Abstract
Background
Human papillomavirus (HPV) 52 is one of the prevalent oncogenic HPV genotypes in East Asia. Chinese women have the highest susceptibility to the HPV52 type, but research data on HPV52 genetic variability and its carcinogenicity in China is lacking.
Methods
The present study aimed to investigate the genetic variability of HPV52 currently circulating among Chinese women by PCR sequencing the entire E6 and E7 oncogenes. HPV52 sequence alignment, genetic heterogeneity analyses and maximum-likelihood phylogenetic tree construction were performed by BioEdit software and MEGA X software.
Results
Between 2016 and 2018, the overall HPV infection rate was 21.3%, of which HPV52 was the most prevalent high-risk type (17.2%) in the Taizhou area, China. A total of 339 single HPV52-positive samples were included in this study. We obtained 27 distinct variation patterns of HPV52 with the accession GenBank numbers ON529577-ON529603. Phylogenetic analysis showed that 96.6% of HPV52 variants belonged to lineage B, which seemed to be uniquely defined by G350T, A379G (K93R) in the E6 gene and C751T, A801G in the E7 gene. Due to the dominance of lineage B in our study population, the results could not be used to assess the association of the HPV52 (sub)lineage with the risk of cervical lesions. In addition, no significant trends were observed between the nucleotide substitutions of HPV52 variants and the risk of cervical carcinogenesis.
Conclusion
Our data showed that HPV52 variants were strongly biased towards lineage B. These results confirmed that cervical lesions in the Taizhou area are highly attributable to HPV52, which may be due to the high infection rate of lineage B in the population.
Similar content being viewed by others
Background
Cervical cancer caused by persistent infection with high-risk human papillomavirus (HPV) continues to be a global public health problem. According to GLOBOCAN 2018, there are approximately 106,400 new cases and 47,700 deaths related to cervical cancer annually in China, accounting for 18.6% and 15.2% of the global data, respectively [1]. To date, over 170 HPV types have been identified and mainly divided into high- and low-risk types based on the malignant progression of the disease [2]. Of the known high-risk HPV types, HPV16 is the most common type worldwide [3], but the infection rate of HPV52 in East Asia (such as China and Japan) is much higher than that in other areas around the world [4]. In our previous Taizhou area HPV studies, the results suggested that Chinese women have the highest susceptibility to the HPV52 type, but the carcinogenic ability of HPV52 was relatively weaker than that of HPV16 or HPV58 [5,6,7]. The genomic characterization of HPV52 has obvious epidemiological characteristics, especially the (sub)lineages and single nucleotide substitutions in the two major oncogenes E6 and E7, which may increase the risk of infection with HPV52 in Chinese women [8,9,10].
HPV52 genomes are approximately 7.9 kb, and their gene structure includes the early regions (E1, E2, E4, E5, E6, and E7), the late regions (L1 and L2) and the long control region (LCR). When HPV infects cervical epithelial cells and integrates into the host genome, the expression of two major E6 and E7 oncoproteins increases uncontrolled, resulting in host cell immortalization, transformation, and carcinogenesis [11]. Based on genomic DNA analysis, HPV52 variants are divided into five main phylogenetic lineages (A, B, C, D, and E) and nine sublineages (A1, A2, B1, B2, B3, C1, C2, D, and E) [12, 13]. The distribution of HPV variants varies with their geographical location and ethnic group, especially HPV52, which is more likely to infect Chinese women. However, there are few research data on HPV52 genetic variability and its carcinogenicity in China. In this study, we investigated the genetic variability in the E6 and E7 genes of HPV52 obtained from exfoliated cervical cells and explored their role in cervical cancer risk among Taizhou women in China.
Methods
Subject recruitment
From April 2016 to December 2018, exfoliated cervical cells were collected from outpatients undergoing cervical cancer screening at the gynaecological clinic of Taizhou Hospital, Zhejiang Province. The inclusion criteria for this study were as follows: single HPV52 infection. The exclusion criteria were as follows: second round of screening or more, received cervical treatment, and had a history of cancer or hysterectomy. The samples in cell preservation medium were stored at − 20 °C before DNA extraction.
According to several years of experience with cervical cancer screening in the Taizhou area, we recommended that HPV52-positive patients undergo colposcopy examination immediately [5]. The suspected cervical tissues were fixed in a 10% formalin solution, and then histological diagnoses were performed by two pathologists. According to the histological criteria of the World Health Organization (WHO), they were divided into cervical intraepithelial neoplasia (CIN) grade 1, CIN2, CIN3, cervical cancer or normal.
DNA extraction and HPV genotyping
Total cellular DNA was extracted using a DNA Extraction Kit (#GK0122, GENEray, China). HPV genotyping was performed using the GP5 + /bioGP6 + -PCR/MPG assay (CFDA Certified No. (2017): 3,404,697). This assay can simultaneously classify 27 different HPV types, including 14 high-risk HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) and 13 low-risk HPV types (HPV6, 11, 26, 40, 42, 43, 44, 53, 55, 61, 81, 82, and 83). The detailed operation process of HPV genotyping has been described in our previous studies [5, 6]. To avoid the influence of other HPV types on HPV52, only single HPV52-positive samples were selected for subsequent DNA amplification, sequencing and phylogenetic analysis in this study.
DNA amplification and sequencing
The primer sequences specific for the E6 and E7 genes of HPV52 were 52E6E7_F 5′-ACCCACAACCACTTTTTTTTAT-3’ and 52E6E7_R 5′-TTGCCTCTACTTCAAACCAGCC-3’, which were cited from a recently published article [8]. Primers were synthesized by BGI company (Hangzhou, China). The 50 µl PCR volumes included 25 µl 2 × PrimeSTAR Max Premix (TaKaRa, Japan), 20 µl nuclease-free water, 2 µl forward primer, 2 µl reverse primer, and 1 µl template DNA. The PCR conditions were as follows: initial denaturation step at 95 °C for 5 min, followed by 35 repeated cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, a final extension step at 72 °C for 10 min, and holding at 4 °C.
The size of the PCR amplification product was 957 bp (nucleotide sites [nt] 7916–930, including the E6 gene nt 102–548 and E7 gene nt 553–852). Subsequently, the PCR amplification products were purified and sequenced on the ABI 3730xl DNA Genetic Analyser at BGI company. To avoid PCR or sequencing errors, all data were confirmed by repeating the PCR and sequencing reactions at least twice.
Phylogenetic analysis
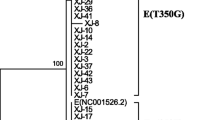
In this study, the HPV52 reference sequence (GenBank: HQ537732) was used as the standard for alignment and nucleotide site numbering. The variations in the E6 and E7 genes in the HPV52 sequences were aligned by BioEdit software. The HPV52 phylogenetic tree was constructed using the maximum-likelihood statistical method by MEGA X software [14]. One thousand bootstrap replicates were used for tree topology evaluation. To construct the phylogenetic branches, HPV52 sequences were downloaded from NCBI (https://www.ncbi.nlm.nih.gov/), including HQ537732 (A1), HQ537739 (A2), HQ537740 (B1), HQ537743 (B2), MZ374448 (B3), HQ537744 (C1), HQ537746 (C2), HQ537748 (D), and MZ374428 (E) [12, 13].
Statistical analysis
The association of cervical lesion risk with HPV52 variants was analysed using the chi-square test or Fisher’s exact test. Odds ratios (ORs) and relative 95% confidence intervals (95% CI) were calculated. All statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL). P values were two-sided, and the results were regarded as statistically significant if the P value was 0.05 or less.
Results
Characteristics of the study population
Between April 2016 and December 2018, the overall HPV infection rate was 21.3% (3287/15429) in women who were screened for cervical cancer in Taizhou Hospital. Among all HPV-positive women, the infection rate of HPV subtypes is shown in Fig. 1. HPV52 was the most prevalent high-risk type (17.2%, 565/3287), followed by HPV58 (12.9%), HPV16 (10.1%), HPV39 (7.0%), HPV18 (6.1%), and HPV51 (6.0%). The top six low-risk HPV types were HPV53 (11.1%), HPV81 (7.7%), HPV61 (6.7%), HPV43 (5.0%), HPV42 (4.2%), and HPV44 (4.2%).
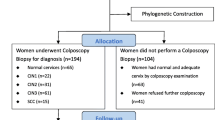
In the present study, 339 women (median age 40.8 years; range 19–75) with a single HPV52 infection were selected (Additional file 1). A total of 325 (95.9%) sequences of the entire E6 and E7 genes from HPV52 were obtained. Fourteen (4.1%) sequences were excluded due to the small number of HPV copies. Among 325 HPV52-infected women, 75 (23.1%) had normal and adequate cervices by colposcopy examination; therefore, no further biopsy diagnosis was performed. Sixty-one (18.8%) women refused further colposcopy examination. The remaining 189 (58.1%) underwent colposcopy biopsy for diagnosis, which was further used for risk association analysis, and 67 were diagnosed with normal cervices after biopsy, 53 with CIN1, 45 with CIN2, 24 with CIN3, and no cases of cervical cancer. The characteristics of the study population categorized by HPV52 (sub)lineage are shown in Table 1.
Variations in the E6 and E7 genes
Compared with the HPV52 reference sequence HQ537732, 98.8% (321/325) of collected HPV52 samples showed nucleotide variations. To visually distinguish the specific nucleotide variations of HPV52 lineage/sublineages, we summarized all of the changes in nucleotide and amino acid sequences in the entire E6 and E7 fragments, as shown in Fig. 2. In this study, we obtained 27 distinct variation patterns denoted 52CNTZ01-52CNTZ27, which were published with the GenBank accession codes ON529577 to ON529603. Of these, 16 (59.3%, 16/27) novel HPV52 variants were detected and are highlighted in bold in Fig. 2. 52CNTZ05 (60.9%, 198/325) was the most common variant in the Taizhou-based population, followed by 52CNTZ12 (21.2%, 69/325) and 52CNTZ17 (3.1%, 10/325).
Genetic variability of HPV52 E6 and E7 nucleotide sequences in Taizhou area, Southeast China. Numbering refers to the first nucleotide of the HPV52 prototype reference sequence (GenBank: HQ537732). Each row indicates the variant identification and the nucleotide sequence alignment compared to the reference. Novel HPV52 variants are highlighted in bold and novel nucleotide substitutions are highlights in gray
In the E6 and E7 genes, a total of 35 single nucleotide substitutions were identified, with 17 (48.6%) nonsynonymous substitutions and 11 (31.4%) novel substitutions. The four most prevalent nucleotide substitutions were G350T (321/325, 98.8%), A379G (K93R) (313/325, 96.3%) in the E6 gene and C751T (314/325, 96.6%), A801G (321/325, 98.8%) in the E7 gene. These four nucleotide substitutions were specific to HPV52 lineage B, but no significant trends in the severity of cervical lesions were observed. Notably, there were three amino acid changes in the 93rd genetic code of the E6 oncoprotein: K93G, K93Q, and K93R. The corresponding nucleotide changes were A378G, A378C, and A379G, which belong to the B lineage in this study. To the best of our knowledge, the nucleotide substitutions of G108C (E3Q), T191C, G267A (D56N), T292G (I64S), T309C, G317A, T329C, and A347G in the E6 gene and A822G, C834T, and A843G in the E7 gene have not been reported in previous studies. However, these novel nucleotide substitutions were detected in only one or two samples, and thus, no statistical analysis was performed.
Phylogenetic construction
The maximum-likelihood phylogenetic tree based on the HPV52 E6-E7 sequences was inferred from 27 obtained HPV52 variants and 9 reference sequences (Fig. 3). According to the phylogenetic tree, the most predominant HPV52 variants belong to lineage B (96.6%, 314/325), followed by sublineages C2 (2.2%, 7/325) and A1 (1.2%, 4/325). Lineage B appeared to be uniquely defined by G350T and A379G (K93R) in the E6 gene and C751T and A801G in the E7 gene. 52CNTZ27 in the A1 sublineage, 52CNTZ19 in the B3 sublineage, and 52CNTZ25 and 52CNTZ26 in the C2 sublineage had proportions of 1.2%, 0.6%, and 2.2%, respectively.
Risk association with cervical lesions
In this study, 189 HPV52-infected women underwent colposcopy biopsy for diagnosis, including 67 normal cervices, 53 with CIN1, 45 with CIN2, and 24 with CIN3. These cases were further used to analyse the association between sequence variations of HPV52 and the risk of cervical lesions. Given the dominance of HPV52 lineage B in our population, the results showed that for HPV52, any association between a specific (sub)lineage and a higher risk of CIN2 or worse lesions could not be well evaluated. Other (sub)lineages were not included in these analyses due to small numbers of samples. Furthermore, no significant difference in the relative risk for cervical carcinogenesis progression was observed among the nucleotide variations in HPV52 E6 and E7 oncogenes (Table 1).
Discussion
Persistent high-risk HPV infection has been identified as a major risk factor for cervical cancer. In China, the infection rates of high-risk HPV, such as HPV16, 18, 52, and 58, increase with the severity of cervical lesions [5, 7]. The overall HPV infection rate (21.3%) of this study was significantly different from that of Yangqu (8.9%), Xinjiang (14.0%), and Shandong (28.4%) but similar to that of other regions in Southeast China, such as Hangzhou (22.4%) and Jiangxi (22.5%) [15,16,17,18,19]. Many factors, including different economic conditions, cultural diversity, HPV vaccine awareness and lifestyles, might be the reasons for the differences in HPV infection rates in different geographical regions [7, 20, 21].
In this study, HPV52 was the most prevalent HPV type, followed by HPV58, 53, 16, 81, 39, 61, and 18. It is worth noting that the Gardasil 9-valent prophylactic vaccine approved for marketing in China in 2016 mainly targets HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58. Therefore, the development of polygenic vaccines containing HPV53, 81, 39, and 61 would be more helpful for Chinese women to prevent HPV infection. Among all HPV types in China, the infection rate of HPV52 was relatively low in the late 1990s but has gradually increased since then [6, 7, 22]. Recently, HPV52 has become the most prevalent type among the Chinese population [6, 8, 9, 17,18,19, 30]. Furthermore, the oncogenicity of HPV is mainly attributable to the combined effects of the E6 and E7 oncoproteins. Accordingly, therapeutic vaccines against HPV E6 and/or E7 have been widely studied [23]. Based on our previous findings, genetic variation in the E6 and E7 genes was found to be highly associated with the risk of cervical cancer [24,25,26]. The genomic characterization of the HPV52 type has obvious epidemiological characteristics, which may be one of the factors that increase the risk of cervical cancer in Chinese women.
We obtained 325 complete sequences of the E6 and E7 genes from HPV52 in the Taizhou area. Most of the HPV52 (96.6%) variants belonged to lineage B, which was consistent with previous reports on HPV52 (sub)lineage distribution in East Asia [27,28,29,30]. It has been reported that lineage B predominated in Asia, while lineage A was the most common lineage in Africa, the Americas and Europe [27]. Given the dominance of HPV52 lineage B detected in our population, the results showed that for HPV52, any association between a specific (sub)lineage and a higher risk of CIN2 or worse lesions could not be well evaluated. Other (sub)lineages were not included in these analyses due to small numbers of samples. In addition to the HPV52 variants of the B lineage, the remaining 52CNTZ27 belongs to the A1 sublineage, and 52CNTZ25 and 52CNTZ26 belong to the C2 sublineage, accounting for 1.2% and 2.2%, respectively. A previous study showed that lineage B had a higher risk of cervical cancer than lineage A (OR: 5.46, 95% CI 2.28–13.07) [27]. Therefore, our results suggested that the reported high attribution of disease to HPV52 in the Taizhou area may be due to the high infection rate of lineage B in our population. However, our existing results could not be used to assess the association of the HPV52 (sub)lineage with the risk of cervical lesions because HPV52 variants were strongly biased towards lineage B. A study investigating the worldwide distribution of HPV52 variants suggested that all lineage B variants identified were sublineage B2, with 89.0% in Asia, 5.5% in Americas, 1.1% in Europe and zero in Afric [27]. Studies from Japan and Korea on HPV52 variants also suggested that all lineage B variants identified were sublineage B2 [29, 31]. During the long history of virus evolution, these HPV52 variants of lineage B may match the characteristics of the East Asia population through unknown viral adaptation mechanisms.
Compared with the HPV52 reference sequence (GenBank: HQ537732), the four most prevalent nucleotide substitutions were G350T (98.8%), A379G (K93R) (96.3%) in the E6 gene and C751T (96.6%), A801G (98.8%) in the E7 gene, which were specific to HPV52 lineage B. Of these, K93R was the only nonsynonymous substitution. Notably, in this study, there were three amino acid changes in the 93rd genetic code of the E6 oncoprotein, K93G, K93Q, and K93R, but no significant trends between the nucleotide substitutions of HPV52 variants and the risk for cervical carcinogenesis were observed. Previous studies have shown that K93R was associated with an increased cervical lesion risk in Korean women, but it has no correlation in Chinese women [31,32,33]. The sequence variants of amino acids may lead to different levels of pathogenicity due to geographical location or ethnic discrepancies. An in vitro study found that although K93R did not increase the immortalization ability of HPV52-positive cells, stronger colony formation and greater cell migration ability were observed when compared to its prototype and other variants [27]. In addition, the roles of synonymous substitutions G350T, C751T and A801G in lineage B on the risk of cervical cancer need to be further studied. Moreover, further functional studies should be conducted to understand the molecular mechanism of how these HPV52 variants of lineage B contribute to enhanced carcinogenicity, together with elucidation of the genetic background of Chinese people, including human leukocyte antigen polymorphisms.
Conclusions
In summary, this is the first comprehensive study to evaluate the genetic variants and phylogenetics of the HPV52 E6 and E7 genes in the Taizhou area, Southeast China. Our study confirmed that the common HPV52 infection in the Taizhou area of China when compared to other areas around the world is largely due to the high infection rate of lineage B in the population.
Availability of data and materials
All data generated during this study are included in this published article. The supplementary materials included the nucleotide variations of the E6 and E7 genes from HPV52 and the follow-up data of patients. In addtion, these sequences have been released to GenBank database with the accession codes of ON529577-ON529603. The links are https://www.ncbi.nlm.nih.gov/nuccore/ON529577 ~ https://www.ncbi.nlm.nih.gov/nuccore/ON529603.
Abbreviations
- bp:
-
Base pair
- CIN:
-
Cervical intraepithelial neoplasia
- DNA:
-
Deoxyribonucleic acid
- HPV:
-
Human papillomavirus
- LCR:
-
Long control region
- LEEP:
-
Loop electrosurgical excision procedure
- nt:
-
Nucleotide sites
- OR:
-
Odds ratios
- ORF:
-
Open reading frames
- PCR:
-
Polymerase chain reaction
- SNP:
-
Single nucleotide polymorphism
- WHO:
-
World Health Organization
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393(10167):169–82. https://doi.org/10.1016/S0140-6736(18)32470-X.
Alejo M, Alemany L, Clavero O, Quiros B, Vighi S, Seoud M, et al. Contribution of Human papillomavirus in neuroendocrine tumors from a series of 10,575 invasive cervical cancer cases. Papillomavirus Res. 2018;5:134–42. https://doi.org/10.1016/j.pvr.2018.03.005.
Guan P, Howell-Jones R, Li N, Bruni L, de Sanjosé S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131(10):2349–59. https://doi.org/10.1002/ijc.27485.
Xu H, Lin A, Shao X, Shi W, Zhang Y, Yan W. Diagnostic accuracy of high-risk HPV genotyping in women with high-grade cervical lesions: evidence for improving the cervical cancer screening strategy in China. Oncotarget. 2016;7(50):83775–83. https://doi.org/10.18632/oncotarget.11959.
Xu HH, Lin A, Chen YH, Dong SS, Shi WW, Yu JZ, et al. Prevalence characteristics of cervical human papillomavirus (HPV) genotypes in the Taizhou area, China: a cross-sectional study of 37 967 women from the general population. BMJ Open. 2017;7(6): e014135. https://doi.org/10.1136/bmjopen-2016-014135.
Xu HH, Wang K, Feng XJ, Dong SS, Lin A, Zheng LZ, et al. Prevalence of human papillomavirus genotypes and relative risk of cervical cancer in China: a systematic review and meta-analysis. Oncotarget. 2018;9(20):15386–97. https://doi.org/10.18632/oncotarget.24169.
Song Z, Cui Y, Li Q, Deng J, Ding X, He J, et al. The genetic variability, phylogeny and functional significance of E6, E7 and LCR in human papillomavirus type 52 isolates in Sichuan, China. Virol J. 2021;18(1):94. https://doi.org/10.1186/s12985-021-01565-5.
Li S, Ye M, Chen Y, Gong Q, Mei B. Genetic variation of E6 and E7 genes of human papillomavirus 52 from Central China. J Med Virol. 2021;93(6):3849–56. https://doi.org/10.1002/jmv.26690.
Wang X, Han S, Li X, Wang X, Wang S, Ma L. Prevalence and distribution of human papillomavirus (HPV) in Luoyang city of Henan province during 2015–2021 and the genetic variability of HPV16 and 52. Virol J. 2022;19(1):37. https://doi.org/10.1186/s12985-022-01759-5.
Rader JS, Tsaih SW, Fullin D, Murray MW, Iden M, Zimmermann MT, et al. Genetic variations in human papillomavirus and cervical cancer outcomes. Int J Cancer. 2019;144(9):2206–14. https://doi.org/10.1002/ijc.32038.
Burk RD, Harari A, Chen Z. Human papillomavirus genome variants. Virology. 2013;445(1–2):232–43. https://doi.org/10.1016/j.virol.2013.07.018.
Bee KJ, Gradissimo A, Chen Z, Harari A, Schiffman M, Raine-Bennett T, et al. Genetic and epigenetic variations of HPV52 in cervical precancer. Int J Mol Sci. 2021;22(12):6463. https://doi.org/10.3390/ijms22126463.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Bio Evol. 2018;35(6):1547–9. https://doi.org/10.1093/molbev/msy096.
Yang J, Wang W, Wang Z, Wang Z, Wang Y, Wang J, et al. Prevalence, genotype distribution and risk factors of cervical HPV infection in Yangqu, China: a population-based survey of 10086 women. Hum Vaccin Immunother. 2020;16(7):1645–52. https://doi.org/10.1080/21645515.2019.1689743.
Wang J, Tang D, Wang K, Wang J, Zhang Z, Chen Y, et al. HPV genotype prevalence and distribution during 2009–2018 in Xinjiang, China: baseline surveys prior to mass HPV vaccination. BMC Womens Health. 2019;19(1):90. https://doi.org/10.1186/s12905-019-0785-3.
Jiang L, Tian X, Peng D, Zhang L, Xie F, Bi C, et al. HPV prevalence and genotype distribution among women in Shandong Province, China: analysis of 94,489 HPV genotyping results from Shandong’s largest independent pathology laboratory. PLoS ONE. 2019;14(1): e0210311. https://doi.org/10.1371/journal.pone.0210311.
Wang L, Yu C, Ni X, Wang F, Wen C, Jin M, et al. Prevalence characteristics of human papillomavirus (HPV) infection among women receiving physical examinations in the Shangcheng District, Hangzhou city, China. Sci Rep. 2021;11(1):16538. https://doi.org/10.1038/s41598-021-96131-y.
Zhong TY, Zhou JC, Hu R, Fan XN, Xie XY, Liu ZX, et al. Prevalence of human papillomavirus infection among 71,435 women in Jiangxi Province, China. J Infect Public Health. 2017;10(6):783–8. https://doi.org/10.1016/j.jiph.2017.01.011.
Wang R, Guo XL, Wisman GB, Schuuring E, Wang WF, Zeng ZY, et al. Nationwide prevalence of human papillomavirus infection and viral genotype distribution in 37 cities in China. BMC Infect Dis. 2015;15:257. https://doi.org/10.1186/s12879-015-0998-5.
Duan R, Qiao Y, Clifford G, Zhao F. Cancer burden attributable to human papillomavirus infection by sex, cancer site, age, and geographical area in China. Cancer Med. 2020;9(1):374–84. https://doi.org/10.1002/cam4.2697.
Chen Z, Wang Q, Ding X, Li Q, Zhong R, Ren H. Characteristics of HPV prevalence in Sichuan Province, China. Int J Gynaecol Obstet. 2015;131(3):277–80. https://doi.org/10.1016/j.ijgo.2015.06.027.
Almeida AM, Queiroz JA, Sousa F, Sousa Â. Cervical cancer and HPV infection: ongoing therapeutic research to counteract the action of E6 and E7 oncoproteins. Drug Discov Today. 2019;24(10):2044–57. https://doi.org/10.1016/j.drudis.2019.07.011.
Xu HH, Zheng LZ, Lin AF, Dong SS, Chai ZY, Yan WH. Human papillomavirus (HPV) 18 genetic variants and cervical cancer risk in Taizhou area, China. Gene. 2018;647:192–7. https://doi.org/10.1016/j.gene.2018.01.037.
Yu JH, Shi WW, Zhou MY, Liu JM, Han QY, Xu HH. Genetic variability and oncogenic risk association of human papillomavirus type 58 E6 and E7 genes in Taizhou area China. Gene. 2019;686:171–6. https://doi.org/10.1016/j.gene.2018.11.066.
Dai MZ, Qiu Y, Di XH, Shi WW, Xu HH. Association of cervical carcinogenesis risk with HPV16 E6 and E7 variants in the Taizhou area, China. BMC Cancer. 2021;21(1):769. https://doi.org/10.1186/s12885-021-08531-y.
Zhang C, Park JS, Grce M, Hibbitts S, Palefsky JM, Konno R, et al. Geographical distribution and risk association of human papillomavirus genotype 52-variant lineages. J Infect Dis. 2014;210(10):1600–4. https://doi.org/10.1093/infdis/jiu310.
Lai TO, Boon SS, Law PT, Chen Z, Thomas M, Banks L, et al. Oncogenicitiy comparison of human papillomavirus type 52 E6 variants. J Gen Virol. 2019;100(3):484–96. https://doi.org/10.1099/jgv.0.001222.
Tenjimbayashi Y, Onuki M, Hirose Y, Mori S, Ishii Y, Takeuchi T, et al. Whole-genome analysis of human papillomavirus genotypes 52 and 58 isolated from Japanese women with cervical intraepithelial neoplasia and invasive cervical cancer. Infect Agent Cancer. 2017;12:44. https://doi.org/10.1186/s13027-017-0155-4.
Zhang Y, Cao M, Wang M, Ding X, Jing Y, Chen Z, et al. Genetic variability in E6, E7, and L1 genes of human papillomavirus genotype 52 from Southwest China. Gene. 2016;585:110–8. https://doi.org/10.1016/j.gene.2016.03.007.
Choi YJ, Ki EY, Zhang C, Ho WC, Lee SJ, Jeong MJ, et al. Analysis of sequence variation and risk association of human papillomavirus 52 variants circulating in Korea. PLoS ONE. 2016;11(12): e0168178. https://doi.org/10.1371/journal.pone.0168178.
Sun Z, Lu Z, Liu J, Wang G, Zhou W, Yang L, et al. Genomic polymorphism of human papillomavirus type 52 in women from Northeast China. Int J Mol Sci. 2012;13(11):14962–72. https://doi.org/10.3390/ijms131114962.
Ding T, Wang X, Ye F, Cheng X, Ma D, Lu W, et al. Distribution of human papillomavirus 58 and 52 E6/E7 variants in cervical neoplasia in Chinese women. Gynecol Oncol. 2010;119(3):436–43. https://doi.org/10.1016/j.ygyno.2010.08.032.
Acknowledgements
We appreciate all the patients for their contribution to this study.
Funding
This work was supported by grants from National Natural Science Foundation of China (No.81901625) and National Natural Science Foundation of Zhejiang province (No.LY20H100004). None of the funders had any influence on the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
H.H.X. designed the experiments, performed analysis and drafted the manuscript. Z.Y., Z.H.H., and Y.Z. performed HPV genotyping. X.H.D. and D.F.Z. carried out the sample collection and PCR amplification. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
THis study was approved by the Institutional Medical Ethics Review Board of Taizhou Hospital of Zhejiang Province (approval #MERB-2017–020), and was carried out in line with the Helsinki Declaration. All participants provided written informed consent for study participation before specimen collection, and the patients’ privacy have been fully protected.
Consent for publication
Written informed consent was obtained from all patients for the publication of their medical data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Clinical data for HPV52 study in Taizhou area, China.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Z., He, ZH., Zhang, Y. et al. Genetic variability in the E6 and E7 oncogenes of HPV52 and its prevalence in the Taizhou area, China. Virol J 19, 194 (2022). https://doi.org/10.1186/s12985-022-01929-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-022-01929-5