Abstract
Background
The evaluation of human papillomavirus (HPV) prevalence rate dynamics and genotype distribution could support the adoption of more targeted prevention and treatment of cervical cancer. We aimed to assess the infection status and genotype characteristics of HPV among gynecological outpatients in Shanghai, China.
Methods
Clinical specimens were collected from patients attending gynaecological department of the Putuo Hospital, Shanghai University of Traditional Chinese Medicine, between January 2015 and December 2019. The cervicovaginal infection of 17 high-risk genotypes and 10 low-risk genotypes were analyzed by Luminex-based multiple assays.
Results
The overall HPV infection rate was 18.81% (95% CI 18.31–19.30%) in Shanghai city, with high-risk, low-risk and mixed high- and low-risk HPV prevalence being 11.65% (95% CI 11.24–12.06%), 4.19% (95% CI 3.94–4.44%) and 2.96% (95% CI 2.74–3.17%), respectively. The five most prevalent high-risk genotypes were HPV-52 (2.95%), HPV-16 (2.34%), HPV-58 (2.07%), HPV-53 (1.67%) and HPV-39 (1.36%). The most common low-risk genotype was HPV-61 (1.52%), followed by HPV-6 (1.29%) and HPV-81 (1.19%). Moreover, the coverage of HPV genotype by nonavalent vaccine was 10.42%, and non-vaccine-covered high-risk genotype was 7.70%. The 15–24 years age group demonstrated the highest HPV prevalence (43.14%), and significant differences were observed among different age groups (P < 0.001).
Conclusions
This study revealed the HPV prevalence and genotype distribution among women in Shanghai city, which could serve as guidance for HPV vaccination and preventative strategies against cervical cancer in this area.
Similar content being viewed by others
Introduction
Human papillomavirus (HPV) is one of most commonly encountered sexually transmitted infection, which mainly causes cervical cancer and other cancers (vaginal, vulvar, anal, penile and oropharyngeal cancer) [1]. More than 200 HPV genotypes have been identified, and they can be classified into high-risk (HR) and low-risk HPV (LR) genotypes based on their carcinogenicity. It has been demonstrated that persistent infection with HR HPV genotype including HPV16/18/31/33/35/39/45/52/58/59 is a major cause of cervical precancerous lesions and cervical cancer [2, 3], while LR-HPVgenotypes such as HPV6/11 are associated with condyloma acuminatum or hyperplastic lesions [4]. Approximately 99% cervical cancers were associated with HPV infection worldwide [5], and there are over 130,000 women suffered from cervical cancer per year in China [6]. Therefore, HPV detection screening is of great importance to reduce the burden of cervical cancer and other HPV-related diseases.
Currently, three licensed HPV vaccines are available, including bivalent (HPV-16 and -18), quadrivalent (HPV-6, -11, -16 and -18) and nonavalent vaccines (HPV-6, -11,-16, -18, -31, -33, -45, -52 and -58) in mainland China. They were launched and approved for use by China Food and Drug Administration (CFDA) in 2016, 2017 and 2018, respectively [7]. However, all these commercially vaccines only provide protection against a few genotypes, which were based on epidemiological data from western countries [8]. Furthermore, the HPV infection rate and genotype distribution vary by countries and regions [9, 10]. For instance, HPV-31 and HPV-33 are more frequent in Europe and America, whereas HPV-52 and -58 are more prevalent in Asia and HPV-35 and -45 in Africa [4]. Recent a meta-analysis indicated the most prevalent genotypes were HPV-16, -52 and -58, followed by HPV-18, -31, -33 and -35 in women with normal uterine cervix in different regions of China [11]. Thus, acquiring updates on prevalence and distribution of HPV genotypes among different areas will provide crucial information for decision on HPV vaccination program and development of new vaccine in China.
Shanghai have 16 districts with over 20 million population in its area. Previous studies have investigated the prevalence and genotype distribution of HPV in Zhoupu [12], Minghang [13] and Songjiang district [14] of Shanghai China. However, there is still limited information on the distribution of HPV infection in Shanghai, China. The primary objective of this study was to investigate the prevalence and genotype distribution of HPV infection among women attending gynecology clinics in Putuo district of Shanghai and to further evaluate the infection patterns in terms of age groups and geographical areas.
Materials and methods
Study participants
From Janauary 2015 to December 2019, women who attended at the Shanghai Putuo Hospital and Liqun Hospital and received HPV DNA genotyping test were included in this restrospective and cross-sectional study. Inclusion criteria for individual were as follows: (1) was ≥ 15 years old; (2) was living in Shanghai city; (3) was first time to receive the test and did not have any treatment; (4) not pregnant and had sexual activity. Finally, a total of 23,866 women with results of genotype-specific HPV were enrolled in this study. This study was approved by the ethics committees of Putuo Hospital, Shanghai University of Traditional Chinese Medicine (PTEC-A-2020-24-1), and the written informed consent was obtained from all the participants at each clinic visit.
Specimen collection
Cervicovaginal cell samples were collected from each participant by professional gynecologists using plastic brushes (Tellgen Life Science, Shanghai, China). The brushes were placed into sterile tubes containing 3 ml of cell preservation solution (Tellgen Life Science, Shanghai, China) and stored at 4 °C, and finally trasported to our clinical lab within one week for HPV DNA genotype testing.
DNA extraction and HPV genotyping
HPV DNA extraction was performed using a domestic commercial available viral DNA extraction kit (Tellgen Life Science, Shanghai, China) according to manufacturers, procedure. HPV detection and genotyping were conducted using Tellgenplex™ HPV DNA Test (Tellgen Life Science, Shanghai, China). The Test is a suspension bead array method that involves PCR, hybridization onto a bead using amplified PCR products and digital singnal processing [8]. In brief, 5 μL of the extracted DNA was used in the 15 μL PCR master mix reaction solution, followed by hybridization with oligonucleotide probes at 95 °C for 5 min, and 48 °C for 30 min.The hybridization product was stained with streptavidin-R-phycoerythrin, and analyzed by Luminex 200. The HPV assay kit detects 27 genotypes, including 17 high-risk (HR) HPV genotypes (HPV-16, -18, -26, -31, -33, -35, -39, -45, -51, -52, -53, -56, -58, -59, -66, -68, and -82) and 10 low-risk (LR) HPV genotypes (HPV-6, -11, -40, -42, -43, -44, -55, -61, -81, and -83).
HPV genotype categories
Beides overall HPV, type-specific, defined HR, LR and mixed HPV prevalence, we also analyzed the prevalence of several different groupings of HPV genotypes including: (1) categories based on licensed vaccine-preventable HPV genotypes: bivalent (2v) (HPV-16/18), quadrivalent (4v) (HPV-6/11/16/18), nonavalent (9v) (HPV-6/11/16/18/31/33/45/52/58) and non-vaccine HR-HPV (HPV-26/35/39/51/53/56/59/66/68/82); (2) categories grouped according infection feature: single and multiple types infection. (3) responsive classification implicated in oncogenic degree (HPV-16/18/31/33/35/39/45/51/52/56/58/59/68). In addition, The participants were divided into six groups by age (≤ 24, 25–34, 35–44, 45–54, 55–64, ≥ 65), and the prevalence of HPV infection in those age groups was calculated. Moreover, the potential impact of current vaccines in different age groups was also evaluated.
Statistical analysis
Excel (version 2010), SPSS software (version 22.0) were used for data processing and analysis. Bubble plots were created with ggplot2 and reshape2 packges, and heatmap plot was conducted with pheatmap package in R (version 4.1.2). The 95% confidence interval (CI) for HPV prevalence was estimated. Considering the impact of 4v and 9v vaccines, McNemar exact test and multiple chi-square test were used to compare the significant difference between two paired percentages and prevalence among different groups, respectively. The prevalence of 17 HR-HPV infection was summarized according geographical division of China based on epidemiological studies published from Janauary 2015 to August 2021. Difference in 17 HR-HPV genotypes distribution were visualized by Non-metric Multi-Dimensional Scaling (NMDS) using PAST software [15]. The linear-by linear association and gamma value were used to evaluate the trend in HPV prevalence over the five years, and P value < 0.05 was considered statistically significant for all analyses.
Results
Overall and type-specific HPV prevalence
There were 23,866 women aged ≥ 15 years included in the study. The overall prevalence of HPV infection was 18.81% (4489 cases, 95% CI 18.31–19.30%). Single and multiple HPV infection accounted for 13.46% (95% CI 13.03–13.89%) and 5.35% (95% CI 5.06–5.63%) of all the participants. In the multiple infection, double HPV genotypes infection was the most common feature (3.62%). The prevalence rates for HR-HPV, LR-HPV and mix-risk HPV were 11.65% (95% CI 11.24–12.06%), 4.19% (95% CI 3.94–4.44%) and 2.96% (95% CI 2.74–3.17%), respectively. Among all of the HR-HPV women, single HR-HPV positive rate was 9.67%, which was significantly higher than that of multiple HR-HPV infection (1.99%, P < 0.001). A similar results was also observed for LR-HPV infection (single HR-HPV: 3.80% vs multiple LR-HPV: 0.40%, P < 0.001). A downward trend of the HPV prevalence was observed based on year (P < 0.001; Tables 1, 2).
The five most common HR-HPV genotypes were HPV-52 (2.95%), HPV-16 (2.34%), HPV-58 (2.07%), HPV-53 (1.67%) and HPV-39 (1.36%). In addition, three most commonly detected LR-HPV genotypes were HPV-61 (1.52%), HPV-6 (1.29%) and HPV-81 (1.19%). We found significant differences for most genotypes from 2015 to 2019, except for HPV-26, HPV-42, HPV-44, HPV-61, HPV-81 and HPV-83 (P > 0.05; Table 2).
Age-specific Prevalence of HPV infection
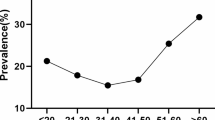
The age-specific prevalence of HPV infection is shown in Fig. 1. There were two peaks in the prevalence of overall HPV infection. The first peak found in women aged ≤ 24 years (43.14%), decreased sharply after the first peak, and maintained a plateau at middle age. The second peak observed at 55–64 years group (18.36%), followed by a moderately decline, and reached lowest prevalence at ≥ 65 years group (14.05%). HR-HPV infection also exhibited similar trend with the infection rates from 8.12% (≥ 65 years) to 20.02% (≤ 24 years). However, LR- and LR- and HR- mixed HPV infection curves showed relatively flat (Fig. 1A). Single HPV infection also peaked at ≤ 24 years, then dropped drastically with age, and stabilized in women aged 35–64 years without significant variation, and then decreased sharply again among ≥ 65 years group. Interestingly, the trend of the dual and multiple infection was similar to that overall HPV infection (Fig. 1B).
Distribution of HPV genotypes
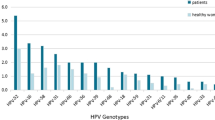
The distribution of HR-HPV and LR-HPV genotypes in different age groups are shown in Fig. 2. In the younger group (≤ 24 years), three most prevalent HR-HPV genotypes were HPV-16, HPV-52 and HPV-59, and the most prominent LR-HPV genotypes were HPV-6, HPV-11 and HPV-43. However, in the older group (≥ 55 years), HPV-52, HPV-16, HPV-58 and HPV-61, HPV-81, HPV-55 were the common HR-HPV and LR-HPV genotype, respectively. There was inconsistent distribution of HPV infection parttern among age groups. The highest percentage of single infection was detected in 35–44 years (77.9% in all HPV positive women). While, both dual and multiple infections were more frequently found in ≤ 24 years group (25.9%; Additional file 1: Table S1). Hierarchical clustering analysis showed that relative young-age group (≤ 34 years) and middle/older group (35–64 years and ≥ 65 years) shared similar distribution of HPV types (Fig. 3).
Prevalence of HPV according to vaccine types
The prevalence of detected genotypes targeted by 2v, 4v, and 9v vaccines were 3.25%, 5.08%, and 10.42%, respectively, whereas the prevalence of non-vaccine HR-HPV genotypes was 7.70% as shown in Table 1. The prevalence of HPV genotypes grouped by age was shown in Fig. 1C. Moreover, we also compared the prevalence of patients with type included in 4v and 9v vaccines among age groups. A significant relationship was observed between vaccines (4v and 9v) and groups (P < 0.001), and a significantly higher coverage in 9v vaccine than in 4v vaccine for each age group (P < 0.001, Additional file 1: Table S2).
Characteristics of HPV infection by geographical regions
We sorted out the reports according to geographical regions: eastern, central and western China based on previous published studies (Additional file 1: Table S3). The prevalence of those studies and NMDS plots of 17 HR-HPV are shown in Fig. 4. The median HPV prevalence in the eastern area of China (21.66%) was relatively higher than in the western (16.95%) and central (18.19%) regions. In general, the most commonly detected HR-HPV genotypes were HPV-16, HPV-52, HPV-58, HPV-53 and HPV-18.
Prevalence and distribution of HPV by using both this study and collected data. A Prevalence of HPV stratified by geographical areas. Solid black lines represent the median value for each geographical group. B Non-metric Multi-Dimensional Scaling (NMDS) of HR-HPV genotypes using the Euclidean similarity index
Discussion
Comparison of the HPV prevalence in Shanghai with other regions of China
HPV prevalence rate varies greatly in different regions of China as it is influenced by multiple factors such as economic levels, living habits, awareness of prevention and screening, and HPV detection method sensitivity. In the current study, we found the overall prevalence of HPV infection was 18.81%, which was consistent with that reported in Hunan (18.71%) [16] and Guangdong (18.34%) [17], lower than in Chongqing (26.15%) [18], Jiangsu (26.92%) [19], Jilin (34.40%) [20] and Fujian (38.3%) [21], but higher than in Liaoning (10.30%) [22], Yunnan (12.90%) [23], Xinjiang (9.34–14.02%) [24, 25] and Huzhou (15.50%) [26]. A recent meta-analysis results showed that the overall prevalence of HR-HPV in mainland Chinese women was 19.0% [7]. HR-HPV prevalence rate in our study was 11.65%, which was 2.78 times the prevalence for LR-HPV infection and consistent with a recent national investigation in 2017 (12.1%) [27], lower than that in Jiangxi (19.53%) [28] and Shandong (24.2%) [29]. It is generally believed that the oncogenic HPV infection rate in developed economic areas was lower than in relatively underdeveloped areas [30]. The feasible reason for the lower HR-HPV prevalence rate in our study may be also associated with relatively developed economy and people,s advanced and better health awareness in Shanghai.
Higher prevalence of HPV in younger women
Many studies have shown that HPV infection were significantly age-specific [31,32,33,34]. In the present study, overall HPV infection rate among young women (≤ 24 years, 43.14%) was much higher than that of other age groups, then the rate of HPV infection reduced sharply, which may be associated with their sexual behavior and attitude. However, decreasing trend stopped and rised slowly at 35–55 years, and slightly increased at 55–64 years (18.36%). Notably, the HR-HPV infection demonstrated the similar pattern. A previous study have been showed that the HR-HPV infection rate of Chinese women demonstrated “two-peak” pattern. The first peak presented at youngest age group (15–19 years), and the second peak observed at 50–60 years group [35]. In this study, the highest HR-HPV infection rate was also observed in the youngest age group (≤ 24 years), and followed by a less obvious peak for the 55–64 years group. While LR-HPV infection did not have the similar distribution. Compared with LR-HPV infection, HR-HPV infection was more likely to be prevalent, persistent and less likely to be cleared [36]. We also presented age-specific prevalence of single, dual and multiple HPV infections. The single genotype infection in aged ≤ 24 years group was higher than that of dual and multiple infections in other age groups, which also observed in northern Henan province of China [37]. Young women are thought to have more frequent sexual activities, more than one partner and relatively inadequate immune response, which makes them have a higher probability of exposure to HPV infection. For menopausal women, immune dysregulation would lead them unable effectively remove and inhibit the virus, may account for viral persistence or reactivation of latent HPV [38]. Therefore, further promotion of vaccination program and preventative screening strategies against cervical cancer for young women susceptible to HPV infection is necessary and urgent for this region. Additionally, cervical cancer screening program like HPV genotyping test is also valuable for perimenopausal women (≥ 55 years).
High frequency of non-vaccine HR-HPV genotypes 53, 39, 56, 51 and 59 in Shanghai women
Knowledge of the genotype distribution of HPV in specific areas will enable the improvement of optimal protective strategies. Previous studies have indicated that HPV-52, -16 and -58 were the most common HPV genotype in many regional of China [16, 23, 39, 40]. In our study, the most common genotype was HPV-52, followed by HPV-16, -58, -53 and -39, which was consistent with the result in Guizhou [41]. HPV-52, -58, -16, -51 and -39 were the five most common HR-HPV genotypes in Yangzhou [42], and HPV-52, -16, -58, -39 and -51 in Wuhan [43]. It has been reported that HPV-52 and -58 were the more prevalent genotypes in Asia, especially in China, and infection with them may have association with the cervical cancer development [38, 44]. HPV-16 and HPV-18 were the most commonly encountered genotype worldwide, accounting for up to 70% of cervical cancers [3]. In our study, HPV-16 ranked second, whereas HPV-18 was only 9th common HR-HPV genotype. In Hangzhou, HPV-16 ranked first, and HPV-18 was 5th most common prevalent genotype [45], and 11th in Shanxi [46]. HPV-16 was the most common genotype coinfection with other types [27, 33]. HPV-18 was more common in other countries than in China [9]. Persistent infection with one or more high risk genotypes of HPV is one of the leading cause for cervical neoplasia [47]. It has been reported that HR-HPV genotypes can be found in more than 90% cervical cancer specimens [48]. HPV vaccination is an effective strategy for the primary prevention of HPV infection and the potential development of cervical neoplasia. Our study showed that bivalent, quadrivalent and nonavalent vaccines only covered 3.25%, 5.08%, and 10.42% HPV genotypes in this area. It was worth noting that in addition to HPV-52, -16 and -58, there was a high prevalence of HPV-53, -39, -56, -51 and -59 in this region. These HR-HPV genotypes are not included in current available vaccines, and may should be taken into account in the future HPV vaccines to reduce the risk of HPV-related cervical cancer development in Shanghai.
Strengths and limitations of this study
This study provides a comprehensive analysis about the characteristics of HPV infection in Shanghai women. There are several limitations of this study. Firstly, this study was a only hospital-based survey including women who visited our hospital and received HPV DNA genotyping from Janauary 2015 to December 2019, which may not represent the general population in Shanghai. Secondly, there is no data of cervical lesion classification due to the inability to obtain complete cytological data of enrolled women. Thirdly, lack information of socieconomic status and sexual behaviors make it difficult to provide practical guidance in prevention of HPV infection.
Conclusions
Our data disclosed a very high prevalence of HPV infection in younger women, suggesting the great necessary of HPV screening and vaccination among younger women. High frequency of non-vaccine-coverd HR-HPV genotypes demands a local epidemiological data-based new HPV vaccine in the future.
Availability of data and materials
The original data that support the findings of this study are available upon reasonable request.
Abbreviations
- HPV:
-
Human papillomavirus
- HR:
-
High-risk
- LR:
-
Low-risk
- CFDA:
-
China food and drug administration
- 95% CI:
-
95% Confidence interval
- 2v:
-
Bivalent
- 4v:
-
Quadrivalent
- 9v:
-
Nonavalent
- NMDS:
-
Non-metric multi-dimensional scaling
References
De Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70.
Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32:16–24.
Crow JM. HPV: the global burden. Nature. 2012;488(7413):S2–3.
De Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin H-R. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56.
Wang X, Huang X, Zhang Y. Involvement of human papillomaviruses in cervical cancer. Front Microbiol. 2018:2896.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32.
Li K, Li Q, Song L, Wang D, Yin R. The distribution and prevalence of human papillomavirus in women in mainland China. Cancer. 2019;125(7):1030–7.
Liao G, Jiang X, She B, Tang H, Wang Z, Zhou H, Ma Y, Xu W, Xu H, Chen W. Multi-infection patterns and co-infection preference of 27 human papillomavirus types among 137,943 gynecological outpatients across China. Front Oncol. 2020;10:449.
De Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, Bosch FX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453–9.
Chen Z, Wang Q, Ding X, Li Q, Zhong R, Ren H. Characteristics of HPV prevalence in Sichuan Province, China. Int J Gynecol Obstet. 2015;131(3):277–80.
Xia C, Li S, Long T, Chen Z, Chan PK, Boon SS. Current updates on cancer-causing types of human papillomaviruses (HPVs) in East, Southeast, and South Asia. Cancers. 2021;13(11):2691.
Li H, Li P, Huang L, Sun L, Ren H, Li P. Prevalence characteristics of cervical human papillomavirus (HPV) infection in the Zhoupu District, Shanghai City, China. Virol J. 2020;17(1):1–8.
Wu J, Li X, Liu X, Gao Z. Human papillomavirus genotype prevalence in the women of Shanghai, China and its association with the severity of cervical neoplasia. Int J Clin Exp Pathol. 2018;11(9):4614.
Zhang C, Zhang C, Huang J, Wu Z, Mei X, Shi W. Prevalence and genotype distribution of human papillomavirus among females in the suburb of Shanghai, China. J Med Virol. 2018;90(1):157–64.
Hammer Ø, Harper D, Ryan P. PAST-palaeontological statistics, ver. 1.89. Palaeontol Electron. 2001;4(1):1–9.
Luo L-P, He P, Liu Q-T, Jiang Y-H, Zhang Y-N, Li Q-Z, Li Q, Li S-T, Yang F, Ling H. Prevalence and genotype distribution of HPV infection among 214,715 women from Southern China, 2012–2018: baseline measures prior to mass HPV vaccination. BMC Infect Dis. 2021;21(1):1–8.
Liu S, Gu X, Weng R, Liu J, Zhong Z. Positivity and prevalence of human papillomavirus among a large population of women in southeastern China. J Int Med Res. 2019;47(12):6171–81.
Tang Y, Zheng L, Yang S, Li B, Su H, Zhang L-P. Epidemiology and genotype distribution of human papillomavirus (HPV) in Southwest China: a cross-sectional five years study in non-vaccinated women. Virol J. 2017;14(1):1–10.
Zhang C, Cheng W, Liu Q, Guan Q, Zhang Q. Distribution of human papillomavirus infection: a population-based study of cervical samples from Jiangsu Province. Virol J. 2019;16(1):1–7.
Hao S, Wang C, Liu S, He J, Jiang Y. HPV genotypic spectrum in Jilin province, China, where non-vaccine-covered HPV53 and 51 are prevalent, exhibits a bimodal age-specific pattern. PLoS ONE. 2020;15(3):e0230640.
Wu C, Zhu X, Kang Y, Cao Y, Lu P, Zhou W, Zhou H, Zhang Y, Song Y. Epidemiology of Humanpapilloma virus infection among women in Fujian, China. BMC Public Health. 2018;18(1):1–8.
Xue H, Lin X, Li T, Yan X, Guo K, Zhang Y. Prevalence and genotype distribution of human papillomavirus infection in asymptomatic women in Liaoning province, China. J Med Virol. 2015;87(7):1248–53.
Li Z, Liu F, Cheng S, Shi L, Yan Z, Yang J, Shi L, Yao Y, Ma Y. Prevalence of HPV infection among 28,457 Chinese women in Yunnan Province, southwest China. Sci Rep. 2016;6(1):1–8.
Wang J, Tang D, Wang K, Wang J, Zhang Z, Chen Y, Zhang X, Ma C. HPV genotype prevalence and distribution during 2009–2018 in Xinjiang, China: baseline surveys prior to mass HPV vaccination. BMC Womens Health. 2019;19(1):1–14.
Yan X, Huang Y, Zhang M, Hu X, Li K, Jing M. Prevalence of human papillomavirus infection and type distribution among Uyghur females in Xinjiang, northwest China. Oncol Lett. 2020;20(4):1–1.
Zhu Y, Qian F, Zou W, Wu X, Liu C, Shen G, Lai S, Yang S. Prevalence and genotype distribution of human papillomavirus infection in Huzhou City, eastern China, 2018–2019. T Roy Soc Trop Med H. 2021;115(1):30–7.
Bao H-L, Jin C, Wang S, Song Y, Xu Z-Y, Yan X-J, Li L-M, Ning Y, Wang H-J. Prevalence of cervicovaginal human papillomavirus infection and genotypes in the pre-vaccine era in China: a nationwide population-based study. J Infection. 2021;82(4):75–83.
Zhong T-Y, Zhou J-C, Hu R, Fan X-N, Xie X-Y, Liu Z-X, Lin M, Chen Y-G, Hu X-M, Wang W-H. Prevalence of human papillomavirus infection among 71,435 women in Jiangxi Province, China. J Infect Public Heal. 2017;10(6):783–8.
Jiang L, Tian X, Peng D, Zhang L, Xie F, Bi C, Wang R, Wang J, Qi D. HPV prevalence and genotype distribution among women in Shandong Province, China: analysis of 94,489 HPV genotyping results from Shandong’s largest independent pathology laboratory. PLoS ONE. 2019;14(1):e0210311.
Niu J, Pan S, Wei Y, Hong Z, Gu L, Di W, Qiu L. Epidemiology and analysis of potential risk factors of high-risk human papillomavirus (HPV) in Shanghai China: a cross-sectional one-year study in non-vaccinated women. J Med Virol. 2022;94(2):761–70.
Luo G, Sun X, Li M, Liu T, Hu G, He Y, Mao L, Yan L, Xie L, Zou H. Cervical human papillomavirus among women in Guangdong, China 2008–2017: implication for screening and vaccination. J Med Virol. 2019;91(10):1856–65.
Hui XuH, Lin A, Hong Chen Y, Shan Dong S, Wu Shi W, Zheng YuJ, Hua Yan W. Prevalence characteristics of cervical human papillomavirus (HPV) genotypes in the Taizhou area, China: a cross-sectional study of 37 967 women from the general population. BMJ Open. 2017;7(6):e014135.
Chen X, Wallin KL, Duan M, Gharizadeh B, Zheng B, Qu P. Prevalence and genotype distribution of cervical human papillomavirus (HPV) among women in urban Tianjin, China. J Med Virol. 2015;87(11):1966–72.
Zhang X, Chen L, Li D, Lyu X, Lu X, Li J, Zhang W, Liu S, Wang J. Epidemiological status of cervical HPV infection in women in Shaanxi province. Chin J Woman Child Health Res. 2017;28:1589–92.
Wang R, Guo X-L, Wisman GB, Schuuring E, Wang W-F, Zeng Z-Y, Zhu H, Wu S-W. Nationwide prevalence of human papillomavirus infection and viral genotype distribution in 37 cities in China. BMC Infect Dis. 2015;15(1):1–10.
Liu H, Wei X, Xie Z, Wang X, Gong X, Ke W, Zou H. Cervical human papillomavirus among 19 753 women attending gynecological department of a major comprehensive hospital in north Anhui China 2013–2016: implication for cervical cancer screening and prevention. J Med Virol. 2019;91(4):698–706.
Wang X, Song Y, Wei X, Wang G, Sun R, Wang M, Zhao L. Prevalence and distribution of human papillomavirus genotypes among women attending gynecology clinics in northern Henan Province of China. Virol J. 2022;19(1):1–7.
Li B, Wang H, Yang D, Ma J. Prevalence and distribution of cervical human papillomavirus genotypes in women with cytological results from Sichuan province, China. J Med Virol. 2019;91(1):139–45.
Zeng Z, Yang H, Li Z, He X, Griffith CC, Chen X, Guo X, Zheng B, Wu S, Zhao C. Prevalence and genotype distribution of HPV infection in China: analysis of 51,345 HPV genotyping results from China’s largest CAP certified laboratory. J Cancer. 2016;7(9):1037.
Ma L, Lei J, Ma L, Cong X, Wang N, Yang H, Liu Q, Yu Y, Cao Y. Characteristics of women infected with human papillomavirus in a tertiary hospital in Beijing China, 2014–2018. BMC Infect Dis. 2019;19(1):1–8.
Chen Z, Li Q, Huang Q, Liu H, Jiang H, Chen Z, An Z, Luo Q. Characteristics of human papillomaviruses distribution in Guizhou Province, China. Virol J. 2019;16(1):1–5.
Li Y, Liu X, Han C, Ren C. Prevalence and genotype distribution of high-risk human papillomavirus in 34 420 cases in Yangzhou city, Jiangsu province, China. J Med Virol. 2021;93(8):5095–102.
Xiang F, Guan Q, Liu X, Xiao H, Xia Q, Liu X, Sun H, Song X, Zhong Y, Yuan CH. Distribution characteristics of different human papillomavirus genotypes in women in Wuhan, China. J Clin Lab Anal. 2018;32(8):e22581.
Baloch Z, Li Y, Yuan T, Feng Y, Liu Y, Tai W, Liu L, Wang B, Zhang A-M, Wu X. Epidemiologic characterization of human papillomavirus (HPV) infection in various regions of Yunnan Province of China. BMC Infect Dis. 2016;16(1):1–14.
Qian L, Zhang Y, Cui D, Lou B, Chen Y, Yu Y, Liu Y, Chen Y. Analysis of epidemiological trends in human papillomavirus infection among gynaecological outpatients in Hangzhou, China, 2011–2015. BMC Infect Dis. 2017;17(1):1–9.
Yang J, Wang W, Wang Z, Wang Z, Wang Y, Wang J, Zhao W, Li D, Liu H, Hao M. Prevalence, genotype distribution and risk factors of cervical HPV infection in Yangqu, China: a population-based survey of 10086 women. Hum Vacc Immunother. 2020;16(7):1645–52.
Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907.
Li N, Franceschi S, Howell-Jones R, Snijders PJ, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128(4):927–35.
Acknowledgements
We thank those authors who provided details of the data in their published articles. We are grateful to the staff from Shanghai Putuo District Central Hospital for their help. Special gratitude to my baby who is about to be born, her arrival gave me a lot of support.
Funding
This work was supported by the Putuo Hospital affiliated with the Shanghai University of Chinese Medicine (Grant No. 2019302).
Author information
Authors and Affiliations
Contributions
X.L. conceived the experiments, analyzed the data and drafted this manuscript; F.X., J.D. and T.Z. performed the experiments; Z.C. and M.Z. collected the samples; X.K. and R.W. contributed a lot to the design of this study. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committees of Putuo Hospital, Shanghai University of Traditional Chinese Medicine (PTEC-A-2020-24-1), and the written informed consent was obtained from all the participants at each clinic visit.
Consent for publication
Not applicable. This manuscript does not contain any persons data in any form.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Prevalence of cervicovaginal human papillomaviruses stratified by age and geographical areas.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, X., Xiang, F., Dai, J. et al. Prevalence of cervicovaginal human papillomavirus infection and genotype distribution in Shanghai, China. Virol J 19, 146 (2022). https://doi.org/10.1186/s12985-022-01879-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-022-01879-y