Abstract
Background
Physical activity combined with virtual reality and exergaming has emerged as a new technique to improve engagement and provide clinical benefit for gait and balance disorders in people with Parkinson’s disease (PD).
Objective
To investigate the effects of a training protocol using a home-based exergaming system on brain volume and resting-state functional connectivity (rs-FC) in persons with PD.
Methods
A single blind randomized controlled trial was conducted in people with PD with gait and/or balance disorders. The experimental (active) group performed 18 training sessions at home by playing a custom-designed exergame with full body movements, standing in front of a RGB-D Kinect® motion sensor, while the control group played using the computer keyboard. Both groups received the same training program. Clinical scales, gait recordings, and brain MRI were performed before and after training. We assessed the effects of both training on both the grey matter volumes (GVM) and rs-FC, within and between groups.
Results
Twenty-three patients were enrolled and randomly assigned to either the active (n = 11) or control (n = 12) training groups. Comparing pre- to post-training, the active group showed significant improvements in gait and balance disorders, with decreased rs-FC between the sensorimotor, attentional and basal ganglia networks, but with an increase between the cerebellar and basal ganglia networks. In contrast, the control group showed no significant changes, and rs-FC significantly decreased in the mesolimbic and visuospatial cerebellar and basal ganglia networks. Post-training, the rs-FC was greater in the active relative to the control group between the basal ganglia, motor cortical and cerebellar areas, and bilaterally between the insula and the inferior temporal lobe. Conversely, rs FC was lower in the active relative to the control group between the pedunculopontine nucleus and cerebellar areas, between the temporal inferior lobes and the right thalamus, between the left putamen and dorsolateral prefrontal cortex, and within the default mode network.
Conclusions
Full-body movement training using a customized exergame induced brain rs-FC changes within the sensorimotor, attentional and cerebellar networks in people with PD. Further research is needed to comprehensively understand the neurophysiological effects of such training approaches.
Trial registration ClinicalTrials.gov NCT03560089.
Similar content being viewed by others
Introduction
Gait and balance disorders are common and debilitating symptoms in people with Parkinson’s disease (PD) [1, 2]. These symptoms worsen over time, becoming resistant to conventional medical treatments and deep brain stimulation [3]. Consequently, they contribute to increased mortality, morbidity, and healthcare costs [4,5,6]. Rehabilitation programs have shown promising results in improving walking and postural abilities in people with PD including increased walking speed, step length, and overall quality of life [7,8,9,10,11]. In recent years, the integration of physical activity with virtual reality combined with task-specific and/or externally cued training, utilizing immersive and nonimmersive environments, has emerged as a novel approach for enhancing motivation and engaging patients' motor and cognitive skills [12,13,14,15,16]. When combined with exercise, virtual reality and exergaming have demonstrated efficacy comparable to that of traditional physiotherapy [12, 17, 18]. Additionally, they offer potential supplementary benefits related to motor disability, automaticity, gait, and balance performance [12, 14, 15, 17, 19,20,21,22,23,24]. Moreover, virtual reality and exergaming training provide the advantage of being adaptable for home use, with or without supervision (telerehabilitation) [18, 25,26,27,28,29,30]. They are also well tolerated and accepted [14, 31, 32], provided they are tailored to the patient’s disability [13, 14].
The underlying mechanisms through which physical training improve motor disability in people with PD are not yet fully understood. Animal models of PD suggest a potential neuroprotective effect of intense motor exercise, involving antioxidant activity, a reduction in inflammation, and the promotion of synaptogenesis [33,34,35,36,37]. Neuroimaging studies in people with PD have revealed compensatory mechanisms that involve increased recruitment of attentional and sensorimotor networks to overcome movement difficulties and improve automaticity [38,39,40,41]. Following training, various significant changes in brain anatomy and functional connectivity have been reported, depending on the specific training programs employed. High-intensity aerobic training primarily restores function in the sensorimotor (primary motor cortex [M1], supplementary motor area [SMA])-cortico-thalamic-striatal pathways, along with possible neuroplastic changes in the nigrostriatal dopaminergic pathway [20, 42]. Goal-directed exercise training strengthens the functional connectivity of premotor areas and increases gray matter volumes in the cerebellum, premotor cortex, parietal cortex, and temporal gyrus [39, 43, 44]. Finally, cognitive-challenging exercise mainly modifies the functioning of the fronto-parietal and SMA-pedunculopontine (PPN) networks and enhances activation of the motor and premotor areas [45,46,47]. Limited neuroimaging studies suggest that integrating virtual reality into physical activity training triggers further brain modification, such as reduced activation in the right anterior prefrontal and inferior frontal gyri and increased activation in the supplementary motor area (SMA) and premotor areas [12, 38, 39].
In this study, we aimed to investigate the effects of a customized exergaming videogame designed to target gait and balance disorders in people with PD and performed at home without direct supervision [48]. The game incorporates auditory and visual cueing, along with real-time feedback, to enhance motor skills [48, 49]. To explore the neural mechanisms underlying the impacts of our exergame training, we conducted a randomized controlled study and examined changes in brain volume and resting-state functional connectivity before and after the exergame intervention. We also explored the relationship between parkinsonian disability and brain resting-state functional connectivity (rs-FC) before training to further interpret the changes induced by our exergame training.
Materials and methods
Participants
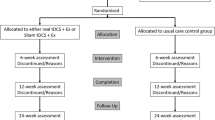
This study was conducted as part of the Parkgame II project, which aimed to evaluate the effectiveness of a home-based customized exergame training system for people with PD with DOPA-resistant gait and/or balance disorders in a controlled randomized trial (Fig. 1) [48]. The Parkgame II project was carried out jointly at two hospitals: the Paris Brain Institute (Paris, France) and the Radboud University Medical Centre (Nijmegen, The Netherlands). For this substudy, between 18 July 2018 and 11 June 2021, we enrolled 23 right-handed people with PD included at the Paris Brain Institute (France) who met the following criteria: (1) aged between 18 and 80 years, (2) idiopathic PD as defined by the UK Parkinson’s Disease Society Brain Bank, and (3) gait and/or balance disorders unresponsive to levodopa treatment (item 12 of the Movement Disorder Society-Unified Parkinson’s Disease Scale (MDS-UPDRS) part 2, gait and balance > 1 and/or item 13, freezing of gait > 1, On-DOPA). Additional inclusion criteria were stable use of dopaminergic medication for at least 1 month prior to study enrollment, no other medical conditions that could interfere with the research study, agreement to participate; and provision of written informed consent and affiliation to a social security scheme. The exclusion criteria were: inability to stand or walk alone; dementia (Mini Mental State Examination score < 24); the presence of impulse control disorders (item 6 of the MDS-UPDRS part 1 score > 2) [50]; and contraindications to MRI. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and approved by the local ethics committee (CPP Est-III: N° 18.04.04). The study was supported by the INSERM (RBM, C17-07, 2017-A02531-52) and registered in a public trial registry (Trial Registration: ClinicalTrials.gov NCT03560089).
Parkinsonian disability, gait and balance assessments
All participants underwent general physical, cognitive and parkinsonian disability assessments at baseline and after the last training session. All assessments were performed on medication approximately two hours after the patients received their usual dopaminergic treatment in the morning. These assessments were conducted by an independent investigator unaware of the patients’ group randomization.
The clinical assessments included (1) the Stand-Walk-Sit test (SWST) duration, (2) the MDS-UPDRS part 3, including the ‘axial’ (sum of the items 3.9 ‘arising from chair, 3.10 ‘gait’, 3.11 ‘freezing of gait’, 3.12 ‘postural stability’ and 3.13 ‘posture’), the ‘upper limb’ (sum of the items 3.3 ‘rigidity’ for upper limbs, 3.4 ‘finger tapping’, 3.5 ‘hand movements’, 3.6 ‘pronation/supination’, 3.15–17 ‘tremor’ for upper limbs), and the ‘lower limb’ (sum of the items 3.3 ‘rigidity’ for lower limbs, 3.7 ‘toe tapping’, 3.8 ‘leg agility’, 3.17 ‘tremor’ for lower limbs) subscores [50]; the Gait and Balance Scale part B (GABS-B) [51]; the Freezing-of-Gait Questionnaire (FOG-Q) [52]; and the Montreal Cognitive Assessment (MoCA) [53].
Additional secondary outcomes included changes in gait kinetics parameters between baseline and post-training using a force plate (0.9°–1.8 m, AMT Inc., LG6–4–1) and a motion capture system (Vicon Nexus, Oxford Metrics, UK). These phases include (1) the anticipatory postural adjustments (APAs) phase, with duration, anteroposterior and mediolateral center of foot pressure displacements during the APA phase, and first step length (eFigure 1), and (2) the mean cadence, stride length, velocity and double stance duration during straight-line forward walking [49].
Training protocols
After baseline assessment, patients were randomly assigned to either the active training program with full-body movements and a Kinect (n = 12, Figs. 1 and 2) or to the control training program with a laptop (n = 11). Both groups performed 18 training sessions over a 6–9 week period (eMethods, eFigure 2) [48]. The Active training group played with full-body movements performed standing in front of a RGB-D Kinect® motion sensor (Version 2, Microsoft, USA), placed below a television screen. The movements consisted of large amplitude and rapid movements of all four limbs, pelvis, and trunk, with lateral, vertical, and forward displacements of the legs, to reinforce foot lifting and postural control. The Control group played seated with a keyboard without the need for any physical effort. The movements controlled the displacements of an avatar in real time, to gain points by collecting coins and avoiding obstacles. Visual (schematic representation of movements) and auditory (rhythmic music) cues were used to encourage movements. Patients received real-time and online feedback while playing, in the form of an auditory or visual stimulus. In addition, their performance was graded at the end of each session.
‘Toap Run’ exergaming. A ‘Toap run’ exergame. Active training involves full-body movements, performed in front of an RGB-D Kinect® motion sensor positioned approximately 2 m away from the patient. These movements encompass large-amplitude rapid movements of all four limbs, pelvis, and trunk, incorporating lateral, vertical, and forward displacements, which are schematically represented for each environment. As the patient moves, a small animal (the avatar) is simultaneously displaced in real time within three different environments: the garden (left), the mine (middle) and the river (right). The objective is for the avatar to collect coins while avoiding obstacles. In contrast, the control training patients underwent seated training, interacting by pushing buttons on the keyboard
MR imaging (MRI)
We acquired anatomical and functional MR images at rest. The MR protocol was carried out using a Siemens Prisma Fit 3Tesla MRI (64-channel head coil, 80 mT/m gradient maximum amplitude) with a magnetization-prepared rapid gradient-echo (MP-RAGE) T1-weighted sequence (TR = 2400 ms, TE = 2.22 ms, FA = 9, 0.8 iso, 256 slices), followed by blood oxygen level-dependent (BOLD) multiecho 3-band T2* for resting-state (rs) fMRI (TR = 1600 ms, TE = [15.20; 37.17; 59.14 ms], FA = 73, 2.5 iso, 54 slices, 300 volumes), with eyes closed.
MRI data preprocessing
Pretraining MRI datasets were acquired for 23 patients, and post-training MRI datasets were acquired for 20 patients. Post-training MRI datasets were not obtained due to the COVID-19 crisis in two patients and due to premature study departure in one patient (medical decision). MRI data from three patients with pretraining MRI scans were excluded from further analysis due to excessive head motion (n = 2) or brain lesions resulting from previous deep brain stimulation (n = 1). Subsequently, we successfully obtained and analyzed pre- and post-training MRI datasets from 16 patients for structural MRI and 17 patients for rs-fMRI. These patients were randomly assigned to the active group (n = 8 for structural MRI and n = 9 for rs-fMRI) or the control group (n = 8) (Fig. 1).
Voxel-based morphometry (VBM)
The Computational Anatomy Toolbox (CAT12) was used for brain tissue segmentation, and the DARTEL algorithm was used to warp the images to the standard Montreal Neurological Institute (MNI) space [54,55,56,57]. Subsequently, brain tissue masks for white matter (WM) and cerebrospinal fluid (CSF) were resliced to a voxel size of 2.5 × 2.5 × 2.5 using FSL [58] to match the voxel size of the BOLD images for further functional image processing.
fMRI
Preprocessing of the multiecho BOLD images was conducted using the AFNI pipeline. The images underwent despiking, slice-timing correction and realignment. The optimized combination of these preprocessed images was obtained using the Tedana algorithm (https://tedana.readthedocs.io/) [59,60,61]. Brain masks were extracted using FSL, and the images were coregistered to T1 images using Statistical Parametric Mapping (SPM12, www.fil.ion.ucl.ac.uk/spm/) [57, 62]. The recombined images were then warped to standard MNI space using DARTEL and smoothed using a 6 mm full-width at half-maximum (FWHM) Gaussian filter kernel. Motion regressors were combined with other noise regressions using the TAPAS physIO pipeline (https://www.translationalneuromodeling.org/tapas) [63], which utilizes individual CSF and WM normalized masks. Aberrant volumes with a Framewise Displacement (FD) greater than 1 were identified and excluded, considering that more than 25% of all volumes had FD > 1 [64]. These regressors were used in a general linear model (GLM) using SPM12 to denoise the smoothed normalized images.
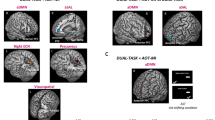
Regions of interest (ROIs) were defined in the MNI space using the Wake Forest University (WFU) Pick Atlas for the human brain (V3.0, ANSIR Laboratory). The selection of ROIs was guided by previous studies investigating connectivity in people with PD with or without gait and balance disorders or FOG and the association between specific brain regions and gait in both healthy subjects and people with PD (Table 1). The following functional regions were identified as the main ensembles of interest, with the basal ganglia serving as a hub and ROIs associated with cueing strategies, locomotion and action observation therapies (AOTs). At the cortical level, the selected ROIs included the primary motor cortex (M1), anterior and posterior SMA, dorsolateral and ventrolateral prefrontal cortex (DLPFC, VLPFC), anterior insula (AI), anterior and posterior cingulate cortex (ACC-BA 32, and PCC), superior parietal cortex, inferior temporal cortex, and precuneus. At the subcortical level, the ROIs encompassed the cerebellar locomotor region (CLR), motor areas (lobes VI, VII and VIII), associative areas (Crus I and Crus II) of the cerebellum, basal ganglia (caudate nucleus, subthalamic nucleus, putamen), thalamus, and the mesencephalic locomotor region (MLR), including the PPN (Table 1, Fig. 3A–F) [65,66,67,68,69,70,71,72,73].
Brain resting-state functional connectivity networks, correlation with the severity of gait and balance disorders and effects of exergaming and control training. Upper Left panel: The images display the networks examined for rs-FC in people with PD before and after training. The networks included A sensorimotor, B basal ganglia, C default mode, D central executive, E dorsal attentional and F ventral attentional networks. In the images, specific regions of interest are labeled: ACC anterior cingulate cortex, CLR cerebellar locomotor region, DLPFC dorsolateral prefrontal cortex, SMA supplementary motor area, M1 primary motor cortex, PCC posterior cingulate cortex, PPN pedunculopontine nucleus, VLPFC ventrolateral prefrontal cortex. Upper Right panel: G The graph illustrates the positive (shown in red) and negative (shown in blue) correlations between the rs-FC and the first component, which reflects the severity of gait and balance disorders. Bottom panels: The graphs represent the significant changes in rs-FC after H active training and I control training. The blue lines represent decreases in rs-FC post-training, while the red lines represent increases in rs-FC. J The graph represents the differences post-training between patient groups. The blue lines represent higher rs-FC in the active group than in the control group, while the red lines represent higher rs-FC in the control group than in the active group
MRI data analysis
To investigate the changes in gray matter (GM) after training, VBM and an anatomical template were generated from WM, grey matter (GM), and CSF individual segmentations using DARTEL [55]. Individual modulated and warped GM masks were then extracted using the GM template. Total intracranial volume (TIV) estimation was conducted using CAT12. Finally, the masks were smoothed with an 8 mm full-width at half-maximum (FWHM) Gaussian filter in SPM 12, ensuring compatibility with subsequent statistical analysis. The pretraining structural images were subtracted from the post-training image using FSL, resulting in individual delta structural images suitable for regression analysis.
To analyze the rs-fMRI data, we measured the amplitude of low-frequency fluctuations (ALFF) for each patient per session. The ALFF was computed using four binary Fourier pairs with a 90° phase lag at frequencies of 0.01, 0.02, 0.04, and 0.08 Hz (100, 50, 25, and 12.5 s) [74]. A classical general linear model (GLM) was used to calculate the main effect of the ALFF, which was subsequently used to extract time courses of the volumes of interest (VOIs). Subsequently, these VOI time courses were employed as regressors in VOI-specific resting-state fMRI GLM, and the positive effect of the VOI contrast was calculated for further statistical analysis.
Statistical analysis
To investigate the relationships between gait and balance disorders and between GM volumes and resting-state connectivity profiles, we conducted a comprehensive analysis. Initially, a principal component analysis (PCA, RStudio) was performed on the clinical and gait parameters obtained before training. This step helped to address any potential co-variability between these parameters. Subsequently, a multiple regression analysis was carried out to establish links between the significant components and GM volumes, with accounting for age and gender as covariates. We employed statistical parametric mapping (SPM) with a probabilistic threshold-free cluster enhancement (pTFCE) correction, setting the p value threshold at p < 0.001 and the minimum cluster size at k = 20.
To assess resting-state connectivity, we filtered the connections based on their correlation with clinical parameters per PCA component using a threshold of rho > 0.5. To identify the most relevant variables and their relationships, we utilized sparse partial least squares (sPLS) regression in the MixOmics toolbox (http://mixomics.org). Two components were used, and the resulting regression models were further filtered based on correlation scores between the model axes and the retained descriptors in each dataset. To ensure strong correlations with sufficient patient variability, we set the filter at 0.6 standard deviation of the rho scores. To evaluate the robustness of each model, we performed bootstrapping, and we assessed the specificity of the models through permutation with 1000 repetitions.
To evaluate the effects of training on GM volume, we employed pTFCE correction with a threshold of p < 0.001 and a minimum cluster size of k = 30 for the VBM data. For the connectivity matrices, tests were conducted for each pair of ROIs with a threshold of p < 0.01. To evaluate changes within each group, Wilcoxon signed-rank tests were utilized for clinical and gait parameters, and paired t tests were performed individually. Additionally, we applied the forced-convergence optimization (ECO) algorithm to identify the most significant changes in gait and balance disorders after training [75]. The algorithm helped filter the patients with the lowest and highest behavioral changes, enabling us to pinpoint the strongest individual brain connectivity network shifts associated with the most significant outcomes.
Results
Demographics, clinical characteristics, severity of gait and balance disorders, and brain imagery in people with PD
Table 2 presents the demographic and clinical characteristics of the patients enrolled in the MRI study. At baseline, both training groups exhibited similar ages, disease durations and severities of gait and balance disorders (Table 2). Patients included in the control group had higher scores on the UPDRS part 3, mainly due to more severe parkinsonian motor signs of the upper limbs (Table 2).
PCA revealed that the first two components accounted for 54% of the variance in baseline clinical and gait parameters (Fig. 4A). The first component primarily captured the severity of gait and balance disorders, encompassing scores from the axial, GABS and FOG-Q assessments, as well as APA CoP displacement and step length, with higher values of this component indicating greater severity of gait and balance disorders. The second component included the MDS-UPDRS 3 and cognition subscale (MoCA score), with higher values indicating a lower severity of motor disability.
Principal components analysis. A The graph reports the results of the principal component analysis including clinical and gait recording data. This analysis revealed two principal components: the first component primarily comprises clinical and gait recording data that reflect the severity of gait and balance disorders, and the second component primarily consists of clinical data that indicate the severity of nonaxial parkinsonian symptoms, including cognition. B The graph reports the rs-FC accounting for interindividual behavioral variability along the two main components, which include clinical and gait recording data. APA. AP anteroposterior anticipatory postural adjustment, GABS Gait and Balance Scale, FOG Freezing of Gait Questionnaire, MOCA Montreal Cognitive Assessment, UPDRS III Unified Parkinson Disease Rating Scale part 3, DS Double stance, HADS Hospital Anxiety and Depression Scale
GM volume analysis revealed that the severity of gait and balance disorders (PC1) at baseline was positively correlated with GM volume in the superior frontal cortex and SMA (R = 0.48, p < 0.05; eFigure 3). For resting state functional connectivity (rs-FC), using the sPLS regression model with the two principal components, we achieved an adjusted R2 = 0.8 for each component (p < 0.001), explaining 69.4% of the remaining variance, and we identified 21 connections that accounted for interindividual behavioral variability along the two main component axes (Figs. 4B and 3G). The severity of gait and balance disorders (PC1) correlated negatively with FC connectivity, meaning that more severe gait and balance disorders were associated with lower FC between these brain areas (Fig. 3G, negative correlation). This included rs-FC between (1) the left temporal inferior cortex and the right primary motor cortex, as well as with the left anterior SMA; (2) the occipital lobe (BA 17–19) and the right cerebellar lobe VIII and CLR; (3) the left CLR and the left posterior cingulum and precuneus bilaterally; and (4) between the right PPN and the left cerebellar lobe VII. In contrast, we observed significant positive correlations between the severity of gait and balance disorders and rs-FC between (1) the right subthalamic nucleus (STN) and the bilateral cerebellar lobes VIII; (2) the left pallidum and the left cerebellar lobe VII and the left anterior SMA; and (3) the ventrolateral prefrontal cortex (BA47) and the right cerebellum (lobe VII and Crus II) (Fig. 3G, positive correlation).
Within-group analysis
Effects of training in the active group
In the active group, significant improvements in gait and balance disorder severity were observed after training compared to before training (Table 2). We found no statistically significant changes in the VBM data (eTable 1, eFigure 4). Conversely, we observed statistically significant changes in the brain rs-FC (Fig. 3H), with significant decreases in the rs-FC between (1) the motor-premotor areas (right M1- left SMA post) and the left temporal inferior cortex; (2) the left DLPFC and the bilateral anterior insula, right frontal inferior and insula; (3) the left parietal superior lobule (PSL) and the right anterior SMA, the posterior cingulate cortex and the right precuneus; (4) the left Crus II and the left cerebellar lobe VI; and (5) the left caudate and the PCC (Fig. 3H). Conversely, significant increases in rs-FC were mainly observed within BGN-CLR areas. This included the rs-FC between the thalamus and the cerebellar areas (right crus I and left lobe VIII), between motor cerebellar regions (right CLR with right lobe VI), and between the CLR and the two pallidum regions (Fig. 3H).
Effects of training in the control group
In the control group, no significant changes in the severity of gait or balance disorders were observed after training (Table 2). Similarly, VBM analysis did not reveal any statistically significant changes in this group (eTable 1, eFigure 4). Conversely, we found statistically significant changes in the rs-FC analysis after Control training, with decreased rs-FC between the right putamen and Crus I and between the left STN and Crus IIB (Fig. 3I), as well as between the right pallidum and insula. Conversely, after control training, there was a significant increase in rs-FC between the right and left putamen, between the right inferior temporal cortex and right cerebellar lobe VI/Crus I areas, and between the right inferior temporal cortex and left motor cerebellar areas (CLR and lobe VIII) (Fig. 3I).
Between-group analysis
We found no significant differences in the VBM between the patient groups post-training. For post-training rs-FC changes, we found lower connectivity in the control group than in the active group between the basal ganglia and motor cortical (M1) and cerebellar areas (CLR, Crus I and Crus II) and between the insula bilaterally and the temporal inferior lobe (Fig. 3J). Conversely, we found greater rs-FC between the PPN and cerebellar areas (lobes VI and VIII), between the temporal inferior lobes and the right thalamus, between the left putamen and DLPFC, and within the DMN (Fig. 3J).
Discussion
In this study, we aimed to investigate the effects of two different training methods on brain rs-FC and motor performance in individuals with PD. The Active group underwent exergaming using full-body movements, while the Control group utilized a computer keyboard.
Before training, we observed correlations between the severity of gait and balance disorders and increased rs-FC between the sensorimotor (SMN, including the SMA and parietal lobe), central executive (CEN, including the precuneus) and cerebellum (CBL, including the CLR) networks; between the MLR-PPN and the CBL (lobe VII), and decreased rs-FC between the cerebellum and both the occipital lobe, precuneus and MLR-PPN areas. These correlations are consistent with previous studies [67, 76,77,78,79,80,81,82,83,84], and suggested that they reflect compensatory mechanisms involving increased cortical and cerebellar involvement. Such mechanisms may overcome the loss of automaticity in gait initiation and postural adaptation while walking in more cognitive/complex situations, and a less efficient utilization of (visual) cueing strategies with reduced activity within the BGN-cerebellar networks.
Following active training, we observed specific changes in rs-FC, along with clinical improvements in gait and balance disorders. Decreased rs-FC was observed between the SMN (M1-SMA), CEN (left inferior temporal cortex, prefrontal cortex), dorsal anterior gyrus (DAN, including the left superior parietal cortex), ventral anterior gyrus (VAN, including the anterior insula), and BGN (left caudate) networks. Conversely, increased rs-FC was observed between the cerebellar (lobe VI and VIII, Crus I, LCR) and basal ganglia (thalamus, pallidum) networks. These changes were not observed in patients who played using the keyboard, and there were no significant changes in the severity of gait or balance disorders post training in these patients. These changes in rs-FC observed in patients in the Active group could thus reflect a restoration, at least in part, of automatic motor control within the SMN and BGN [39], along with reduced involvement of attentional-executive demands in gait control within the DAN and VAN networks, leading to an improvement in gait. These findings are consistent with reduced rs-FC within the posterior mid-cingulate cortex and left precuneus with the BGN, the bilateral thalamus within the CEN, and the bilateral cuneus and left lingual gyrus within the fronto-striatal network (FSN) reported after treadmill training [38, 46]. The integration of virtual reality, including action observation and feedback, as proposed in this study, could also promote better outcomes with greater engagement of the patient and different changes in brain activity. Action observation has been associated with increased activation in motor and premotor areas [45], and treadmill training combined with VR has been associated with increased rs-FC in cortical areas such as the SMA, right precentral gyrus, and middle frontal gyrus [38]. In our study, we did not observe additional rs-FC increases in these cortical areas but rather within the CBL and BGN networks. Notably, increased cerebellar GM volume has been reported after balance training in people with PD [44], and greater activation in the MLR and cerebellar motor region has been found after adapted resistance training with instability in patients with FOG, consistent with clinical improvements [85]. These findings suggest that our active training approach, which also targets balance control and includes learning motor skills, could improve the functional connectivity between the cerebellum and basal ganglia [86, 87].
Fewer changes in rs-FC were found in patients of the Control group who played with the keyboard than in patients who played with body movements (Active group). Resting-state FC within the BGN-anterior insula (VAN) network and striato-cerebellar network was modified, with decreased rs-FC between the visual cerebellar areas and the BGN and increased rs-FC with the right inferior temporal cortex, without clinical improvement in gait and balance disorders. In both groups, rs-FC also increased within the visuomotor cerebellar network (CLR and lobe VI). These changes are consistent with findings in healthy individuals playing video games, which have been associated with decreased activation in visuospatial and attentional networks, potentially reflecting automatization of videogame playing [88, 89]. Increased mesolimbic network activity, particularly during winning trials, has also been reported in young healthy individuals [90, 91], potentially linked to dopamine release in the limbic striatum [92, 93], which was recently also reported in people with PD during videogame playing [94].
Limitations
The present study has several limitations. These limitations include the relatively small sample size for each group, which may limit the generalizability of the findings. Additionally, the small number of patients hindered our ability to examine the relationships between clinical effects and changes in brain activity following training. Another limitation is the baseline group difference in non-axial motor signs, with more severe symptoms in the control group. However, we addressed this disparity by incorporating all variables into our statistical model using multifactor analysis and extracting principal component factors. Furthermore, both groups received gaming programs, preventing us from studying the effects of active exergaming training on brain activity relative to no training or training without gaming.
Conclusions
Our study showed that exergaming interventions can lead to distinct changes in rs-FC and gait performance in individuals with PD. These findings contribute to our understanding of the neural mechanisms underlying the benefits of exergaming interventions in people with PD. Utilizing such a gaming approach, that incorporates virtual reality and action observation, may also increase patient engagement and motivation. Further investigation is warranted to confirm these results in a larger population of people with PD and elucidate the underlying mechanisms contributing to individual variations in clinical outcomes and at an earlier stage of the disease to assess the possible neuroprotective effects, as reported after intensive rehabilitation programs.
Availability of data and materials
All relevant data are within the article. Requests for anonymized data should be sent to M.L. Welter at the Paris Brain Institute, 75013 Paris, France.
Abbreviations
- ACC:
-
Anterior cingulate cortex
- AFI:
-
Analysis of functional neuroimages
- AI:
-
Anterior insula
- APAs:
-
Anticipatory postural adjustments
- ALFF:
-
Amplitude of low-frequency fluctuations
- AOT:
-
Action observation therapies
- BGN:
-
Basal ganglia network
- CAT-12:
-
Computational anatomy toolbox
- CBL:
-
Cerebellar network
- CEN:
-
Central executive network
- CLR:
-
Cerebellar locomotor region
- CSF:
-
Cerebrospinal fluid
- DAN:
-
Dorsal anterior network
- DLPFC:
-
Dorsolateral prefrontal cortex
- FD:
-
Framewise displacement
- fMRI:
-
Functional magnetic resonance imaging
- FOG-Q:
-
Freezing-of-Gait questionnaire
- FSN:
-
Fronto-striatal network
- GABS-B:
-
Gait and balance scale part B
- GLM:
-
General linear model
- GM:
-
Gray matter
- M1:
-
Primary motor cortex
- MDS-UPDRS:
-
Movement disorders society unified parkinson’s disease rating scale
- MoCA:
-
Montreal cognitive assessment
- MLR:
-
Mesencephalic locomotor region
- MNI:
-
Montreal neurological institute
- PCA:
-
Principal component analysis
- PCC:
-
Posterior cingulate cortex
- PD:
-
Parkinson’s disease
- PPN:
-
Pedunculopontine nucleus
- r s-FC:
-
Resting-state functional connectivity
- r s-fMRI:
-
Resting-state functional magnetic resonance imaging
- SMA:
-
Supplementary motor area
- STN:
-
Subthalamic nucleus
- VLPFC:
-
Ventrolateral prefrontal cortex
- WM:
-
White matter
References
Mirelman A, Bonato P, Camicioli R, Ellis TD, Giladi N, Hamilton JL, et al. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019;18:697–708.
Nutt JG, Horak FB, Bloem BR. Milestones in gait, balance, and falling. Mov Disord. 2011;26:1166–74.
Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol. 2015;11:98–110.
Bäckström D, Granåsen G, Domellöf ME, Linder J, Jakobson Mo S, Riklund K, et al. Early predictors of mortality in parkinsonism and Parkinson disease: a population-based study. Neurology. 2018;91:e2045–56.
Kempster PA, Williams DR, Selikhova M, Holton J, Revesz T, Lees AJ. Patterns of levodopa response in Parkinson’s disease: a clinico-pathological study. Brain. 2007;130:2123–8.
Lau B, Meier N, Serra G, Czernecki V, Schupbach M, Navarro S, et al. Axial symptoms predict mortality in PD patients with subthalamic stimulation. Neurology. 2019;92:2559–70.
Conradsson D, Löfgren N, Nero H, Hagströmer M, Ståhle A, Lökk J, et al. The effects of highly challenging balance training in elderly with Parkinson’s disease: a randomized controlled trial. Neurorehabil Neural Repair. 2015;29:827–36.
Jung SH, Hasegawa N, Mancini M, King LA, Carlson-Kuhta P, Smulders K, et al. Effects of the agility boot camp with cognitive challenge (ABC-C) exercise program for Parkinson’s disease. NPJ Parkinsons Dis. 2020;6:31.
Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, et al. Cueing training in the home improves gait-related mobility in Parkinson’s disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–40.
Radder DLM, Silva L, de Lima A, Domingos J, Keus SHJ, van Nimwegen M, Bloem BR, et al. Physiotherapy in Parkinson’s disease: a meta-analysis of present treatment modalities. Neurorehabil Neural Repair. 2020;34:871–80.
Tomlinson CL, Patel S, Meek C, Clarke CE, Stowe R, Shah L, et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev. 2012;7:002817.
Canning CG, Allen NE, Nackaerts E, Paul SS, Nieuwboer A, Gilat M. Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nat Rev Neurol. 2020;16:409–25.
Gallou-Guyot M, Nuic D, Mandigout S, Compagnat M, Welter ML, Daviet JC, et al. Effectiveness of home-based rehabilitation using active video games on quality of life, cognitive and motor functions in people with Parkinson’s disease: a systematic review. Disabil Rehabil. 2022;44:8222–33.
Laar A, Silva de Lima AL, Maas BR, Bloem BR, de Vries NM. Successful implementation of technology in the management of Parkinson’s disease: barriers and facilitators. Clin Park Relat Disord. 2023;8:100188.
Mirelman A, Maidan I, Deutsch JE. Virtual reality and motor imagery: promising tools for assessment and therapy in Parkinson’s disease. Mov Disord. 2013;28:1597–608.
Nieuwboer A, De Weerdt W, Dom R, Truyen M, Janssens L, Kamsma Y. The effect of a home physiotherapy program for persons with Parkinson’s disease. J Rehabil Med. 2001;33:266–72.
Dockx K, Bekkers EM, Van den Bergh V, Ginis P, Rochester L, Hausdorff JM, et al. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst Rev. 2016;12:010760.
Garcia-Agundez A, Folkerts A-K, Konrad R, Caserman P, Tregel T, Goosses M, et al. Recent advances in rehabilitation for Parkinson’s disease with exergames: a systematic review. J Neuroeng Rehabil. 2019;16:17.
Da Silva KG, Nuvolini RA, Bacha JMR, De Freitas TB, Doná F, Torriani-Pasin C, et al. Comparison of the effects of an exergame-based program with conventional physiotherapy protocol based on core areas of the European guideline on postural control, functional mobility, and quality of life in patients with Parkinson’s disease: randomized clinical trial. Games Health J. 2023;12:228–41.
Ferrazzoli D, Ortelli P, Zivi I, Cian V, Urso E, Ghilardi MF, et al. Efficacy of intensive multidisciplinary rehabilitation in Parkinson’s disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2018;89:828–35.
Mirelman A, Rochester L, Maidan I, Del Din S, Alcock L, Nieuwhof F, et al. Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): a randomised controlled trial. Lancet. 2016;388:1170–82.
Ribas CG, Alves da Silva L, Corrêa MR, Teive HG, Valderramas S. Effectiveness of exergaming in improving functional balance, fatigue and quality of life in Parkinson’s disease: a pilot randomized controlled trial. Parkinsonism Relat Disord. 2017;38:13–8.
Wang B, Shen M, Wang Y-X, He Z-W, Chi S-Q, Yang Z-H. Effect of virtual reality on balance and gait ability in patients with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil. 2019;33:1130–8.
Yen CY, Lin KH, Hu MH, Wu RM, Lu TW, Lin CH. Effects of virtual reality-augmented balance training on sensory organization and attentional demand for postural control in people with Parkinson disease: a randomized controlled trial. Phys Ther. 2011;91:862–74.
Burdea GC. Virtual rehabilitation–benefits and challenges. Methods Inf Med. 2003;42:519–23.
Chuang C-S, Chen Y-W, Zeng B-Y, Hung C-M, Tu Y-K, Tai Y-C, et al. Effects of modern technology (exergame and virtual reality) assisted rehabilitation vs conventional rehabilitation in patients with Parkinson’s disease: a network meta-analysis of randomised controlled trials. Physiotherapy. 2022;117:35–42.
dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, da Guedes Silva K, Oliveira Tde P, Peterson Zomignani A, et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson’s disease–effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy. 2012;98:217–23.
Ellis T, Latham NK, Deangelis TR, Thomas CA, Saint-Hilaire M, Bickmore TW. Feasibility of a virtual exercise coach to promote walking in community-dwelling persons with Parkinson disease. Am J Phys Med Rehabil. 2013;92:472–85.
Gandolfi M, Geroin C, Dimitrova E, Boldrini P, Waldner A, Bonadiman S, et al. Virtual reality telerehabilitation for postural instability in Parkinson’s disease: a multicenter, single-blind, randomized. Controlled Trial Biomed Res Int. 2017;2017:7962826.
Song J, Paul SS, Caetano MJD, Smith S, Dibble LE, Love R, et al. Home-based step training using videogame technology in people with Parkinson’s disease: a single-blinded randomised controlled trial. Clin Rehabil. 2018;32:299–311.
Marotta N, Calafiore D, Curci C, Lippi L, Ammendolia V, Ferraro F, et al. Integrating virtual reality and exergaming in cognitive rehabilitation of patients with Parkinson disease: a systematic review of randomized controlled trials. Eur J Phys Rehabil Med. 2022;58:818–26.
Prosperini L, Tomassini V, Castelli L, Tacchino A, Brichetto G, Cattaneo D, et al. Exergames for balance dysfunction in neurological disability: a meta-analysis with meta-regression. J Neurol. 2021;268:3223–37.
da Silva PGC, Domingues DD, de Carvalho LA, Allodi S, Correa CL. Neurotrophic factors in Parkinson’s disease are regulated by exercise: evidence-based practice. J Neurol Sci. 2016;363:5–15.
Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch Phys Med Rehabil. 2003;84:1109–17.
Machado S, Teixeira D, Monteiro D, Imperatori C, Murillo-Rodriguez E, da Silva Rocha FP, et al. Clinical applications of exercise in Parkinson’s disease: what we need to know? Expert Rev Neurother. 2022;22:771–80.
Petzinger GM, Walsh JP, Akopian G, Hogg E, Abernathy A, Arevalo P, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–300.
Smith AD, Zigmond MJ. Can the brain be protected through exercise? Lessons from an animal model of parkinsonism. Exp Neurol. 2003;184:31–9.
Droby A, Maidan I, Jacob Y, Giladi N, Hausdorff JM, Mirelman A. Distinct effects of motor training on resting-state functional networks of the brain in Parkinson’s disease. Neurorehabil Neural Repair. 2020;34:795–803.
Maidan I, Rosenberg-Katz K, Jacob Y, Giladi N, Hausdorff JM, Mirelman A. Disparate effects of training on brain activation in Parkinson disease. Neurology. 2017;89:1804–10.
Tessa C, Lucetti C, Diciotti S, Baldacci F, Paoli L, Cecchi P, et al. Decreased and increased cortical activation coexist in de novo Parkinson’s disease. Exp Neurol. 2010;224:299–306.
Wai Y-Y, Wang J-J, Weng Y-H, Lin W-Y, Ma H-K, Ng S-H, et al. Cortical involvement in a gait-related imagery task: comparison between Parkinson’s disease and normal aging. Parkinsonism Relat Disord. 2012;18:537–42.
Ferrazzoli D, Ortelli P, Cucca A, Bakdounes L, Canesi M, Volpe D. Motor-cognitive approach and aerobic training: a synergism for rehabilitative intervention in Parkinson’s disease. Neurodegener Dis Manag. 2020;10:41–55.
Baglio F, Pirastru A, Bergsland N, Cazzoli M, Tavazzi E. Neuroplasticity mediated by motor rehabilitation in Parkinson’s disease: a systematic review on structural and functional MRI markers. Rev Neurosci. 2021. https://doi.org/10.1515/revneuro-2021-0064.
Sehm B, Taubert M, Conde V, Weise D, Classen J, Dukart J, et al. Structural brain plasticity in Parkinson’s disease induced by balance training. Neurobiol Aging. 2014;35:232–9.
Agosta F, Gatti R, Sarasso E, Volonté MA, Canu E, Meani A, et al. Brain plasticity in Parkinson’s disease with freezing of gait induced by action observation training. J Neurol. 2017;264:88–101.
Ding H, Droby A, Anwar AR, Bange M, Hausdorff JM, Nasseroleslami B, et al. Treadmill training in Parkinson’s disease is underpinned by the interregional connectivity in cortical-subcortical network. NPJ Parkinsons Dis. 2022;8:153.
King LA, Mancini M, Smulders K, Harker G, Lapidus JA, Ramsey K, et al. Cognitively challenging agility boot camp program for freezing of gait in Parkinson disease. Neurorehabil Neural Repair. 2020;34:417–27.
Nuic D, van de Weijer S, Cherif S, Skrzatek A, Zeeboer E, Olivier C, et al. Home-based exergaming to treat gait and balance disorders in patients with Parkinson’s disease: a phase II randomized controlled trial. Eur J Neurol. 2024;31:e16055.
Nuic D, Vinti M, Karachi C, Foulon P, Van Hamme A, Welter M-L. The feasibility and positive effects of a customised videogame rehabilitation programme for freezing of gait and falls in Parkinson’s disease patients: a pilot study. J Neuroeng Rehabil. 2018;15:31.
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement disorder society-sponsored revision of the Unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–70.
Thomas M, Jankovic J, Suteerawattananon M, Wankadia S, Caroline KS, Vuong KD, et al. Clinical gait and balance scale (GABS): validation and utilization. J Neurol Sci. 2004;217:89–99.
Giladi N, Tal J, Azulay T, Rascol O, Brooks DJ, Melamed E, et al. Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov Disord. 2009;24:655–61.
Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–25.
Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113.
Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51.
Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. Neuroimage. 2013;65:336–48.
Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Stuttgart; New York: G. Thieme; New York: Thieme Medical Publishers; 1988.
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–90.
DuPre E, Luh W-M, Spreng RN. Multi-echo fMRI replication sample of autobiographical memory, prospection and theory of mind reasoning tasks. Sci Data. 2016;3:160116.
Kundu P, Voon V, Balchandani P, Lombardo MV, Poser BA, Bandettini PA. Multi-echo fMRI: a review of applications in fMRI denoising and analysis of BOLD signals. Neuroimage. 2017;154:59–80.
Kundu P, Inati SJ, Evans JW, Luh W-M, Bandettini PA. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage. 2012;60:1759–70.
Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2:166–72.
Frässle S, Aponte EA, Bollmann S, Brodersen KH, Do CT, Harrison OK, et al. TAPAS: an open-source software package for translational neuromodeling and computational psychiatry. Front Psychiatry. 2021;12:680811.
Seto E, Sela G, McIlroy WE, Black SE, Staines WR, Bronskill MJ, et al. Quantifying head motion associated with motor tasks used in fMRI. Neuroimage. 2001;14:284–97.
Dalla Volta R, Fasano F, Cerasa A, Mangone G, Quattrone A, Buccino G. Walking indoors, walking outdoors: an fMRI study. Front Psychol. 2015;6:1502.
de Lima-Pardini AC, Coelho DB, Nucci MP, Boffino CC, Batista AX, de Azevedo Neto RM, et al. Brain networks associated with anticipatory postural adjustments in Parkinson’s disease patients with freezing of gait. Neuroimage Clin. 2020;28:102461.
Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, et al. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS ONE. 2014;9:e100291.
Jahn K, Deutschlander A, Stephan T, Kalla R, Wiesmann M, Strupp M, et al. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage. 2008;39:786–92.
Lewis SJG, Shine JM. The next step: a common neural mechanism for freezing of gait. Neuroscientist. 2016. https://doi.org/10.1177/1073858414559101.
Marchal V, Sellers J, Pélégrini-Issac M, Galléa C, Bertasi E, Valabrègue R, et al. Deep brain activation patterns involved in virtual gait without and with a doorway: an fMRI study. PLoS ONE. 2019. https://doi.org/10.1371/journal.pone.0223494.
Prodoehl J, Rafferty MR, David FJ, Poon C, Vaillancourt DE, Comella CL, et al. Two-year exercise program improves physical function in Parkinson’s disease: the PRET-PD randomized clinical trial. Neurorehabil Neural Repair. 2015;29:112–22.
Shine JM, Matar E, Ward PB, Bolitho SJ, Gilat M, Pearson M, et al. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease. Brain. 2013;136:1204–15.
Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, et al. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain. 2011;134:59–72.
Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41.
De Vico FF, Latora V, Chavez M. A topological criterion for filtering information in complex brain networks. PLoS Comput Biol. 2017;13:e1005305.
Fasano A, Herman T, Tessitore A, Strafella AP, Bohnen NI. Neuroimaging of freezing of gait. J Parkinsons Dis. 2015;5:241–54.
Tessitore A, Amboni M, Esposito F, Russo A, Picillo M, Marcuccio L, et al. Resting-state brain connectivity in patients with Parkinson’s disease and freezing of gait. Parkinsonism Relat Disord. 2012;18:781–7.
Bardakan M, Fink GR, Zapparoli L, Bottini G, Paulesu E, Weiss PH. Imaging the neural underpinnings of freezing of gait in Parkinson’s disease. NeuroImage Clin. 2022. https://doi.org/10.1016/j.nicl.2022.103123.
Canu E, Agosta F, Sarasso E, Volontè MA, Basaia S, Stojkovic T, et al. Brain structural and functional connectivity in Parkinson’s disease with freezing of gait. Hum Brain Mapp. 2015;36:5064–78.
Lench DH, Embry A, Hydar A, Hanlon CA, Revuelta G. Increased on-state cortico-mesencephalic functional connectivity in Parkinson disease with freezing of gait. Parkinsonism Relat Disord. 2020;72:31–6.
Sigurdsson HP, Yarnall AJ, Galna B, Lord S, Alcock L, Lawson RA, et al. Gait-related metabolic covariance networks at rest in Parkinson’s disease. Mov Disord. 2022;37:1222–34.
Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage. 2007;35:222–33.
Lenka A, Naduthota RM, Jha M, Panda R, Prajapati A, Jhunjhunwala K, et al. Freezing of gait in Parkinson’s disease is associated with altered functional brain connectivity. Parkinsonism Relat Disord. 2016;24:100–6.
Yu Q, Li Q, Fang W, Wang Y, Zhu Y, Wang J, et al. Disorganized resting-state functional connectivity between the dorsal attention network and intrinsic networks in Parkinson’s disease with freezing of gait. Eur J Neurosci. 2021;54:6633–45.
Silva-Batista C, de Lima-Pardini AC, Nucci MP, Coelho DB, Batista A, Piemonte MEP, et al. A randomized, controlled trial of exercise for Parkinsonian individuals with freezing of gait. Mov Disord. 2020;35:1607–17.
Duchesne C, Gheysen F, Bore A, Albouy G, Nadeau A, Robillard ME, et al. Influence of aerobic exercise training on the neural correlates of motor learning in Parkinson’s disease individuals. Neuroimage Clin. 2016;12:559–69.
Lehéricy S, Benali H, Van de Moortele P-F, Pélégrini-Issac M, Waechter T, Ugurbil K, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA. 2005;102:12566–71.
Lu AS, Baranowski T, Thompson D, Buday R. Story immersion of videogames for youth health promotion: a review of literature. Games Health J. 2012;1:199–204.
Voss MW, Prakash RS, Erickson KI, Boot WR, Basak C, Neider MB, et al. Effects of training strategies implemented in a complex videogame on functional connectivity of attentional networks. Neuroimage. 2012;59:138–48.
Cole SW, Yoo DJ, Knutson B. Interactivity and reward-related neural activation during a serious videogame. PLoS ONE. 2012;7:e33909.
Kätsyri J, Hari R, Ravaja N, Nummenmaa L. The opponent matters: elevated FMRI reward responses to winning against a human versus a computer opponent during interactive video game playing. Cereb Cortex. 2013;23:2829–39.
Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, et al. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–8.
Kühn S, Gleich T, Lorenz RC, Lindenberger U, Gallinat J. Playing super mario induces structural brain plasticity: gray matter changes resulting from training with a commercial video game. Mol Psychiatry. 2014;19:265–71.
Toldo JMP, Arjona M, Campos Neto GC, Vitor T, Nogueira SA, Amaro E, et al. Virtual rehabilitation in parkinson disease: a dopamine transporter imaging study. Am J Phys Med Rehabil. 2021;100:359–66.
Acknowledgements
The authors would like to warmly acknowledge the dedication with which our patients participated in this research and are grateful to François-Xavier Lejeune for help with the statistical analysis.
Financial Disclosure in the preceding 12 Months
A Skzratek, D Nuic, S Cherif, C Gallea, B Beranger, and E Bardinet have no conflicts of interest to declare. ML Welter reports scientific grants from Boston Scientific, France Parkinson Association, personal fees from BIAL for scientific consulting, and travel grants from Pfizer.
Funding
This study was supported by the France Parkinson Association, Eurostars programme (Grant EUROSTARS E! 10634), Agence Nationale de la Recherche (Grant ANR LabCom N° ANR-13-LAB1-0003-01/ANR1 18-LCCO-0004-01) and the Fondation d’entreprise IRCEM. The funding source had no role in the analysis or interpretation of the data, the writing of the report or the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
Conceptualization: M-L Welter, C Gallea, E Bardinet; Investigation: M-L Welter, A Skrzatek, S Cherif, D Nuic; Formal Analysis: A Skrzatek, D Nuic, S Cherif, C Gallea, B Beranger, E Bardinet; Writing: original draft: A Skrzatek, E Bardinet, M-L Welter; Review and editing: A Skrzatek, D Nuic, S Cherif, C Gallea, B Beranger, E Bardinet, M-L Welter. Project administration: M-L Welter, E Bardinet; Funding acquisition: M-L Welter.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and approved by the local ethics committee (CPP Est-III: N° 18.04.04). The study was promoted by the INSERM (RBM, C17-07, 2017-A02531-52) and registered in a public trial registry (Trial Registration: ClinicalTrials.gov NCT03560089). All patients had agreed to participate and provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Skrzatek, A., Nuic, D., Cherif, S. et al. Brain modulation after exergaming training in advanced forms of Parkinson’s disease: a randomized controlled study. J NeuroEngineering Rehabil 21, 133 (2024). https://doi.org/10.1186/s12984-024-01430-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12984-024-01430-w