Abstract
Background
COVID-19, the coronavirus disease that emerged in December 2019, caused drastic damage worldwide. At the beginning of the pandemic, available data suggested that the infection occurs more frequently in adults than in infants. In this review, we aim to provide an overview of SARS-CoV-2 infection in children before and after B.1.617.2 Delta and B.1.1.529 Omicron variants emergence in terms of prevalence, transmission dynamics, clinical manifestations, complications and risk factors.
Methods
Our method is based on the literature search on PubMed, Science Direct and Google Scholar. From January 2020 to July 2022, a total of 229 references, relevant for the purpose of this review, were considered.
Results
The incidence of SARS-CoV-2 infection in infants was underestimated. Up to the first half of May, most of the infected children presented asymptomatic or mild manifestations. The prevalence of COVID-19 varied from country to another: the highest was reported in the United States (22.5%). COVID-19 can progress and become more severe, especially with the presence of underlying health conditions. It can also progress into Kawasaki or Multisystem Inflammatory Syndrome (MIS) manifestations, as a consequence of exacerbating immune response. With the emergence of the B.1.617.2 Delta and B.1.1.529 Omicron variants, it seems that these variants affect a large proportion of the younger population with the appearance of clinical manifestations similar to those presented by adults with important hospitalization rates.
Conclusion
The pediatric population constitutes a vulnerable group that requires particular attention, especially with the emergence of more virulent variants. The increase of symptomatic SARS-CoV-2 infection and hospitalization rate among children highlights the need to extend vaccination to the pediatric population.
Similar content being viewed by others
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), has spread rapidly around the world since its emergence in Wuhan, China, late 2019 [1, 2]. By January 2020, the virus has been isolated and sequenced [3, 4], revealing close relationships to coronaviruses such as SARS-CoV [5] and MERS-CoV [6]. On March 11, 2020, the World Health Organization (WHO) announced COVID-19 as a pandemic [7] causing 430,257,564 confirmed cases of COVID-19, including 5,922,047 deaths up to 25 February 2022 [8].
SARS-CoV-2 is an enveloped, positive RNA virus [9]. It belongs to the family of Coronaviridae, the subfamily of Orthocoronavirinae, the genera of Betacoronavirus and subgenus of Sarbecovirus [10, 11]. It is responsible for severe lower respiratory tract infections in humans [12].
SARS-CoV-2 causes pneumonia, characterized by fever, cough, shortness of breath, and bilateral infiltration on chest imaging [2, 12]. It can also induce fatal lung damage, multiple organ failure and death [9, 13]. The clinical manifestations can be classified into four severity of illness categories: asymptomatic, mild, moderate and severe clinical [14]. In contrast with adults who can develop the four types of clinical manifestations, children are mainly asymptomatic or present mild infection and, in some cases, they can develop severe post-COVID-19 manifestations such as Kawasaki-like symptoms [15,16,17,18]. After a year into the COVID-19 pandemic, new variants emerged and spread rapidly across the continents [19]. In the United Kingdom, the B.1.617.2 Delta variant, initially identified in India in October 2020, spreads rapidly through schools [20]. Children seem to be the most affected category [19, 21, 22]. The B.1.1.529 Omicron variant first detected in South Africa, in 2021, appeared to be more contagious than the Delta variant, associated with a significant increase in the number of pediatric and adults SARS-CoV-2 infections [23,24,25,26,27,28].
This review gives an overview of SARS-CoV-2 infection in children before and after B.1.617.2 Delta and B.1.1.529 Omicron variants emergence. We analyzed current knowledge on the prevalence, clinical manifestations and complications among immunocompetent and immunodepressive children as well as risk factors for the severity of COVID-19.
Methods
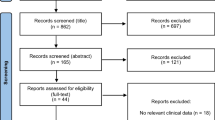
Original research studies published on COVID-19/SARS-CoV-2 among children in English, between January 2020 and July 2022, were identified using PubMed, Science Direct and Google Scholar. The search used combinations of the keywords “COVID-19,” “SARS-CoV-2,” “clinical manifestations” “prevalence”, “Transmission”, “pediatric population,” “child”, “SARS-CoV-2 variants”, “Delta variant”, ”B.1.617.2”, “Omicron variant”, “B.1.1.529”, “Risk factors” and “COVID-19 vaccines” (Fig. 1). In addition, the reference lists of the retrieved articles were checked for other relevant articles. Moreover, 229 references were considered relevant to the aim of this review. An additional table file shows more details about these references (see Additional file 1: Table S1).
Epidemiology of COVID-19 in infants and children
Epidemiology of COVID-19 in infants before the Delta and Omicron era
At the beginning of the COVID-19 epidemic, the available data during the period February 26, 2020, to June 10, 2020, suggested that the infection occurs more frequently in adults and seems to be unusual in infants (1.7–2% of the diagnosed cases of COVID-19) [29, 30]. On February 11, 2020, the Chinese Center for Disease Control and Prevention (China CDC) showed that among 72,314 cases less than 1% and 1% of the cases were in children younger than 10 years and 10 to 19 years, respectively [31]. In the United States, data concerning 149,760 laboratory-confirmed COVID-19 cases obtained between February 12 and April 2, 2020, showed that 1.7% were in children aged less than 18 years [32]. From March 5 to April 8, 2020, the Chicago Department of Public Health reported that among 6369 laboratory-confirmed cases, 1.0% were children aged 0–17 years [30].
Afterward, it appeared that the true incidence of infection in infants was underestimated [33]. A retrospective study based on Nationwide case series of 2135 COVID-19 pediatric patients (< 18 years) with COVID-19 reported to the China CDC, from January 16, 2020, to February 8, 2020, indicated that children of all ages appeared to be susceptible to COVID-19 [33]. In this study, 731 (34.1%) children had laboratory-confirmed COVID-19. The Center for Disease Control and Prevention (CDC) showed an increase in the rate of COVID-19 pediatric cases, from March to July 2020, in the USA: 7.3% of all COVID-19 cases compared to 1.7% of COVID-19 pediatric cases obtained during the period 12 February to 2 April [34, 35]. It seems that children and adults acquired infection at similar rates but develop different clinical manifestations [33]. In China, several studies including population varied from 731 to 1391 children showed only a few numbers of infants (1–2%) developed symptomatic forms [31,32,33,34,35,36]. Also, a systematic review conducted by Bhuiyan et al. [37] showed that among 1214 confirmed COVID-19 pediatric cases younger than five years nearly half of the cases were asymptomatic.
Globally, the prevalence of COVID-19-positive infants was underreported. The number of COVID-19-positive infants was reported only in a few studies in which, it is indicated among other positive cases [38, 39]. Figure 2 shows the prevalence of COVID-19-positive infants in different regions of the world based on the reported data before the emergence of the delta and omicron variants. The prevalence of COVID-19 in infants varied from country to country (1.1–22.5%) (Fig. 2). The highest percentages of COVID-19 positive infants were from United States (22.5%), Saudi Arabia (13.2%), Spain (12.6%) and Nigeria (11.7%) [40,41,42,43]. Lower percentages were found in Brazil (8.4%), Canada (7.3%), Korea (6.5%), India (5.9%), Norway (5.0%), Congo (4.5%), Germany (2.7%), Australia (2.6%), United Kingdom (2.2%), China (1.6–2.2%), Iran (1.7%), Italy (1.5%) and Netherland (1.1%) [37, 39, 44,45,46,47,48]. These differences may be explained by the possible country-level influence on COVID-19 such as the testing strategy of suspected cases, the original population structure of each country, and whether the control measures (social distance, wearing masks, washing hands, etc.) were strictly followed by the public or not and also by the period of the state of the epidemic. However, it is necessary to point out that the true prevalence of COVID-19 in infants remains underestimated due to the lack of stated data and the high frequency of asymptomatic forms [49].
Epidemiology of COVID-19 in infants during the Delta and Omicron era
During Delta era
Since its emergence in December 2020, the delta variant (B.1.617.2) seems to affect a large proportion of the younger population with the appearance of clinical signs similar to those presented by adults [21, 22, 50]. Main data was obtained from the UK, after April 2021, where this variant became a dominant strain and was spread rapidly through schools [20]. Public Health England confirmed an increased number of outbreaks and clusters at primary and secondary schools from May to June 2, 2021 [20]. Reports from different areas such as Bolton indicated that cases were growing fastest among school-age children, with an increased number of cases at any point during the pandemic [51]. Also, data from the Office for National Statistics of UK for the weeks of 29th May and 1st June, showed an increase in the numbers of cases in school children aged 7–11 years [20, 52]. In the United States, it was suggested that the delta variant was highly transmissible in indoor sports settings and households, which might lead to higher attack rates among exposed persons especially children aged 5–19 years [53]. Overall, in the epidemiological analysis of COVID-19 in infants during the Delta variant era, there was a significant increase in pediatric cases and hospitalizations [54,55,56]. Data suggested that these increases are related to increased Delta COVID-19 incidence rather than increased Delta virulence in infants [54, 55, 57].
During Omicron era
The emergence of the Omicron variant was also linked to an increasing number of SARS-CoV-2 infections and hospital admissions of infants and adults. Available data showed rising in pediatric SARS-CoV-2 hospitalizations in many countries [23,24,25,26,27,28]. In the UK, according to the data from the COVID-19 Clinical Information Network study, the proportion of infants aged less than 1 year admitted to hospital was 42.2% (period from 14 December 2021 to 12 January 2022), much higher than earlier in the pandemic (Fig. 3) [23, 24]. However, reports from Scotland, the US, South Africa, and England showed lower rates of hospitalization following Omicron infection compared with the Delta variant infection [58,59,60,61]. The national data from a Scotland cohort analysis suggested that Omicron is associated with a two-thirds reduction in the risk of COVID-19 hospitalization when compared to Delta [59]. In the US, a retrospective cohort study conducted on 577,938 patients during the period when Delta and Omicron variants predominate showed that the outcomes in children under 5 years old are mildest after the emergence of the Omicron variant [61]. The risk for hospitalization and the risk for emergency department admission that occurred after the emergence of the Omicron variant was one-third (Fig. 3) and less than one-fifth of that during the Delta variant period, respectively [61]. These findings were also observed for children aged 5–11 and 12–17 years old (Fig. 3) [61]. The increased rate of infections and hospitalizations caused by the Omicron variant compared with the Delta variant seems to be attributed to differences in the prevalence of the virus variants.
Transmission dynamic among infants and adults
Transmission dynamic Before Delta and Omicron era
The need to understand the impact of children in the SARS-CoV-2 pandemic sighted researchers to study the transmission dynamics of SARS-CoV-2 between infant-infant and infant-adult. From February 2020 to January 2021, the obtained data were controversial. Many studies reported low transmissibility of SARS-CoV2 among children and also from children to adults [36, 38, 62, 63]. For instance, when investigating SARS-CoV-2 transmissibility in 23 clusters, Maltezou et al. showed that the transmission of infection within families including children occurs more frequently from adult to child [63]. Low transmissibility in children was also reported by other studies conducted in China and United States, which it has shown that young children often acquired infections through community sources [35, 37]. Bhuiyan et al. [37], found that among 1214 children younger than five years, the majority (> 95%) had a community source of infection. However, in the same period, other studies suggested an important role of children in the transmission dynamics of SARS-CoV-2 [30, 64]. A household transmission pattern study, in the United States, demonstrated that children were the potential source of further transmission in approximately 20% of households, with possible child-to-adult transmission in 20% and possible child-to-child transmission in 17% [64]. Also, Mannheim et al. suggested both child-to-child and child-to-adult transmission is estimated at 13% [30]. In India, based on the contact-tracing data from the Indian states of Tamil Nadu and Andhra Pradesh by 1 August 2020, Laxminarayan et al. [1] showed that the transmission occurs more frequently among children who contact cases from their own age. The enhanced infection risk among individuals exposed to similar-age cases was also apparent among adults [1]. The authors concluded that children may play a key role in the transmission dynamics of SARS-CoV-2 in middle and low incomes countries where social, cultural, and economic conditions favor close contact between children and also with adults [1, 65].
Transmission dynamic during the Delta and Omicron era
During Delta era
The emergence of novel variants much more transmissible raised questions about the change in the relative risk of SARS-CoV-2 child–adult transmissibility [66]. Loenenbach et al. [67] 2021 showed that the susceptibility and infectiousness of children having the SARS-CoV-2 B.1.1.7 variant are substantially higher compared with the pre-variants of concern (VOC) period (39% vs. 7.9%). The SARS-CoV-2 B.1.617.2 variant seems to be around 60% more transmissible than the SARS-CoV-2 B.1.1.7 variant [68, 69]. It is spreading rapidly, particularly through primary and secondary schools as well as indoor sports settings and households, which might lead to higher attack rates among children [20, 21, 53].
During Omicron era
A rapid increase in COVID-19 cases was reported from the beginning of December 2021, as a result of the Omicron variant spreading. The transmission dynamics of this new variant was assessed by an in-silico analysis. Data from this study showed that the infectivity of Omicron might be more than tenfold higher than that of the original virus and approximately twice as high as that of Delta [70]. According to reported data, it is estimated that the Omicron variant infects six times as many people as Delta over the same time and that the Rt of Omicron is 1.4- to 3.1-fold higher than that of Delta [25, 26, 71]. In Gauteng South Africa, Viana et al. [28] estimated that Omicron had a growth advantage of 0.24 per day over Delta, corresponding to a 5.4-fold weekly increase in cases compared to Delta. Data on the rate in children in the US showed that the rate of infected children admitted to hospital from 26 December 2021 to 1 January 2022 was nearly double the rate reported in early December before the Omicron variant began to take hold [27]. Current data shows that the Omicron variant is more contagious than the Delta variant. However, evidences from other international studies are needed to highlight the role of SARS-CoV-2 variants in increasing outbreak size.
Incubation period
The incubation period is the duration from the exposure of the pathogen to the onset of symptoms. Studies report a median incubation period of 5 to 7.5 days, before the emergence of the B.1.617.2 variant. Shen et al. [72] showed that the median incubation period was 7.5 days in six children ranging from 1 to 16 days. In another study, the median incubation period of children was 5 days (ranging from 3 to 12 days) [73]. It was shown that the incubation period of SARS-CoV-2 differs from variant to another (Table 1). The incubation period for the Delta variant, to the best of our knowledge, was only reported in four studies from China and Japan [74,75,76,77]. It was significantly shorter (3.7, 4.0 and 4.4 days) than for non-Delta strains (4.9–6.0 days) [74,75,76]. However, in another study, a longer incubation period was reported (6.0 days) [77]. After the emergence of the Omicron variant, preliminary information suggests that the median incubation period may be shorter at around 2–3 days in children and adults [78].
Common clinical manifestations
Before the Delta and Omicron era
At the onset of the SARS-CoV-2 pandemic up to May 2021, compared to adults, children get less COVID-19 and had less severe cases [33, 48, 62,63,64]. Unlike adults, infants develop more likely asymptomatic [33, 36, 72, 79,80,81,82,83] or mild forms [16, 31, 33, 36, 64, 84, 85]. Symptomatic children reported fever and dry cough as the most common symptoms. They were reported at 36–73% and 19–54% of pediatric cases, respectively [32, 35, 48, 66, 67, 79, 86,87,88,89,90,91,92,93]. The body temperature varied mainly between 37.5 and 38.5 [86]. These symptoms can be accompanied by headache, fatigue, myalgia, nasal congestion, sneezing, [28, 48, 64, 68, 69, 79, 86, 88, 89, 94]. Headache was reported in almost 25–79% of cases [48, 64, 70, 71].
Regarding nasal congestion, some studies, in the USA and China reported an important proportion of 50–68% of pediatric cases with rhinorrhea [64, 94]. In contrast, other studies based on a limited number of cases, reported fewer proportions ranging from 2.8 to 16% of cases with rhinorrhea and nose congestion [86, 87, 89, 91, 95].
While adult patients rarely developed intestinal signs, symptoms such as nausea, vomiting, abdominal pain, constipation, and diarrhea are frequent among pediatric populations [96, 97]. A wide variety of manifestations can appear before, with, or after the development of respiratory symptoms [67,68,69, 79, 96, 98,99,100]. Anorexia or poor appetite was frequent (34–67%), followed by diarrhea (2–32.5%), vomiting (1–28.3%), abdominal pain and discomfort (1–11.9%) and nausea (1–11.1%). [9, 79, 96, 99, 101,102,103,104,105,106,107,108,109,110].
Compared with symptomatic adults, children were less likely to report loss of taste and loss of smell [48, 64] and more likely to report sore throat [64, 83, 86, 88, 89]. In Taiwan, up to April, 7, among 24 children, 12.5% had a loss of smell and/or taste [48]. Other studies reported also the development of sore throat. According to Laws RL and colleagues in a series of 19 children, 68% had presented sore throat [64]. However sore throat seems low in other studies: 1 case in a total of 20 [88], 2 cases of sore throat among 31 [89].
Clinical manifestations during the Delta and Omicron era
During Delta era
The Delta variant infection appeared to cause a similar common outcome [56, 111]. A retrospective study in China investigated the clinical features of delta-infected patients and non-delta ones and showed that cough and fever are still predominant signs, but gastrointestinal symptoms have become much less frequent [111]. In South Korea, a retrospective study showed that there was no significant difference in COVID-19 symptoms in delta and non-delta patients except for the lower frequencies of rhinorrhea (25% vs. 10.5%, P = 0.003), nasal stuffiness (34.8% vs. 15.4%, P = 0.001) and sore throat (23.9% vs. 12.6%, P = 0.02) [56]. Additionally, it was shown that there was no statistically significant difference between the two groups in the frequency of pneumonia (2.2% vs. 0.7%, P = 0.56) and hospital transfer (5.4% vs. 2.1%, P = 0.27) [56].
During Omicron era
The same common manifestations were observed with Delta and Omicron variants. A study comparing the outcome of COVID-19 infection in pediatrics and adults before and after the emergence of the Omicron variant showed that both Delta and Omicron variants induce relatively stable outcomes [61]. However, higher manifestations rates were associated with the Omicron variant due to its higher infectivity compared to the other variants [61].
Complications of COVID-19
Complications before the Delta and Omicron era
Before the emergence of the Delta variant, complications among positive COVID-19 children cases were less frequent. They were especially reported among patients with underlying medical conditions. Required hospitalization was in 11% to 60% of cases and admission to the Intensive Care Unit (ICU) in almost 10% of cases, the majority presented medical underlying conditions [32, 93, 112, 113].
Severe cases presented hypoxia, tachypnea and oxygen saturation less than 92% [33, 93]. Critical cases can rapidly progress into acute respiratory distress syndrome or respiratory failure and present shock, encephalopathy, myocardial injury, heart failure, coagulation dysfunction, acute kidney injury and Organ dysfunction [33, 87, 94].
Complications during the Delta and Omicron era
During Delta era
After the emergence of the Delta and Omicron variants of SARS-CoV-2, available information reported high hospitalization rates among children [19, 114,115,116]. Furthermore, a study conducted in Scotland and England showed that the risk of COVID-19 hospitalization was approximately double in those with Delta VOC compared with Alpha VOC and that, particularly in those with comorbidities [117, 118]. However, other studies showed that the increased rate of hospitalization during the Delta emergence was not associated with a severe outcome with a similar rate of Intensive Care Unit admission (Table 1) [69, 119, 120]. In Japan, the intensive care unit admission rate was higher during the Delta VOC era than in the pre-Delta VOC era (1.4% [n = 5] vs. 0.1% [n = 1], P = 0.006), among pediatric patients with COVID-19 (Table 1), but no patient in either group died or required mechanical ventilation or extracorporeal membrane oxygenation [54]. According to these data, it appears that the Delta variant does not cause worse clinical outcomes compared to prior lineages. Furthermore, a retrospective study investigating imaging characteristics of children infected with the Delta variant, showed that the imaging-based severity was significantly milder than that in cases in 2020 [121].
During Omicron era
For the Omicron variant, based on a multicenter nationwide database in the US, no major changes in hospitalization were observed during the two-week that immediately preceded the emergence of the Omicron variant (11/16/2021–11/30/2021) and the 10-week Delta variant period before it (9/1/2021–11/15/2021) [61]. A lower rate of emergency department visits was found among children and adults compared to the Delta variant period (Table 1) [61]. In the UK, it was shown that the rate of admission to intensive care was less than that detected during the delta period (Table 1) [23].
Immunodepressive children
Immunodepressive children before the Delta and Omicron era
As COVID-19 can trigger complications of an underlying disease, patients with immune deficits need to be monitored carefully during the COVID-19 pandemic [122]. Nevertheless, at the beginning of the pandemic, studies about COVID-19 among Primary Immunodeficiency (PID) patients were controversial. According to the Joint Forces of International Societies for Immunodeficiencies, most patients present a mild disease [122, 123]. For instance, In Iran, a case report of an eight-year-old boy who had Specific Antibody Deficiency (SAD), presented wet cough and rhinorrhea [124]. In general, patients with severe disease evolution, additionally, have comorbidities or complications of their immunodeficiency [122, 123].
Other studies considered that patients with immunodeficiency are at increased risk of developing severe COVID-19 [125,126,127]. PID patients presented: shortness of breath, a drop in oxygen saturation, respiratory distress, tachypnea, seizure, cardiomegaly, cardiac, and pulmonary arrest, requiring hospitalization and admission to intensive care units [128,129,130]. For instance, among an international series of 94 COVID-19 PID patients, 18 were admitted to intensive care units, and nine patients died [129]. Another study reported up to 50% hospitalization rate among PID requiring mechanical ventilation [130,131,132]. Fatal outcomes were also reported among PID and Secondary Immunodeficiency (SID) patients. It was estimated up to 35.3% and 44% respectively [130].
It was expected that prolonged carriage of SARS-CoV-2 is possible such as in the case of viruses belonging to the family of Picornaviridae: poliovirus [133], non-polio enteroviruses [134, 135] and cosavirus [136]. Data reported shedding of infectious SARS-CoV-2 and genomic RNA by PID occurred for many months [137]. They excreted the virus up to 120 days after initial detection (70–120 days) [138]. In these cases, Primary Immunodeficient (PIDs) and Secondary Immunodeficient (SIDs) patients, may constitute an increased risk of transmission to the community with the persistence of a reservoir for the virus and the potential emergence of mutated strains.
Immunodepressive children during the Delta and Omicron era
The available data on immunocompromised patients infected with the delta variant is limited. According to the study of Hadjadj and colleagues, the emergence of SARS-CoV-2 strains like the Delta variant (B.1.617.2) with increased viral form and immune escape potential, raises concerns among immunocompromised patients [139, 140]. Recently, a new variant of concern (VOC) called Omicron was first identified on November 24, 2021, in an immunocompromised patient from Johannesburg, South Africa as a consequence of prolonged replication among PID patients [141,142,143,144,145]. However, no more data has been published on the behavior of omicron in severely immunocompromised individuals [146].
Kawasaki-like and Multisystem Inflammatory Syndrome COVID-19 manifestations
SARS-CoV-2 can induce a systemic inflammatory response affecting multiple organs, with severely affected lungs. The extrapulmonary manifestations can include systemic vasculature, similar to Kawasaki disease (KD) or Multisystem Inflammatory Syndrome [18, 147]. KD is an acute systemic inflammatory disease of medium- and small-sized vessels that mostly involve children under 5 years old [148,149,150,151,152]. In the acute phase of the disease, patients might have haemo-dynamic instability, a condition known as Kawasaki Disease Shock Syndrome (KDSS) [153]. The cause of KD is unknown, but it is generally accepted that viral agents can trigger the disease [18]. The diagnosis is clinical and based on a combination of fever for at least five days and generalized inflammation involving lymph nodes, skin, and mucous membranes [147, 154, 155]. It can also induce conjunctivitis, coronary artery dilation/aneurysms and bilateral bulbar, [18, 156, 157]. Several cases have shown that SARS-CoV-2 stimulates an immune reaction mimicking KD [87, 156, 158,159,160,161,162,163,164,165,166, 185]. Association to COVID-19 was mainly confirmed by serology test since clinical manifestations occur during the post-COVID-19 period in Children [87, 147, 156, 159,160,161,162,163,164,165,166]. Children can present classical form of the Kawasaki disease or incomplete (atypical) form [156, 158,159,160, 162, 167,168,169,170,171,172,173,174,175].
Multisystem Inflammatory Syndrome in Children (MIS-C) is a pediatric hyperinflammation disease (cytokine storm) that was associated with SARS-CoV-2 infection [18, 158]. In general, it manifests 2–4 weeks following their initial COVID-19 infection as a consequence of an excessive immune response [167]. The clinical presentation of MIS-C includes high fever, inflammation and multi-organ dysfunction including gastrointestinal, cardiovascular (high frequency of myocarditis up to 80%), hematological, mucocutaneous, and respiratory [18, 168,169,170,171,172,173]. MIS-C appears to share clinical features with KD, toxic shock syndrome and macrophage activation syndrome (MAS) [18, 170]. Despite this, MIS-C differs from KD in the involvement of greater age, gastrointestinal, myocarditis and/or cardiogenic shock, heart failure requiring inotropic support and circulatory assistance [18, 170]. On the other hand, comorbidity was associated with MIS-C manifestation including asthma and overweight. [175].
Outcomes
In the pediatric population, disease duration is relative to the severity of the disease. Indeed, the duration is over 10 days across all patients and over 20 days in critically ill patients [13, 88, 89, 91, 94].
The length of hospital stay has varied between different studies: the minimum and maximum averages reported have been 8.3 and 15.3 days respectively [72, 91]. In the case of hospitalization, the mean time was 14 days [72, 86]. Qiu et al. [86] concluded that patients with the moderate clinical type spent more days in hospital compared with those with a mild clinical type. Delta variant patients seem to have a significantly longer median (5.7) hospital length of stay [176]. In general, children who are admitted to PICU recovered without any adverse outcomes [87]. Fatal outcomes were also reported, generally in patients with underlying health conditions [32, 92, 177].
Factors impacting lower susceptibility of children to COVID-19 infection
There are several hypotheses on the mechanisms underlying the lower susceptibility of children to COVID-19 infection than adults [178, 179]. First, there is a lower expression of the Angiotensin-Converting Enzyme 2 (ACE2) receptor to which the virus would bind to enter cells [180]. ACE2 is a receptor for SARS-CoV-2 and the key region responsible for the interaction [106, 181]. Differences in the distribution of ACE2 in the developing phase of childhood are a possible reason for milder SARS-CoV-2 infection [29]. Second, an “immaturity” and consequently loss of functioning of the ACE2 receptors, makes it difficult, for the virus to enter the body [182]. Third, kids present a great expression of the innate immune response, which is more effective against this virus than adults [179, 183]. Children seem also to present a more efficient immune response due to the stimulation given by typical age and vaccinations [184]. According to the literature, some vaccines such as HBV, Tetanus, Measles, Mumps, Rubella and BCG may have a protective effect against COVID-19 [185,186,187].
Factors impacting the severity of COVID-19 in children
Before the Delta and Omicron era
There are many risk factors associated with the progression and severity of COVID19 in children such as age, male gender, ethnicity, comorbidity, coinfection and virus type [29].
In the United States, Bandi S and colleagues reported that the mean age of COVID-19–positive children was significantly higher than those testing negative (9.72 vs. 4.85 years) [188]. In another study, severe and critical cases were reported mainly in children aged less than 1 year [36]. However, DeBiasi RL and colleagues showed that adolescents and young adults were more commonly critically ill than younger children [189].
Ethnicity was examined in the study of Bandi et al. [188]. African American children had a significantly higher rate of positive tests for COVID-19: 6.8% versus 1.7% of white children. Among 58 children, in the United Kingdom, race (black or Asian) was described as a risk factor for COVID-19 [190].
Male gender is a risk factor for severe coronavirus disease in adults [191]. A predominance of boys was reported in all age subgroups among 2490 pediatric cases of COVID-19 in a series in the United States [32]. In a cross-sectional study of 48 children with COVID-19 admitted in the USA and Canadian intensive care units (ICUs), 52% were boys [192].
Underlying medical comorbidity may be a risk factor for severe disease in childhood [32, 80, 88, 92, 177, 192, 193].
The rate of patients with preexisting morbidity, developing severe COVID-19 manifestations, varied between 23 and 83% [193]. Among underlying diseases, chronic lung disease was the most common followed by cardiovascular disorder and immunosuppressive disease [32]. Malnutrition, cancers and kidney diseases were also implicated [178]. Respiratory syncytial virus and Enterobacter aerogenes were implicated [87, 194].
During the Delta and Omicron era
During Delta era
The emergence of the delta variant B.1.617.2 confirmed the impact of virus strains on the severity of the disease. B.1.617.2 was considered as the most dominant SARS-CoV-2 variant before the appearance of the Omicron variant and it was rapidly spread around the world [195, 196]. The Delta variant harbors different mutations in the Spike gene that helped the virus to be more transmissible, escape immune response, easily break into cells and efficiently replicate (T19R, L452R, T478K, D614G, P681R, and d960N, G142D, R158G with deletions at positions 157 and 158) [195, 197, 198].
For instance, D614G interrupts certain molecular interactions in the spike protein facilitating virus propagation. L452R can enhance the interaction between ACE2 and the spike protein, which makes the virus more susceptible to infect cells. T478K is unique to the delta variant. Its role has not yet been described and like L452R, it may help enhance the maintenance of spike proteins on ACE2 [197, 199, 200].
During Omicron era
After this wave, a new variant B.1.1.529 was first reported to the WHO in South Africa on November 24, 2021, called Omicron [141]. This variant contains a large number of mutations. It included more than 30 mutations in the spike protein alone compared to delta variant mutations in addition to others in proteins such as NSP12 and NSP14 which enhanced the viral replication [145, 195, 201,202,203]. If vaccine coverage is still insufficient in any region of the world, it will be possible in the future that other varieties will emerge with significant changes in transmissibility, infectivity and pathogenicity.
SARS-COV-2 vaccines in children
After the emergence of the Delta variants, children of all ages seem to be more vulnerable to develop severe cases. Thus, they should benefit from vaccination to protect themselves from severe and long-term consequences and, also, to restrain virus circulation among the population. Two types of mRNA vaccines, Pfizer-BioNTech (BNT162b2) and Moderna, were allowed for children [204,205,206,207,208,209]. The Pfizer vaccine was authorized, in May 2021, for children aged more than 12 years, and in December 2021, for children aged 5–11 years [205]. In July 2021, the European Medicines Agency (EMA) authorized the Moderna vaccine for those aged 12–17 years, while vaccination of kids aged 5–11 years is still under-investigation [210]. Both vaccines have been applied, in children over the age of 12, in American, European, African and Asian countries [211,212,213,214,215,216,217,218,219,220,221,222]. While only Pfizer has been implemented for those aged 5–11 years, in Italy, the United States, Israel, United Kingdom, Germany, Ireland, Poland, France, Spain and Denmark. [210,211,212,213, 218, 223,224,225]. Several studies have shown that vaccines were safe and effective in reducing virus transmission and protecting them against re-infection with SARS-CoV-2 [205, 226,227,228]. However, they may cause mild to moderate adverse reactions, such as fever, fatigue and irritability, with a low risk of myocarditis, especially for children aged 5–11 years [205, 225,226,227].
Conclusion
The pediatric population constitutes a vulnerable group that requires particular attention during the SARS-CoV2 pandemics and especially with the emergence of more transmissible and virulent SARS-CoV-2 variants. Although children are less susceptible to COVID-19, and the clinical picture in childhood is often distinct from that in adults, in both age groups chronic underlying medical problems, immunodeficiency and virus virulence can predispose to severe disease. In contrast with adults, in whom older age is an independent risk factor for severity and mortality, very young age is considered a risk factor for severity in children, The care of children with allergies or immune conditions is being adapted to the current situation, with more remote working and guiding children to reduce the likelihood of infection in children who would be deemed at higher risk of severe COVID-19 disease [122]. The current COVID-19 pandemic might also pose a risk to pediatric patients with secondary immunodeficiencies, such as patients on immunosuppressive therapy for autoimmune or severe allergic diseases [122]. With the emergence of the Delta and Omicron variants, infection in children seems to be more frequent and severe. The increase of symptomatic and severe SARS-CoV-2 infections highlights the need to extend vaccination to the pediatric population. The Pfizer and Moderna vaccines were authorized for children since they were found to be safe, immunogenic, and efficacious.
Availability of data and materials
The present study was a systemic review based on published original research articles. All data are publicly available within the manuscript. We have accessed the published articles.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- COVID-19:
-
Coronavirus disease 2019
- WHO:
-
World Health Organization
- ICU:
-
Intensive Care Unit
- KD:
-
Kawasaki Disease
- KDSS:
-
Kawasaki Disease Shock Syndrome RNA: Ribonucleic Acid
- MAS:
-
Macrophage Activation Syndrome
- MIS-C:
-
Multisystem Inflammatory Syndrome in Children
- NSP12:
-
Non Structural Protein 12
- NSP14:
-
Non Structural Protein 14
- HBV:
-
Hepatitis B Virus
- PICU:
-
Pediatric Intensive Care Unit
- PID:
-
Primary Immunodeficiency
- SAD:
-
Specific Antibody Deficiency
- SID:
-
Secondary Immunodeficiency
- VOC:
-
Variants of Concern
- USA:
-
United States of America
References
Laxminarayan R, Wahl B, Dudala SR, Gopal K, Mohan BC, Neelima S, Jawahar Reddy KS, Radhakrishnan J, Lewnard JA. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020. https://doi.org/10.1126/science.abd7672.
Rathore JS, Ghosh C. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a newly emerged pathogen: an overview. Pathog Dis. 2020;78(6):ftaa042.
Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3.
Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33.
Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76.
Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–20.
World Health Organization. Coronavirus disease (COVID-19) outbreak. https://www.who.int.
WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China The Lancet févr. 2020;395(10223):497–506.
Wang H. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur J Clin Microbiol Infect Dis. 2020. https://doi.org/10.1007/s10096-020-03899-4.
Wassenaar TM, Zou Y. 2019_nCoV/SARS-CoV-2: rapid classification of betacoronaviruses and identification of Traditional Chinese Medicine as potential origin of zoonotic coronaviruses. Lett Appl Microbiol. 2020;70(5):342–8.
Lotfi M, Rezaei N. SARS-CoV-2: a comprehensive review from pathogenicity of the virus to clinical consequences. J Med Virol. 2020;92(10):1864–74.
Zhang T, Cui X, Zhao X, Wang J, Zheng J, Zheng G, et al. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J Med Virol. 2020;92(7):909–14.
World Health Organization (2020) Clinical management of COVID-19: interim guidance, 27 May 2020. World Health Organization. https://apps.who.int/iris/handle/10665/332196.
Jahangir M, Nawaz M, Nanjiani D, Siddiqui MS. Clinical manifestations and outcomes of COVID-19 in the paediatric population: a systematic review. Hong Kong Med J. 2020. https://doi.org/10.12809/hkmj208646.
Rezaei N. COVID-19 affects healthy pediatricians more than pediatric patients. Infect Control Hosp Epidemiol sept. 2020;41(9):1106–7.
Shen K, Yang Y, Wang T, Zhao D, Jiang Y, Jin R, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J Pediatr. 2020;16(3):223–31.
Gkoutzourelas A, Bogdanos DP, Sakkas LI. Kawasaki disease and COVID-19. Mediterr J Rheumatol. 2020;31(Suppl 2):268–74.
The Public Health England (PHE) Variant Technical Group. SARS-CoV-2 variants of concern and variants under investigation in England: Technical briefing 10. London: PHE. [Accessed: 15 Jun 2021]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/984274/Variants_of_Concern_VOC_Technical_Briefing_10_England.pdf
Torjesen I. Covid-19: Delta variant is now UK’s most dominant strain and spreading through schools. BMJ. 2021;373:n1445.
Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet Lond Engl. 2021;397(10293):2461–2.
O’Dowd A. Covid-19: Cases of Delta variant rise by 79%, but rate of growth slows. BMJ. 2021;373:n1596. https://doi.org/10.1136/bmj.n1596.
Torjesen I. Covid-19: Omicron variant is linked to steep rise in hospital admissions of very young children. BMJ. 2022;14(376):o110. https://doi.org/10.1136/bmj.o110.
Scientific Advisory Group for Emergencies. CO-CIN: Child admissions and severity by epoch CO-CIN update January 2022, 6–14 Jan 2022. https://www.gov.uk/government/publications/co-cin-child-admissions-and-severity-by-epoch-co-cin-update-january-2022-6-january-2022
Tian D, Sun Y, Xu H, Ye Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol. 2022. https://doi.org/10.1002/jmv.27643.
Callaway E, Ledford H. How bad is Omicron? Nature. 2021;600:197–9.
McNiff S. Covid hospitalizations rising in kids too young for vaccine. US News. 13 Jan 2022. https://www.usnews.com/news/health-news/articles/2022-01-13/covid-hospitalizations-rising-in-kids-too-young-for-vaccine
Viana R, Moyo S, Amoako DG, Tegally H, Scheepers C, Althaus CL, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022. https://doi.org/10.1038/s41586-022-04411-y.
Tsabouri S, Makis A, Kosmeri C, Siomou E. Risk factors for severity in children with Coronavirus Disease 2019: a comprehensive literature review. Pediatr Clin North Am. 2021;68(1):321–38. https://doi.org/10.1016/j.pcl.2020.07.014.
Mannheim J, Gretsch S, Layden JE, Fricchione MJ. Characteristics of hospitalized pediatric Coronavirus Disease 2019 cases in Chicago, Illinois, March–April 2020. J Pediatric Infect Dis Soc. 2020;9(5):519–22. https://doi.org/10.1093/jpids/piaa070.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
CDC COVID-19 Response Team. Coronavirus Disease 2019 in Children-United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422–6.
Dong Y, Mo X, Hu Y. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145(6):e20200702.
CDC COVID-19 Response Team. Infections among children: Covid-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/pediatrichcp.html. Accessed 9 Jan 2020
Borrelli M, Corcione A, Castellano F, Fiori Nastro F, Santamaria F. Coronavirus Disease 2019 in children. Front Pediatr. 2021;9:668484.
Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020;145:e20200702.
Bhuiyan MU, Stiboy E, Hassan MZ, Chan M, Islam MS, Haider N, Jaffe A, Homaira N. Epidemiology of COVID-19 infection in young children under five years: a systematic review and meta-analysis. Vaccine. 2021;39(4):667–77. https://doi.org/10.1016/j.vaccine.2020.11.078.
Pan A, Liu L, Wang C, Guo H, Hao X, Wang Q, Huang J, He N, Yu H, Lin X, Wei S, Wu T. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323(19):1915–23. https://doi.org/10.1001/jama.2020.6130.
Public Health Agency of Canada COVID-19; Surveillance; Epidemiology Team1. Descriptive epidemiology of deceased cases of COVID-19 reported during the initial wave of the epidemic in Canada, January 15 to July 9, 2020. Can Commun Dis Rep. 2020;46(10):344–8. https://doi.org/10.14745/ccdr.v46i10a06.
Elimian KO, Ochu CL, Ilori E, Oladejo J, Igumbor E, Steinhardt L, et al. Descriptive epidemiology of coronavirus disease 2019 in Nigeria, 27 February–6 June 2020. Epidemiol Infect. 2020;11(148): e208. https://doi.org/10.1017/S095026882000206X.
Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–44. https://doi.org/10.1016/S0140-6736(20)31483-5.
Leidman E, Duca LM, Omura JD, Proia K, Stephens JW, Sauber-Schatz EK. COVID-19 trends among persons aged 0–24 years-United States, March 1–December 12, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(3):88–94. https://doi.org/10.15585/mmwr.mm7003e1.
Alahmari AA, Khan AA, Elganainy A, Almohammadi EL, Hakawi AM, Assiri AM, Jokhdar HA. Epidemiological and clinical features of COVID-19 patients in Saudi Arabia. J Infect Publ Health. 2021;14(4):437–43. https://doi.org/10.1016/j.jiph.2021.01.003.
Nachega JB, Ishoso DK, Otokoye JO, Hermans MP, Machekano RN, Sam-Agudu NA, et al. Clinical characteristics and outcomes of patients hospitalized for COVID-19 in Africa: early insights from the Democratic Republic of the Congo. Am J Trop Med Hyg. 2020;103(6):2419–28. https://doi.org/10.4269/ajtmh.20-1240.
Poustchi H, Darvishian M, Mohammadi Z, Shayanrad A, Delavari A, Bahadorimonfared A, et al. SARS-CoV-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: a population-based cross-sectional study. Lancet Infect Dis. 2021;21(4):473–81. https://doi.org/10.1016/S1473-3099(20)30858-6.
Jakhmola S, Baral B, Jha HC. A comparative analysis of COVID-19 outbreak on age groups and both the sexes of population from India and other countries. J Infect Dev Ctries. 2021;15(3):333–41. https://doi.org/10.3855/jidc.13698.
Korea Disease Control and Prevention Agency (2020) Weekly report on the COVID-19 situation in the Republic of Korea As of April (As of April 4, 2020). Available: http://www.kdca.go.kr/filepath/boardDownload.es?bid=0031&list_no=366798&seq=1. Accessed: 12 April 2020.
Jeng MJ. Coronavirus disease 2019 in children: current status. J Chin Med Assoc. 2020;83(6):527–33. https://doi.org/10.1097/JCMA.0000000000000323.
Sola AM, David AP, Rosbe KW, Baba A, Ramirez-Avila L, Chan DK. Prevalence of SARS-CoV-2 infection in children without symptoms of Coronavirus Disease 2019. JAMA Pediatr. 2021;175(2):198–201. https://doi.org/10.1001/jamapediatrics.2020.4095.
World Health Organization. (2021). COVID-19 weekly epidemiological update, edition 76, 25 January 2022.
National Association of Head Teachers. Government data on COVID variant cases linked to schools should be published “immediately,” say education unions. 27 May 2021. https://www.naht.org.uk/news-and-opinion/pressroom/government-data-on-covid-variant-cases-linked-to-schools-shouldbe-published-immediately-say-education-unions.
Office for National Statistics. Coronavirus (Covid-19) infection survey, UK. 4 Jun 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/4june2021#age-analysis-of-the-number-of-people-who-had-covid-19.
Dougherty K, Mannell M, Naqvi O, Matson D, Stone J. SARS-CoV-2 B.1.617.2 (Delta) variant COVID-19 outbreak associated with a gymnastics facility-Oklahoma, April–May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(28):1004–7. https://doi.org/10.15585/mmwr.mm7028e2.
Shoji K, Akiyama T, Tsuzuki S, Matsunaga N, Asai Y, Suzuki S, Iwamoto N, Funaki T, Ohmagari N. Comparison of the clinical characteristics and outcomes of COVID-19 in children before and after the emergence of Delta variant of concern in Japan. J Infect Chemother. 2022;S1341–321X(22):00022–8. https://doi.org/10.1016/j.jiac.2022.01.009.
Edward PR, Lorenzo-Redondo R, Reyna ME, Simons LM, Hultquist JF, Patel AB, Ozer EA, Muller WJ, Heald-Sargent T, McHugh M, Dean TJ, Dalal RM, John J, Manz SC, Kociolek LK. Severity of Illness Caused by Severe Acute Respiratory Syndrome Coronavirus 2 Variants of Concern in Children: A Single-Center Retrospective Cohort Study. medRxiv [Preprint]. 2021 Oct 26:2021.10.23.21265402. doi: https://doi.org/10.1101/2021.10.23.21265402
Ryu BH, Hong SI, Lim SJ, Cho Y, Hong KW, Bae IG, Cho OH. Features of COVID-19 among children and adolescents without risk factors before and after the Delta variant outbreak in South Korea. Pediatr Infect Dis J. 2022;41(1):e34–5. https://doi.org/10.1097/INF.0000000000003394.
Siegel DA, Reses HE, Cool AJ, et al. MAPW1. Trends in COVID-19 cases, emergency department visits, and hospital admissions among children and adolescents aged 0–17 years–United States, August 2020–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1249–54.
Wolter N, Jassat W, Walaza S, et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa. bioRxiv. Published online December 21, 2021. doi :https://doi.org/10.1101/2021.12.21.21268116
Sheikh A, Kerr S, Woolhouse M, McMenamin J, Robertson C. Severity of Omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. Published online December 22, 2021. Accessed December 23, 2021. https://www.pure.ed.ac.uk/ws/files/245818096/Severity_of_Omicron_variant_of_concern_and_vaccine_effectiveness_against_symptomatic_disease.pdf
Report 50 - Hospitalisation risk for Omicron cases in England. Accessed 23 Dec 2021. https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-50-severity-Omicron/
Wang L, Berger NA, Kaelber DC, Davis PB, Volkow ND, Xu R. Comparison of outcomes from COVID infection in pediatric and adult patients before and after the emergence of Omicron. medRxiv [Preprint]. 2022 Jan 2:2021.12.30.21268495. doi: https://doi.org/10.1101/2021.12.30.21268495. PMID: 35018384; PMCID: PMC8750707.
Lee B, Raszka WV Jr. COVID-19 transmission and children: the child is not to blame. Pediatrics. 2020;146(2):e2020004879. https://doi.org/10.1542/peds.2020-004879.
Maltezou HC, Vorou R, Papadima K, et al. Transmission dynamics of SARS-CoV-2 within families with children in Greece: a study of 23 clusters. J Med Virol. 2021;93(3):1414–20. https://doi.org/10.1002/jmv.26394.
Laws RL, Chancey RJ, Rabold EM, Chu VT, Lewis NM, et al. Symptoms and transmission of SARS-CoV-2 among children-Utah and Wisconsin, March–May 2020. Pediatrics. 2021;147(1):e2020027268. https://doi.org/10.1542/peds.2020-027268.
Kitano T, Kitano M, Krueger C, Jamal H, Al Rawahi H, Lee-Krueger R, et al. The differential impact of pediatric COVID-19 between high-income countries and low- and middle-income countries: a systematic review of fatality and ICU admission in children worldwide. PLoS ONE. 2021;16(1):e0246326. https://doi.org/10.1371/journal.pone.0246326.
Pascarella S, Ciccozzi M, Zella D, Bianchi M, Benetti F, Benvenuto D, et al. SARS-CoV-2 B.1.617 Indian variants: are electrostatic potential changes responsible for a higher transmission rate? J Med Virol. 2021. https://doi.org/10.1002/jmv.27210.
Loenenbach A, Markus I, Lehfeld AS, An der Heiden M, Haas W, Kiegele M, Ponzi A, Unger-Goldinger B, Weidenauer C, Schlosser H, Beile A, Buchholz U. SARS-CoV-2 variant B.1.1.7 susceptibility and infectiousness of children and adults deduced from investigations of childcare centre outbreaks, Germany, 2021. Euro Surveill. 2021;26(21):2100433. https://doi.org/10.2807/1560-7917.ES.2021.26.21.2100433.
Singh J, Rahman SA, Ehtesham NZ, Hira S, Hasnain SE. SARS-CoV-2 variants of concern are emerging in India. Nat Med. 2021. https://doi.org/10.1038/s41591-021-01397-4.
Callaway E. Delta coronavirus variant: scientists brace for impact. Nature. 2021;595(7865):17–8. https://doi.org/10.1038/d41586-021-01696-3.
Schmidt F, Weisblum Y, Rutkowska M, et al. High genetic barrier to SARS-CoV-2 polyclonal neutralizing antibody escape. Nature. 2021;600(7889):512–6.
CDC. COVID Data Tracker [Internet]. [cited 2021 Dec 23]; Available from: https://covid.cdc.gov/covid-data-tracker/#variant-proportions
Shen Q, Guo W, Guo T, Li J, He W, Ni S, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol. 2020;55(6):1424–9.
Han Y, Feng Z, Sun L, Ren X, Wang H, Xue Y, et al. A comparative-descriptive analysis of clinical characteristics in 2019-coronavirus-infected children and adults. J Med Virol. 2020;92(9):1596–602.
Ogata T, Tanaka H, Irie F, Hirayama A, Takahashi Y. Shorter incubation period among unvaccinated Delta variant Coronavirus Disease 2019 patients in Japan. Int J Environ Res Public Health. 2022;19(3):1127. https://doi.org/10.3390/ijerph19031127.
Wang Y, Chen R, Hu F, et al. Transmission, viral kinetics and clinical characteristics of the emergent SARS-CoV-2 Delta VOC in Guangzhou. China EClinicalMedicine. 2021;40:101129. https://doi.org/10.1016/j.eclinm.2021.101129.
Zhang M, Xiao J, Deng A, Zhang Y, Zhuang Y, Hu T, Li J, Tu H, Li B, Zhou Y, Yuan J, Luo L, Liang Z, Huang Y, Ye G, Cai M, Li G, Yang B, Xu B, Huang X, Cui Y, Ren D, Zhang Y, Kang M, Li Y. Transmission dynamics of an outbreak of the COVID-19 Delta variant B.1.617.2-Guangdong Province, China, May–June 2021. China CDC Wkly. 2021;3(27):584–6. https://doi.org/10.46234/ccdcw2021.148.
Li L, Han ZG, Qin PZ, Liu WH, Yang Z, Chen ZQ, Li K, Xie CJ, Ma Y, Wang H, Huang Y, Fan SJ, Yan ZL, Ou CQ, Luo L. Transmission and containment of the SARS-CoV-2 Delta variant of concern in Guangzhou, China: a population-based study. PLoS Negl Trop Dis. 2022;16(1):e0010048. https://doi.org/10.1371/journal.pntd.0010048.
Jansen L, Tegomoh B, Lange K, et al. Investigation of a SARS-CoV-2 B.1.1.529 (Omicron) variant cluster–Nebraska, November–December 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1782–4.
Su L, Ma X, Yu H, et al. The different clinical characteristics of corona virus disease cases between children and their families in China—the character of children with COVID-19. Emerg Microbes Infect. 2020;9:707–13.
Feng K, Yun YX, Wang XF, et al. Analysis of CT features of 15 children with 2019 novel coronavirus infection [in Chinese]. Zhonghua Er Ke Za Zhi. 2020;58:E007.
Ma X, Su L, Zhang Y, Zhang X, Gai Z, Zhang Z. Do children need a longer time to shed SARS-CoV-2 in stool than adults? J Microbiol Immunol Infect. 2020;53:373–6.
Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–11.
Li W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol. 2020;50:796–9.
Yang P, Liu P, Li D, et al. Corona Virus Disease 2019, a growing threat to children? J Infect. 2020;80(6):671–93.
Yu N, Li W, Kang Q, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20:559–64.
Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–96.
Zheng F, Liao C, Fan QH, et al. Clinical characteristics of children with Coronavirus Disease 2019 in Hubei. China Curr Med Sci. 2020;40(2):275–80.
Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55:1169–74.
Wang D, Ju XL, Xie F, et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China [in Chinese]. Zhonghua Er Ke Za Zhi. 2020;58:269–74.
Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323:1313–4.
Liu W, Zhang Q, Chen J, et al. Detection of COVID-19 in children in early January 2020 Wuhan. China N Engl J Med. 2020;382:1370–1.
Lu X, Zhang L, Du H, et al. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382(17):1663–5.
Parri N, Lenge M. Children with Covid-19 in Pediatric Emergency Departments in Italy. N Engl J Med. 2020;383(2):187.
Sun D, Li H, Lu XX, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr. 2020;16(3):251–9.
Zhou Y, Yang GD, Feng K, et al. Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children [in Chinese]. Zhongguo Dang Dai Er Ke ZaZhi. 2020;22:215–20.
Al-Beltagi M, Saeed NK, Bediwy AS, El-Sawaf Y. Paediatric gastrointestinal disorders in SARS-CoV-2 infection: epidemiological and clinical implications. World J Gastroenterol. 2021;27(16):1716–27.
Han YN, Feng ZW, Sun LN, et al. A comparative-descriptive analysis of clinical characteristics in 2019-coronavirus infected children and adults. J Med Virol 2020;Apr 6. Epub ahead of print.
Aguila EJT, Cua IHY, Dumagpi JEL, Francisco CPD, Raymundo NTV, Sy-Janairo MLL, Cabral-Prodigalidad PAI, Lontok MAD. COVID-19 and its effects on the digestive system and endoscopy practice. JGH Open. 2020;4:324–31. https://doi.org/10.1002/jgh3.12358.
Jin X, Lian JS, Hu JH, Gao J, Zheng L, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–9. https://doi.org/10.1136/gutjnl-2020-320926].
Jahangir M, Nawaz M, Nanjiani D, Siddiqui MS. Clinical manifestations and outcomes of COVID-19 in the paediatric population: a systematic review. Hong Kong Med J [Internet]. 30 Sept 2020 [cited July 31 2021]; Available from: https://www.hkmj.org/abstracts/v27n1/35.htm
Pan L, Mu M, Ren HG, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, crosssectional, multicenter study. 2020. Available from URL: https://journals.lww.com/ajg/Documents/COVID_Digestive_Symptoms_AJG_Preproof.pdf.
Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in Fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020;S0016–5085(20):30448.
Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single centered, retrospective, observational study. Lancet Gastroenterol Hepatol. 2020;8:475–81.
Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet Gastroenterol Hepatol. 2020;395:507–13.
Wang Y, Liu Y, Liu L, Wang X, Luo N, Ling L. Clinical outcome of 55 asymptomatic cases at the time of hospital admission infected with SARS-Coronavirus-2 in Shenzhen, China. Oxf Univ Press Infect Dis Soc Am. 2020. https://doi.org/10.1093/infdis/jiaa119/5807958.
Xu X, Wu X, Jiang X, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606.
Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Eng J Med. 2020;382:1708–20.
Young B, Ong S, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488.
Han C, Duam C, Zhang S et al. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020 Epub ahead of print: 1.
Giacomet V, Barcellini L, Stracuzzi M, Longoni E, Folgori L, Leone A, Zuccotti GV, COVID-19 Pediatric network. Gastrointestinal symptoms in severe COVID-19 children. Pediatr Infect Dis J. 2020;39:e317–20. https://doi.org/10.1097/INF.0000000000002843.
Hu Z, Huang X, Zhang J, Fu S, Ding D, Tao Z. Differences in clinical characteristics between Delta variant and Wild-Type SARS-CoV-2 infected patients. Front Med (Lausanne). 2022;3(8):792135. https://doi.org/10.3389/fmed.2021.792135.
Italian National Health Institute (Istituto Superiore di Sanità). Coronavirus epidemic: situation report. March 26, 2020. (In Italian) (https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_26-marzo%202020.pdf)
Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2020. https://doi.org/10.1001/jamapediatrics.2020.1346.
Mahase E. Delta variant: What is happening with transmission, hospital admissions, and restrictions? BMJ. 2021;373:n1513.
Ryu B-H, et al. Features of COVID-19 Among children and adolescents without risk factors before and after the Delta variant outbreak in South Korea. Pediatr Infect Dis J. 2022;41:e34–5. https://doi.org/10.1097/INF.0000000000003394.
Wang L, Berger NA, Kaelber DC, Davis PB, Volkow ND, Xu R. COVID infection severity in children under 5 years old before and after Omicron emergence in the US. medRxiv [Preprint]. 2022 Jan 13:2022.01.12.22269179. doi: https://doi.org/10.1101/2022.01.12.22269179. PMID: 35043116; PMCID: PMC8764724
Sheikh A, McMenamin J, Taylor B, Robertson C, Public Health Scotland, the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2. https://doi.org/10.1016/S0140-6736(21)01358-1.
Twohig KA, Nyberg T, Zaidi A, et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 Delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2021;S1473–3099(21):00475–8. https://doi.org/10.1016/S1473-3099(21)00475-8.
Murillo-Zamora E, Trujillo X, Huerta M, Ríos-Silva M, Baltazar-Rodríguez LM, Guzmán-Esquivel J, Benites-Godínez V, Ortega-Ramírez AD, Mendoza-Cano O. Decreased risk of COVID-19 pneumonia in children and adolescents during the Delta variant emergence. Public Health. 2021;204:9–11. https://doi.org/10.1016/j.puhe.2021.12.017.
Delahoy MJ, Ujamaa D, Whitaker M, et al. Hospitalizations associated with COVID-19 among children and adolescents-COVID-NET, 14 states, March 1, 2020-August 14, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1255–60.
Cheng QR, Fan MX, Hao J, Hu XC, Ge XH, Hu ZL, Li Z. Chest CT features of children infected by B.1.617.2 (Delta) variant of COVID-19. World J Pediatr. 2022;18(1):37–42. https://doi.org/10.1007/s12519-021-00484-3.
Brough HA, Kalayci O, Sediva A, Untersmayr E, Munblit D, Rodriquez Del Rio P, et al. Managing childhood allergies and immunodeficiencies during respiratory virus epidemics-the 2020 COVID-19 pandemic. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. 2020. Epub 2020/04/23.
Al Yazidi LS, Al Rawahi H, Al Busaidi I, Al TS. COVID-19 and primary immunodeficiency: one-year experience. J Paediatr Child Health. 2021;57(4):594.
Ahanchian H, Moazzen N, Faroughi MSD, Khalighi N, Khoshkhui M, Aelami MH, et al. COVID-19 in a Child with Primary Specific Antibody Deficiency [Internet]. In Review; 2020 May [cited August 23 2021]. Available from: https://www.researchsquare.com/article/rs-28155/v1.
CDC. Coronavirus Disease 2019 (COVID-19). Cent Dis Control Prev. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/asthma.html. Accessed April 2, 2020
Liu BM, Hill HR. Role of host immune and inflammatory responses in COVID-19 cases with underlying primary immunodeficiency: a review. J Interferon Cytokine Res. 2020;40(12):549–54.
Babaha F, Rezaei N. Primary immunodeficiency diseases in COVID-19 pandemic: a predisposing or protective factor? Am J Med Sci. 2020;360(6):740–1.
Delavari S, Abolhassani H, Abolnezhadian F, Babaha F, Iranparast S, Ahanchian H, et al. Impact of SARS-CoV-2 pandemic on patients with primary immunodeficiency. J Clin Immunol. 2021;41(2):345–55.
Meyts I, Bucciol G, Quinti I, Neven B, Fischer A, Seoane E, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147(2):520–31.
Shields AM, Burns SO, Savic S, Richter AG, Anantharachagan A, Arumugakani G, et al. COVID-19 in patients with primary and secondary immunodeficiency: the United Kingdom experience. J Allergy Clin Immunol. 2021;147(3):870-875.e1.
Quinti I, Lougaris V, Milito C, Cinetto F, Pecoraro A, Mezzaroma I, et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146(1):211–3.
Soresina A, Moratto D, Chiarini M, Paolillo C, Baresi G, Foca E, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. 2020. Epub 2020/04/23.
Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol. 2005;59:587–635.
Driss N, Ben-Mustapha I, Mellouli F, Ben Yahia A, Touzi H, Bejaoui M, et al. High susceptibility for enterovirus infection and virus excretion features in Tunisian patients with primary immunodeficiencies. Clin Vaccine Immunol CVI. 2012;19(10):1684–9.
Driss N, Mellouli F, Ben Yahia A, Touzi H, Barbouche MR, Triki H, et al. Sequential asymptomatic enterovirus infections in a patient with major histocompatibility complex class II primary immunodeficiency. J Clin Microbiol. 2014;52(9):3486–9.
Lamari A, Triki H, Driss N, Touzi H, Meddeb Z, Ben Yahia A, et al. Iterative excretion of human Cosaviruses from different genotypes associated with combined immunodeficiency disorder. Intervirology. 2018;61(5):247–54.
Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL, et al. Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell. 2020;183(7):1901-1912.e9.
Tarhini H, Recoing A, Bridier-Nahmias A, Rahi M, Lambert C, Martres P, et al. Long term SARS-CoV-2 infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J Infect Dis. 2021;223:1522.
Hadjadj J, Planas D, Ouedrani A, Buffier S, Delage L, Nguyen Y, et al. Immunogenicity of BNT162b2 vaccine against the Alpha and Delta variants in immunocompromised patients with systemic inflammatory diseases. Ann Rheum Dis. January 2022; annrheumdis-2021-221508.
Chappell H, Patel R, Driessens C, Tarr AW, Irving WL, Tighe PJ, et al. Immunocompromised children and young people are at no increased risk of severe COVID-19. J Infect. 2022;84(1):31–9.
WHO Coronavirus disease (COVID-19). Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
CDC. Omicron Variant: What You Need to Know. https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html.
Wang L, Cheng G. Sequence analysis of the emerging SARS-CoV-2 variant Omicron in South Africa. J Med Virol. 2022;94(4):1728–33.
Bansal N, Raturi M, Bansal Y. SARS-CoV-2 variants in immunocompromised COVID-19 patients: The underlying causes and the way forward. Transfus Clin Biol. December 2021; S1246782021005322.
Gao S-J, Guo H, Luo G. Omicron variant (B.1.1.529) of SARS-CoV-2, a global urgent public health alert! J Med Virol. 2022;94(4):1255–6.
Singhal T. The emergence of Omicron: challenging times are here again! Indian J Pediatr. 2022. https://doi.org/10.1007/s12098-022-04077-4.
Fang Y, Aravamudan VM, Sridharan GK, Mehta KK, Sekhar R, Senguttuvan NB, Venkatachalam I, Abid MB. Kawasaki like illness due to COVID-19: a review of the literature. J Infect Dev Ctries. 2021;15:630–8. https://doi.org/10.3855/jidc.14185.
Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936–41. https://doi.org/10.1136/ard.2005.046300.
McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH. Diagnosis, Treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. 2017;135(17):e927–99.
Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–6.
Kato H, Sugimura T, Akagi T, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996;94:1379–85.
Xu S, Chen M, Weng J. COVID-19 and Kawasaki disease in children. Pharmacol Res. 2020;159:104951.
Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123:e783–9.
Dietz SM, van Stijn D, Burgner D, et al. Dissecting Kawasaki disease: a state-of-the-art review. Eur J Pediatr. 2017;176:995-1009.9.
McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–99.
Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D’Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicenter of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020. https://doi.org/10.1016/S0140-6736(20)31103-X.
Saguil A, Fargo M, Grogan S. Diagnosis and management of kawasaki disease. Am Fam Physician. 2015;91:365–71.
Kabeerdoss J, Pilania RK, Karkhele R, Kumar TS, Danda D, Singh S. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int. 2020;21:1–14.
Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020. https://doi.org/10.1136/bmj.m2094.
Jones VG, Mills M, Suarez D. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10(6):537–40. https://doi.org/10.1542/hpeds.2020-0123.
Rivera-Figueroa E, Santos R, Simpson S, et al. Incomplete Kawasaki disease in a child with COVID-19. Indian Pediatr. 2020;57(7):680–1. https://doi.org/10.1007/s13312-020-1900-0.
Pouletty M, Borocco C, Ouldali N, et al. Pediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. https://doi.org/10.1136/annrheumdis-2020-217960.
Ramcharan T, Nolan O, Lai CY, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARSCoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK Tertiary Paediatric Hospital. Pediatr Cardiol. 2020;12:1–11. https://doi.org/10.1007/s00246-020-02391-2.
Ouldali N, Pouletty M, Lokmer J et al (2020) Response to: ‘Correspondence on ‘Paediatric multisystem infammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort’by Pouletty et al’by Pino et al. Ann Rheum Dis. DOI: https://doi.org/10.1136/annrheumdis-2020-218614
Lee PY, Day-Lewis M, Henderson LA, et al. Distinct clinical and immunological features of SARS-COV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;23:141113. https://doi.org/10.1172/JCI141113.
Pino R, Izurieta AC, Ríos-Barnés M, et al. Correspondence on: ‘Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort’by Pouletty et al. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-218538.
Thompson LA, Kelly MN. Return to play after COVID-19 infection in children. JAMA Pediatr. 2021;175(8):875–875.
Centers for Disease Control and Prevention. Emergency preparedness and response: multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Health advisory (https://emergency.cdc.gov/han/2020/han00432.asp).
Nakra N, Blumberg D, Herrera-Guerra A, Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: review of clinical presentation, hypothetical pathogenesis, and proposed management. Children. 2020;7(7):69.
Riollano-Cruz M, Akkoyun E, Briceno-Brito E, Kowalsky S, Posada R, Sordillo EM, et al. Multisystem inflammatory syndrome in children (MIS-C) related to COVID-19: a New York city experience. J Med Virol. 2020. https://doi.org/10.1002/jmv.26224.
Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–46.
Miller J, Cantor A, Zachariah P, et al. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology. 2020. https://doi.org/10.1053/j.gastro.2020.05.079.
Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, et al. Multisystem inflammatory syndrome in children during the Coronavirus 2019 pandemic: a case series. J Pediatr Infect Dis Soc. 2020;9(3):393–8.
Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10(1):69.
Belhadjer Z, Me’ot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020. https://doi.org/10.1161/CIRCULATIONAHA.120.048360.
Christensen PA, Olsen RJ, Long SW, Subedi S, Davis JJ, et al. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. Am J Pathol. 2022;192(2):320–31. https://doi.org/10.1016/j.ajpath.2021.10.019.
Oualha M, Bendavid M, Berteloot L, et al. Severe and fatal forms of COVID-19 in children. Arch Pediatr. 2020;27(5):235–8.
De Jacobis IT, Vona R, Cittadini C, Marchesi A, Cursi L, Gambardella L, et al. Clinical characteristics of children infected with SARS-CoV-2 in Italy. Ital J Pediatr. 2021;47(1):90.
Pecoraro L, Carbonare LD, Franceschi LD, Piacentini G, Pietrobelli A. The psychophysical impact that COVID-19 has on children must not be underestimated. Acta Paediatr. 2020;109(8):1679–80.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80. https://doi.org/10.1016/j.cell.2020.02.052.
Wang Q, Zhang Y, Wu L, et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894-904.e9.62.
Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID-19? J Microbiol Immunol Infect. 2020;53(3):371–2. https://doi.org/10.1016/j.jmii.2020.02.011.
Be’ne’teau-Burnat B, Baudin B, Morgant G, et al. Serum angiotensin-converting enzyme in healthy and sarcoidotic children: comparison with the reference interval for adults. Clin Chem. 1990;36(2):344–6.
Shen K, Yang Y, Wang T, Zhao D, Jiang Yi, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J Pediatr. 2020;16(3):223–31.
Gold JE, Baumgartl WH, Okyay RA, Licht WE, Fidel PL, Noverr MC, et al. Analysis of Measles-Mumps-Rubella (MMR) titers of recovered COVID-19 patients. MBio. 2020;11(6):e02628-e2720.
Escobar LE, Molina-Cruz A, Barillas-Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc Natl Acad Sci U S A. 2020;117(30):17720–6.
Haddad-Boubaker S, Othman H, Touati R, Ayouni K, Lakhal M, Ben Mustapha I, et al. In silico comparative study of SARS-CoV-2 proteins and antigenic proteins in BCG, OPV, MMR and other vaccines: evidence of a possible putative protective effect. BMC Bioinform. 2021;22:163.
Bandi S, Nevid MZ, Mahdavinia M. African American children are at higher risk of COVID‐19 infection. Genuneit J, éditeur. Pediatr Allergy Immunol. 2020;31(7):861–4. https://doi.org/10.1111/pai.13298.
DeBiasi RL, Song X, Delaney M, et al. Severe COVID-19 in children and young adults in the Washington. DC Metropolitan Region J Pediatr. 2020;223:199-203.e1.
Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020. https://doi.org/10.1001/jama.2020.10369.
Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020. https://doi.org/10.1001/jama.2020.4683.
Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus Disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020. https://doi.org/10.1001/jamapediatrics.2020.1948.
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Shi B, Xia Z, Xiao S, et al. Severe pneumonia due to SARS-CoV-2 and respiratory syncytial virus infection: a case report. Clin Pediatr (Phila). 2020;59(8):823–6.
Aleem A, Akbar Samad AB, Slenker AK. Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 [cited August 7 2021]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK570580/
Galloway SE, Paul P, MacCannell DR, et al. (2021) Emergence of SARS-CoV-2 B.1.1.7 lineage—United States, December 29, 2020–January 12. MMWR Morb Mortal Wkly Rep. 2021;70(3):95–9.
Shiehzadegan S, Alaghemand N, Fox M, Venketaraman V. Analysis of the Delta variant B.1.617.2 COVID-19. Clin Pract. 2021;11(4):778–84.
Sanches PRS, Charlie-Silva I, Braz HLB, Bittar C, Freitas Calmon M, Rahal P, et al. Recent advances in SARS-CoV-2 Spike protein and RBD mutations comparison between new variants Alpha (B.1.1.7, United Kingdom), Beta (B.1.351, South Africa), Gamma (P.1, Brazil) and Delta (B.1.617.2, India). J Virus Erad. 2021;7(3):100054.
He X, He C, Hong W, Zhang K, Wei X. The challenges of COVID‐19 Delta variant: Prevention and vaccine development. MedComm. 2021;2(4):846–54.
Hesman Saey T, Garcia de Jesus E. Why the coronavirus’s delta variant dominated 2021. https://www.sciencenews.org/article/covid-delta-variant-mutations-coronavirus-life-cycle-2021.
He X, Hong W, Pan X, Lu G, Wei X. SARS-CoV-2 Omicron variant: characteristics and prevention. MedComm. 2021;2(4):838–45.
GISAID. Covariants. 2021. https://covariants.org/variants/21K.Omicron. Accessed 7 Dec 2021.
Sun Y, Lin W, Dong W, Xu J. Origin and evolutionary analysis of the SARS-CoV-2 Omicron variant. J Biosaf Biosecurity. 2022;4(1):33–7.
COVID-19 Vaccine recommendations for children and teens [Internet]. [cited 19 July 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccines-children-teens.html
Ladhani SN. COVID-19 vaccination for children aged 5–11 years. Lancet. 2022;400(10346):74–6.
Pfizer-BioNTech COVID-19 vaccine (also known as COMIRNATY): overview and safety-Pfizer [Internet]. [cited 18 July 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/Pfizer-BioNTech.html
The Pfizer BioNTech (BNT162b2) COVID-19 vaccine: What you need to know [Internet]. [cited 20 July 2022]. Available from: https://www.who.int/news-room/feature-stories/detail/who-can-take-the-pfizer-biontech-covid-19--vaccine-what-you-need-to-know
Stay up to date with your COVID-19 vaccines-Moderna [Internet]. [cited 18 July 2022]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html#moderna-12-17
Covid-19 : le vaccin Spikevax® de Moderna peut être utilisé à partir de l’âge de 12 ans [Internet]. [cited 20 July 2022]. Available from: https://www.has-sante.fr/jcms/p_3280559/fr/covid-19-le-vaccin-spikevax-de-moderna-peut-etre-utilise-a-partir-de-l-age-de-12-ans#voirAussi
COVID vaccines: Which countries are vaccinating children over 5 and how do they compare? [Internet]. [cited 18 July 2022]. Available from: https://www.euronews.com/next/2022/02/25/covid-vaccine-for-children-who-in-europe-is-leading-the-race
Woodworth KR, Moulia D, Collins JP, Hadler SC, Jones JM, Reddy SC, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5–11 years—United States, November 2021. Morb Mortal Wkly Rep. 2021;70(45):1579–83.
Dorabawila V, Hoefer D, Bauer UE, Bassett MT, Lutterloh E, Rosenberg ES. Effectiveness of the BNT162b2 vaccine among children 5–11 and 12–17 years in New York after the emergence of the Omicron variant. Epidemiology. 2022. https://doi.org/10.1101/2022.02.25.22271454.
Sacco C, Del Manso M, Mateo-Urdiales A, Rota MC, Petrone D, Riccardo F, et al. Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5–11 years in Italy: a retrospective analysis of January–April, 2022. Lancet Lond Engl. 2022;400(10346):97–103.
Zimmermann P, Pittet LF, Finn A, Pollard AJ, Curtis N. Should children be vaccinated against COVID-19? Arch Dis Child. 2022;107(3):e1.3-e8.
Tunis [Internet]. [cité July 18 2022]. Available from: https://www.allodocteurs.africa/la-tunisie-autorise-le-vaccin-anti-covid-de-pfizer-pour-les-enfants-de-5-a-11-ans-6960.html
Thompson LA, Rasmussen SA. Children and COVID-19 Vaccines. JAMA Pediatr. 2021;175(8):876.
Zou X, Cao B. COVID-19 vaccines for children younger than 12 years: are we ready? Lancet Infect Dis [Internet]. June 28 2021 [cited August 24 2021]; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8238447/
https://www.ouest-france.fr/sante/virus/coronavirus/covid-19-etats-unis-allemagne-israel-ces-pays-ou-la-vaccination-des-ados-a-deja-commence-7302089 (consulted on 21/09/2021).
COVID-19 vaccine for children [Internet]. [cited July 20, 2022]. Available from: https://u.ae/en/information-and-services/justice-safety-and-the-law/handling-the-covid-19-outbreak/vaccines-against-covid-19-in-the-uae/covid19-vaccine-for-children.
Mallapaty S, Callaway E, Kozlov M, Ledford H, Pickrell J, Van Noorden R. How COVID vaccines shaped 2021 in eight powerful charts. Nature. 2021;600(7890):580–3.
Latest on the region’s Covid-19 recovery [Internet]. MEED. 2022 [cited 20 July 2022]. Available from: https://www.meed.com/latest-news-on-the-pandemics-economic-impact
FDA authorizes COVID vaccines for the littlest kids: what the data say [Internet]. [cited 20 July 2022]. Available from: https://www.nature.com/articles/d41586-022-01689-w
Khatatbeh M, Albalas S, Khatatbeh H, Momani W, Melhem O, Al Omari O, et al. Children’s rates of COVID-19 vaccination as reported by parents, vaccine hesitancy, and determinants of COVID-19 vaccine uptake among children: a multi-country study from the Eastern Mediterranean Region. BMC Public Health. 2022;22(1):1375. https://doi.org/10.1186/s12889-022-13798-2.
Sam-Agudu NA, Quakyi NK, Masekela R, Zumla A, Nachega JB. Children and adolescents in African countries should also be vaccinated for COVID-19. BMJ Glob Health. 2022;7(2):e008315.
Gerber JS, Offit PA. COVID-19 vaccines for children. Science. 2021;374(6570):913–913.
Hause AM, Shay DK, Klein NP, Abara WE, Baggs J, Cortese MM, Fireman B, Gee J, Glanz JM, Goddard K, Hanson KE. Safety of COVID-19 vaccination in United States children ages 5 to 11 years. Pediatrics. 2022;150(2):11.
Kamidani S, Rostad CA, Anderson EJ. COVID-19 vaccine development: a pediatric perspective. Curr Opin Pediatr. 2021;33(1):144–51.
Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022. https://doi.org/10.1056/NEJMoa2116298.
Acknowledgements
This study was conducted in the Laboratory of Virus, Host and Vectors, Institut Pasteur de Tunis, University of Tunis El Manar, Tunis, Tunisia (LR 20 IPT 02), funded by the Tunisian Ministry of High education and research.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
SH-B conceptualized the systematic review. HK and KA conducted the search, data extraction and first draft writing under the supervision of SH-B. All authors contributed to finalizing the draft paper. HT and SH-B contributed to the revision and approval for submission. All authors have read and agreed to the submitted version of the manuscript.
Authors’ information
Haifa Khemiri: Ph.D. student in Virology from Faculty of Sciences of Tunis (FST), University of Tunis El Manar, Tunisia. She is a member of the Laboratory of Virus, Host and Vectors, Institut Pasteur de Tunis, Tunisia (LR 20 IPT 02). Her research interests include omics data analysis of Human viruses, especially SARS-CoV2 and enteroviruses.
Kaouther Ayouni: Ph.D. in Virology from Faculty of Sciences of Tunis (FST), University of Tunis El Manar, Tunisia. Currently, she has a post-Doctoral position at the Laboratory of Virus, Host and Vectors, Institut Pasteur de Tunis, University of Tunis El Manar, Tunis, Tunisia (LR 20 IPT 02). Her research interests include molecular characterization and omics data analysis of Human viruses, especially hepatitis viruses and SARS-CoV2.
Henda Triki: MD. She is a Professor in Virology 2006 at the Faculty of Medicine of Tunis (FMT). She is the head of the Laboratory of Clinical Virology, which acts as the WHO Regional Reference Laboratory for Poliomyelitis and Measles in the EMR region. In 2018, she was assigned as Director of the Clinical Investigation Center entitled: "Transmissible diseases: Natural history and innovated tools for diagnostic, prevention and treatment" at Pasteur Institute of Tunis.
Sondes Haddad-Boubaker: PhD in Virology. She obtained the Habilitation to Conduct Research (HDR) in 2020. She is an Assistant Professor at the Laboratory of Clinical Virology, Pasteur Institute of Tunis, which acts as the WHO Regional Reference Laboratory for Poliomyelitis and Measles in the EMR region. Her research interest includes molecular characterization and omics data analysis of Human viruses, especially poliovirus and other enteric viruses and SARS-CoV2.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As the data came from publicly available published articles, this study did not require ethical approval.
Consent for publication
All authors have read and agreed to the submitted version of the manuscript.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
General information about the included studies and their references.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khemiri, H., Ayouni, K., Triki, H. et al. SARS-CoV-2 infection in pediatric population before and during the Delta (B.1.617.2) and Omicron (B.1.1.529) variants era. Virol J 19, 144 (2022). https://doi.org/10.1186/s12985-022-01873-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-022-01873-4