heterogeneity was identified (i.e., I2 statistic > 50%); otherwise, a fixed-effects model was used. Sensitivity analyses of adverse events were conducted based on study types to confirm the reliability of pooled analyses. We used Egger’s test and funnel plots to assess publication bias (Supplementary Figs. 1–2 [Additional file 1]). Statistical significance was set at p < 0.05. All statistical analyses were performed using R (version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
Progress of selection and characteristics of study
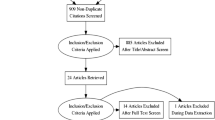
A total of 184 articles were retrieved from the included databases (Supplementary Table 1 [Additional file 1]), and we finally included 7746 patients from 17 studies after selection (Fig. 1). The main characteristics of the study were summarized in Table 1 [9,10,11,12,13,14,15, 20,21,22,23,24,25,26,27,28,29]. We in total enrolled 3 RCTs, and 14 retrospective cohort studies. Among them, 4 studies compared the efficacy and safety of azvudine and Paxlovid in fighting against COVID-19, while remaining 13 studies compared azvudine with placebo, or no specific antiviral therapies. Except two RCTs conducted in Brazil, other studies were progressed and reported in China. We employed Cochrane risk of bias tool for RCTs, and ROBIN-I tools for cohort studies to evaluate bias of studies. Most studies were classified into low or moderated bias, except one RCT conducted by Ren et al. [9] without blinding, one study with only abstract and unclear information [28] and another retrospective study conducted by Shao et. al [21] without confounding adjustment. All aforementioned results were showed in Supplementary Tables 2–3 [Additional file 1], and illustrated in Supplementary Fig. 3 [Additional file 1] as well.
The efficacy of azvudine
We collected four clinical outcomes for efficacy evaluation, including mortality, nucleic acid negative conversion time, ICU admission, and progression to IMV/ECMO.
Mortality
A pooled analysis of 7 studies involving 4421 patients showed that azvudine significantly reduced mortality in patients with COVID-19 compared with no antiviral drugs (OR: 0.41, 95%CI 0.31–0.54, p < 0.001) (Table 2; Fig. 2a). We further repeated the analysis in COVID-19 patients with special characteristics. Three and five studies, respectively, compared mortality risks between azvudine and no antiviral therapies, in patents of mild to moderate state, and severe form of COVID-19. Pooled analyses showed azvudine were still associated with decreased mortality risks no matter the disease severity (mild to moderate: OR 0.28, 95%CI 0.13–0.58, p < 0.001; severe: OR 0.43, 95%CI 0.30–0.63, p < 0.001) (Fig. 2b, Supplementary Fig. 4 [Additional file 1]). Pooled analysis of 4 studies showed azvudine associated with a 52% reduction of mortality risks in COVID-19 patients over 65 years old, compared with no antiviral therapies (OR 0.48, 95%CI 0.34–0.66, p < 0.001). Meanwhile, another 2 studies did not identify such associations in patients no more than 65 years old [12, 25]. However, similar relationships were not showed in patients with hypertension, diabetes mellitus, or cardiovascular diseases (Table 2; Fig. 2b).
Two studies compared the efficacy of azvudine and Paxlovid on mortality risks of COVID-19 patients. A study conducted by Deng et al. [14] showed a lower mortality risk in azvudine (OR 0.38, 95%CI 0.15–0.98, p = 0.04), while another retrospective study found no significant difference between azvudine and Paxlovid (OR 1.27, 95%CI 0.47–3.42, p = 0.63) [15]. Besides, another study only contrasted mortality rate between 2 groups, which also showed mortality rates were comparable between the two groups (azvudine n = 4 vs. Paxlovid n = 11, p = 0.18) [26].
Nucleic acid negative conversion time
Three RCTs and one retrospective study reported time to first nucleic-acid negative conversion of COVID-19 patients using azvudine compared to controls, all demonstrating that azvudine significantly shortened the time to nucleic acid conversion [9,10,11, 24]. Another two studies compared the nucleic acid negative conversion time of COVID-19 patients receiving either azvudine and Paxlovid. The two articles both reported longer nucleic acid negative conversion time in azvudine group (median time 10 days vs. 5.8 days, and 16.5 days vs. 13 days, respectively) [15, 27].
ICU admission and progression to IMV/ECMO
Pooled results of three retrospective cohort studies enrolling 570 patients did not find benefit on ICU admission rates in COVID-19 patients receiving azvudine, compared with those with no antiviral therapy (OR: 0.90, 95%CI 0.47–1.72, p = 0.74). Similarly, a pooled analysis of four studies showed no significant difference between two groups as for the incidence of IMV/ECMO (OR 0.94, 95%CI 0.54–1.62, p = 0.82). The results were also illustrated in Fig. 3.
Safety evaluation of Azvudine
A total of six studies reported 497 adverse events (AEs). Relevant AEs could be classified into gastrointestinal symptoms, elevation of liver enzymes or serum creatinine, neurological symptoms, chest discomfort, rash, and decline of leukocytes or platelet.
We employed random-effect model (I2 85.1%, p < 0.001) for pooled analysis, and found no significant difference in the incidence of adverse events between using azvudine and no antiviral therapy (OR 1.26, 95%CI 0.59–2.70, p = 0.56). In addition, the incidences of gastrointestinal symptoms (OR: 2.44, 95%CI 0.70–8.48, p = 0.16), elevated liver enzymes (OR: 0.90, 95%CI 0.46–1.75, p = 0.76), elevated creatinine (OR: 0.68, 95%CI 0.34–1.36, p = 0.27), neurological symptoms (OR: 1.02, 95%CI 0.22–4.71, p = 0.98) and chest discomfort (OR: 1.29, 95%CI 0.59–2.82, p = 0.52) did not differ between two groups (Fig. 4, Supplementary Fig. 5 [Additional file 1]).
We also did sensitive analyses including different study types, and the results were listed in Table 3. The digestive symptoms were more likely to appear in Azvudine group when the analysis was restricted in 3 cohort studies (OR: 4.84, 95% CI 0.85–27.7, p = 0.08), with high heterogeneity between studies. The differences were not significance in meta-analyses of total 6 studies (OR: 2.44, 95% CI 0.70–8.48, p = 0.16) and 3 RCTs (OR: 1.08, 95% CI 0.49–2.35, p = 0.85).
Discussion
Azvudine was approved in emergency for fighting against COVID-19 since the epidemic of Omicron strain last winter in China [6]. The relationship of its administration and different clinical outcomes have been reported in several studies, without a determined conclusion. We therefore quantitatively review the efficacy and safety of this antinucleotide drug. To our knowledge, this is the first meta-analysis demonstrated that azvudine could reduce mortality risk in total patients. No significant associations were identified between azvudine and ICU admission, IMV/ECMO incidence, and rates of adverse events.
The importance of antiviral therapy was well recognized. Treating with specific antiviral drugs in time could help prevent the progression of COVID-19 to severe, or even critical state [30, 31]. Besides, recent meta-analyses found Paxlovid can reduce the mortality rate of COVID-19, compared with placebo, or no antiviral treatment [32, 33]. Similarly, our pooled analysis also showed reduced mortality risks in COVID-19 patients receiving azvudine, compared with those using placebo, or without specific antiviral therapies. This significant associations were also validated in patients with different disease severity, and patients older than 65 years old. The aforementioned results reinforced the necessity of using specific antiviral therapy, especially in patients over 65 years old, no matter the state of disease. Besides older age, COVID-19 patients with comorbidities were also in high risks of disease progression [34]. However, our meta-analysis did not identify survival benefit of using azvudine, in patients with hypertension, diabetes, or cardiovascular diseases. One possible explanation might be the statistical power was weakened due to decreased sample sizes and study numbers. And this might also explain why we did not identify associations between application of azvudine and ICU admission rates, or possibility of IMV/ECMO, compared with no antiviral therapies.
Head-to-head comparisons between azvudine and other antiviral drugs fighting against COVID-19 were not enough. We therefore could not employ quantitative pooled analysis, but summarized the results instead. Various studies showed azvudine could shorten the nucleic acid negative conversion time, but its efficacy seemed to be inferior to Paxlovid [9,10,11, 15, 24, 27]. However, the superiority of Paxlovid were not identified for mortality in COVID-19 patients. In addition, one study even found survival benefit in COVID-19 patients receiving azvudine, compared with those using Paxlovid [14]. Inactive viral debris that has lost pathogenicity may also lead to nucleic acid positivity [35]. This could partly explain the inconsistency of different outcomes.
A key concern for Paxlovid administration was its effect on cytochrome system and the resulting drug interactions [36]. Azvudine therefore had its advantages in patients with comorbidities with combined therapies. Future studies comparing the efficacy of these two drugs in fighting against COVID-19, especially in specific populations are still needed, to better illustrate whether azvudine could be an alternative choice, or just a supplementation to Paxlovid, and the suitable scenarios for its application.
The safety of azvudine has previously been demonstrated in the treatment of acquired immune deficiency syndrome [37]. As a nucleoside analogue, its liver and kidney impairment are a matter of concern. Although occasionally reported, we did not identify safety concerns of azvudine in relevant cohort studies. Similarly, our pooled analysis showed that the total adverse event rates of azvudine was similar to that of the control group, which was consistent with the findings of a previously published meta-analysis [16]. Besides, there was no significant difference between the two groups in the incidence of gastrointestinal symptoms, neurological symptoms and elevation of liver enzymes and creatinine. These evidences indicated that azvudine was safe for the treatment of COVID-19 patients.
There are some limitations in our meta-analysis. Firstly, most studies included for meta-analysis were retrospective studies. We tried to reduce the risk of bias by choosing results calculated after propensity score matching or multivariable adjustment. Second, heterogeneity was showed in the analysis of mortality risks in patients with cardiovascular disease, as well as the analysis of adverse events. Publication bias was also existed in studies mentioning patients with cardiovascular diseases. We therefore employed random-effect model for these analyses. In addition, we performed sensitive analyses for adverse events, and the results remained when only RCTs included for reduced heterogeneity. In summary, the pooled results should be interpreted with caution and further validated in future by enrolling high-quality prospective studies.
Conclusions
The current meta-analysis found azvudine was effective in reducing mortality risks of COVID-19 patients, compared with no antiviral therapies, especially in patients older than 65 years, no matter the severity of disease. Meanwhile, the incidence of adverse events of patients using azvudine was comparable to those with no antiviral treatment. High-quality prospective studies are required to confirm our findings. We also expect future evidences centered on comparison of azvudine and other antiviral drugs, and application of azvudine in specific populations.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AE:
-
Adverse event
- CI:
-
Confidence interval
- COVID-19:
-
Coronavirus disease 2019
- L.OVE:
-
Living Overview of Evidence
- FNC:
-
Azvudine
- ECMO:
-
Extracorporeal membrane oxygenation
- HR:
-
Hazards ratio
- ICU:
-
Intensive care unit
- IMV:
-
Invasive mechanical ventilation
- L.OVE:
-
Living Overview of Evidence
- OR:
-
Odds radio
- Paxlovid:
-
Nirmatrelvir/Ritonavir
- RCTs:
-
Randomized controlled studies
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- R:
-
Retrospective cohort
- RN:
-
Ritonavir-Nirmatrelvir
- T FNANC :
-
Time to first nucleic-acid negative conversion
- NA:
-
Not available
References
WHO COVID-19 Dashboard. Geneva: World Health Organization; 2020. Available online: https://covid19.who.int/
Keni R, Alexander A, Nayak PG, Mudgal J, Nandakumar K. COVID-19: emergence, spread, possible treatments, and global burden. Front Public Health. 2020;8:216.
Joint prevention and control Mechnism of The State Council for the Novel Coronavirus Pneumonia. Notice on further optimizing and implementing the prevention and control measures of the novel coronavirus. [http://www.gov.cn/xinwen/2022-12/07/content_5730443.htm]
Tian D, Sun Y, Xu H, Ye Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol. 2022;94:2376–83.
Lamontagne F, Agarwal A, Rochwerg B, Siemieniuk RA, Agoritsas T, Askie L, Lytvyn L, Leo YS, Macdonald H, Zeng L, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379.
General Office of the National Health Commission. Notice on including azovudine tablets into the diagnosis and treatment protocol for COVID-19 in China. [https://www.gov.cn/zhengce/zhengceku/2022-08/10/content_5704788.htm]
Zhang JL, Li YH, Wang LL, Liu HQ, Lu SY, Liu Y, Li K, Liu B, Li SY, Shao FM, et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct Target Ther. 2021;6:414.
Yu B, Chang J. Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduct Target Therapy. 2020;5.
Ren Z, Luo H, Yu Z, Song J, Liang L, Wang L, Wang H, Cui G, Liu Y, Wang J et al. A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv Sci. 2020;7.
da Silva RM, Cabral PGA, de Souza SB, Arruda RF, Cabral SPF, de Assis ALEM, Martins YPM, Tavares CAdA, Viana Junior AB, Chang J et al. Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19. Front Med. 2023;10.
Cabral P, de Souza S, Silva R, Arruda R, Cabral S, de Assis A, Júnior A, Degrave W, Moreira A, Silva C et al. Serial viral load analysis by Ddpcr to evaluate Fnc efficacy and safety in the treatment of moderate cases of Covid-19. ResearchSquare. 2022.
Zong K, Zhou H, Li W, Jiang E, Liu Y, Li S. Azvudine reduces the in-hospital mortality of COVID-19 patients: a retrospective cohort study. Acta Pharm Sinica B. 2023.
Shen M, Xiao C, Sun Y, Li D, Wu P, Jin L, Wu Q, Dian Y, Meng Y, Zeng F et al. Real-world effectiveness of azvudine in hospitalized patients with COVID-19: a retrospective cohort study. medRxiv. 2023.
Deng G, Li D, Sun Y, Jin L, Zhou Q, Xiao C, Wu Q, Sun H, Dian Y, Zeng F et al. Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study. J Med Virol. 2023;95.
Zhao Q, Zheng B, Han B, Feng P, Xia Z, Jiang H, Ying Y, Zhu J, Fei C, Xiang J, et al. Is azvudine comparable to nirmatrelvir-ritonavir in real-world efficacy and safety for hospitalized patients with COVID-19? A retrospective cohort study. Infect Dis Ther. 2023.
Chen Z, Tian F. Efficacy and safety of azvudine in patients with COVID-19: a systematic review and meta-analysis. Heliyon. 2023;9:e20153.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Yang H, Wang Z, Jiang C, Zhang Y, Zhang Y, Xu M, Zhang Y, Wang Y, Liu X, An Z, et al. Oral azvudine for mild-to-moderate COVID-19 in high risk, nonhospitalized adults: results of a real-world study. J Med Virol. 2023;95:e28947.
Shao J, Fan R, Guo C, Huang X, Guo R, Zhang F, Hu J, Huang G, Cao L. Composite interventions on outcomes of severely and critically ill patients with COVID-19 in Shanghai, China. Microorganisms. 2023;11.
Chen R, Guo Y, Deng S, Wang J, Gao M, Han H, Wang L, Jiang H, Huang K. All-cause mortality in moderate and severe COVID-19 patients with myocardial injury receiving versus not receiving azvudine: a propensity score-matched analysis. Cardiol Plus. 2023;8:103–10.
Shang S, Fu B, Geng Y, Zhang J, Zhang D, Xiao F, Sheng Z, Zhai J, Li W, Chen X, et al. Azvudine therapy of common COVID-19 in hemodialysis patients. J Med Virol. 2023;95:e29007.
Chen W, Xu H, Hong L, Yang R, Peng C, Wang G, Li W. Oral azvudine (FNC) tablets in patients infected with SARS-CoV-2 Omicron variant. A retrospective cohort study. 2023.
Han X, Han X, Wang Y, Wang Z, Cui J, Zhao W, Mo G, Liu Y, Zheng M, Xie F et al. Effectiveness and optimal timing of azvudine in COVID-19 patients: a multi-center retrospective study in Beijing, China. ResearchSquare. 2023.
Dian Y, Meng Y, Sun Y, Deng G, Zeng F. Azvudine versus paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities. J Infect. 2023;87:E24–7.
Gao Y, Luo Z, Ren S, Duan Z, Han Y, Liu H, Gao Z, Zhang X, Hu Z, Ma Y. Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19. J Infect. 2023;86:E158–60.
Zhou Y, Liu Y, Jiang L, Zhang R, Zhang H, Shi Q, Yang Z, Mao Y, Liu S, Yang Z, et al. Azvudine and nirmatrelvir–ritonavir in hospitalised patients with moderate–to–severe covid–19: emulation of a randomised target trial. SSRN; 2023.
Sun Y, Jin L, Dian Y, Shen M, Zeng F, Chen X, Deng G. Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. Eclinicalmedicine. 2023;59.
Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400:1213–22.
Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22:1681–93.
Amani B, Amani B. Efficacy and safety of nirmatrelvir/ritonavir (paxlovid) for COVID-19: a rapid review and meta-analysis. J Med Virol. 2023;95:e28441.
Cheema HA, Jafar U, Sohail A, Shahid A, Sahra S, Ehsan M, Athar F, Shah J, Sah R. Nirmatrelvir-Ritonavir for the treatment of COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2023;95:e28471.
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369m1985.
Chu VT, Schwartz NG, Donnelly MAP, Chuey MR, Soto R, Yousaf AR, Schmitt-Matzen EN, Sleweon S, Ruffin J, Thornburg N, et al. Comparison of home antigen testing with RT-PCR and viral culture during the course of SARS-CoV-2 infection. JAMA Intern Med. 2022;182:701–9.
Lemaitre F, Grégoire M, Monchaud C, Bouchet S, Saint-Salvi B, Polard E. Management of drug-drug interactions with nirmatrelvir/ritonavir in patients treated for Covid-19: guidelines from the French society of pharmacology and therapeutics (SFPT). Therapie. 2022;77:509–21.
Sun L, Peng Y, Yu W, Zhang Y, Liang L, Song C, Hou J, Qiao Y, Wang Q, Chen J, et al. Mechanistic insight into antiretroviral potency of 2’-Deoxy-2’-β-fluoro-4’-azidocytidine (FNC) with a long-lasting effect on HIV-1 prevention. J Med Chem. 2020;63:8554–66.
Acknowledgements
We thank Dingding Zhang for guidance on constructing the search strategy and methodology for this systematic review and meta-analysis.
Funding
This work was supported by the National Key Research and Development Program of China (grant number 2023YFC3041900) and Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (grant number 2021-I2M-1-048). The funding sources have no role in the study design or execution, analyses, interpretation of the data, or decision to submit results.
Author information
Authors and Affiliations
Contributions
X.T., J.F., L.W., H.Z. and J.W. designed the study. Y.W. and H.X. extracted data, assessed the bias and did statistical analysis. Y.W. and H.X. drafted manuscript and X.T. revised manuscript. All authors reviewed and approved the the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Supplementary Table 1
. Search strategy and original results from database. Supplementary Table 2. Risk of bias of included cohort studies. Supplementary Table 3. Risk of bias of included randomized controlled studies. Supplementary Fig. 1. Publication bias evaluated by funnel plots for efficacy of azvudine. Supplementary Fig. 2. Publication bias evaluated by funnel plots for adverse events of azvudine. Supplementary Fig. 3. Risk of bias of included studies. Supplementary Fig. 4. Effectiveness of azvudine on mortality in COVID-19 patients. Supplementary Fig. 5. Adverse events of azvudine compared with controls
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, Y., Xie, H., Wang, L. et al. Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and meta-analysis. Virol J 21, 46 (2024). https://doi.org/10.1186/s12985-024-02316-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02316-y