Abstract
Background
Stroke is a significant contributor of worldwide disability and morbidity with substantial economic consequences. Rehabilitation is a vital component of stroke recovery, but inpatient stroke rehabilitation programs can struggle to meet the recommended hours of therapy per day outlined by the Canadian Stroke Best Practices and American Heart Association. Mobile applications (apps) are an emerging technology which may help bridge this deficit, however this area is understudied. The purpose of this study is to review the effect of mobile apps for stroke rehabilitation on stroke impairments and functional outcomes. Specifically, this paper will delve into the impact of varying mobile app types on stroke rehabilitation.
Methods
This systematic review included 29 studies: 11 randomized control trials and 18 quasi-experimental studies. Data extrapolation mapped 5 mobile app types (therapy apps, education apps, rehab videos, reminders, and a combination of rehab videos with reminders) to stroke deficits (motor paresis, aphasia, neglect), adherence to exercise, activities of daily living (ADLs), quality of life, secondary stroke prevention, and depression and anxiety.
Results
There were multiple studies supporting the use of therapy apps for motor paresis or aphasia, rehab videos for exercise adherence, and reminders for exercise adherence. For permutations involving other app types with stroke deficits or functional outcomes (adherence to exercise, ADLs, quality of life, secondary stroke prevention, depression and anxiety), the results were either non-significant or limited by a paucity of studies.
Conclusion
Mobile apps demonstrate potential to assist with stroke recovery and augment face to face rehabilitation, however, development of a mobile app should be carefully planned when targeting specific stroke deficits or functional outcomes. This study found that mobile app types which mimicked principles of effective face-to-face therapy (massed practice, task-specific practice, goal-oriented practice, multisensory stimulation, rhythmic cueing, feedback, social interaction, and constraint-induced therapy) and education (interactivity, feedback, repetition, practice exercises, social learning) had the greatest benefits.
Protocol registration PROPSERO (ID CRD42021186534). Registered 21 February 2021
Similar content being viewed by others
Background
Stroke continues to be a leading cause of worldwide disability and morbidity amongst all cardiovascular diseases [1]. From 1990 to 2019, strokes had a global rise in prevalence reaching 101 million people and causing a loss of 143 million disability-adjusted life years [1] at a cost of billions of dollars per year to North American economies [2, 3]. Stroke rehabilitation includes an organized interdisciplinary team approach to stroke specific therapy, and is a critical component of recovery and successful re-integration into society [4]. Compared with other acute stroke interventions, stroke rehabilitation has been found to be as effective or superior to thrombolysis or aspirin [5, 6]. On a per dollar value, the clinical benefits of stroke rehabilitation have been shown to outweigh its costs significantly [7].
Current Canadian Stroke Best Practices and American Heart Association guidelines state that inpatient stroke rehabilitation should provide task-specific therapy (defined as physiotherapy, occupational therapy, and speech and language therapy), for at least 3 h per day of 5 days per week [8, 9]. Evidence supports that more therapy results in improved outcomes [10]. Unfortunately, many institutions struggle to provide this rehabilitation intensity. A 2018 Canadian study found inpatients in a stroke rehabilitation participated in 8.5 h per week of therapy, much below the guideline recommendations of 15 h per week [11]. Outside of therapy, stroke rehabilitation inpatients spend most of their days sedentary [11,12,13]. There are numerous barriers for meeting current stroke rehabilitation guidelines, including insufficient staff, timing mismatch with other patient activities such as investigations for stroke work-up (CTs, echocardiograms, Holter monitors), and a rise in the number of patients requiring stroke rehabilitation [14, 15].
Mobile applications (apps) for stroke rehabilitation have become an emerging area of interest because of their mobility, multi-functional capabilities such as reminders and videos, and their ability to give patients autonomy over therapy [16,17,18]. A 2018 systematic review defined several mobile apps for stroke rehabilitation with the potential to be clinically effective [19]. For example, there are mobile apps designed as games to improve finger dexterity, programs to increase exercise adherence, home exercise programs for upper limb rehabilitation, and mirror therapy for facial paresis [16,17,18, 20]. Mobile apps can target different aspects of stroke rehabilitation. The purpose of this study is to review the effect of mobile apps for stroke rehabilitation on stroke-related impairments and functional outcomes. Specifically, this paper will delve into the effect of varying mobile app types on stroke rehabilitation outcomes.
Method
Search strategy
A search of all studies prior to May 31, 2020 was completed in the following databases: MEDLINE, EMBASE, Cochrane Library, CINAHL, SCOPUS, COMPENDEX, and IEEE Xplore. Keywords were identified using PUBMED MeSH terms of “mobile applications”, “stroke”, and “rehabilitation”. Search strings were created using Boolean operators “OR” and “AND” to combine the keywords. See Additional file 1: Fig. S1 for the sample search strategy used in MEDLINE. Supplementary searching via pearl growing was completed in the included studies.
Study selection
Studies were included if they were in English and met the following criteria: (1) Randomized control trials (RCTs), quasi-experimental clinical trials, or qualitative studies, (2) study population were adult (18 + years of age) stroke survivors who underwent rehabilitation, and (3) the primary intervention studied was a mobile app (phone, tablet, or PC) on any operating system (e.g., iOS, Android, Windows).
Studies were excluded if they were: (1) reviews, protocols, abstracts, case reports/series, or descriptions of mobile apps, (2) study population were children (< 18 years old) or adults with neurological deficits not secondary to a stroke, or (3) studied mobile apps designed secondarily for another technological tool (e.g., mobile app designed to control robotics devices, functional electrical stimulation, virtual reality headset, telerehabilitation, brain-computer interfaces) or mobile apps part of a larger rehabilitative system requiring additional equipment.
Screening process
Results from the initial search were imported into Covidence, a systematic review software (Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org). After duplicates were removed by Covidence, two investigators (S.G.S. and H.W.) independently screened the titles and abstracts through the inclusion and exclusion criteria. The remaining studies were then read in full and assessed for final inclusion eligibility. At the full-text screen phase, reasons for exclusion were recorded and Cohen’s kappa coefficient for inter-rater reliability was calculated. Cohen's kappa coefficient results of ≤ 0 represented poor agreement, 0.01–0.20 was slight agreement, 0.21–0.40 was fair agreement, 0.41– 0.60 was moderate agreement, 0.61–0.80 was substantial agreement, and 0.81–1.00 is almost perfect agreement [21]. At each step, disagreements were discussed between S.G.S. and H.W. before a final decision was made. Prior to screening, it was decided that disagreements that cannot be resolved between S.G.S and H.W would be brought to the remaining authors for a deciding vote. However, this was ultimately not needed.
Outcomes
Of all the outcomes identified, only those explored in more than 1 study were included in this review. This included three stroke impairments classified as neurological deficits because of a stroke (motor paresis, aphasia, neglect) and five functional outcomes classified as functional improvements that patients make during recovery from a stroke (adherence to exercise, activities of daily living (ADLs), quality of life, secondary stroke prevention, and depression and anxiety).
Quality evaluation
Risk of bias in the RCTs was assessed using the Cochrane Risk of Bias tool [22]. Bias was divided into low, unclear, or high risk of bias.
The methodological quality of each RCT was analyzed using the modified Downs and Black checklist [23, 24]. RCT methods with a score of 25–27 were considered excellent quality, 19–24 were considered good quality, 14–18 were considered fair quality, and 13 or less were considered poor quality. Since subjects could not be blinded to the intervention, the ‘Intervalidity-Bias’ section had 1 point removed.
Data extraction
All eligible studies for analysis had data extracted and added to study summary tables. We classified mobile app types into 5 different categories: therapy app, education app, rehab videos, reminders, or a combination. Therapy apps have users interact with the device to complete activities which mimic therapy exercises, often in the form of a game such as finger baseball where users flick their finger on the screen to hit an incoming baseball. Education apps provide a virtual platform for users to learn about stroke and its management. Rehab videos are videos of therapists demonstrating rehabilitation exercises which users can watch and use as a mobile guide to practice with at their leisure. Reminders include messaging texts to remind users to encourage compliance. See Additional file 1: Table S1 for further detailed description of app types. Combinations of mobile apps types that use multiple interventions were also found in these studies. Outcome summary tables were created from RCT extrapolated data, to summarize results based on each outcome and matched to one of the mobile app types.
Results
Study selection
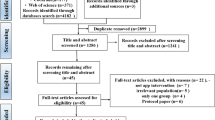
The search identified 1529 possible studies for screening, 99 studies underwent a full text review, and ultimately 11 RCTs [16,17,18, 20, 25,26,27,28,29,30,31] and 18 quasi-experimental studies [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49] met the eligibility criteria for inclusion in this manuscript (Fig. 1). No qualitative study met all the inclusion criteria and thus no qualitative studies were included. A calculated Cohen’s kappa coefficient of 0.75 demonstrated substantial agreement on which studies to include or exclude between reviewers.
Study quality
Eleven RCTs were assessed for risk of bias (Additional file 1: Fig. S2) and methodological quality (Additional file 1: Table S2). Bias assessment showed that random sequence generation was identified in 7 RCTs, method of allocation concealment was explained in 5 RCTs, blinding of outcome assessments was clear in 7 RCTs, and a low risk of attrition bias was demonstrated in 6 RCTs (Additional file 1: Fig. S3). When comparing methods and pre-published study protocol, only 3 studies were found to have a low risk of reporting bias [17, 20, 30]. In every RCT, participants were not blinded to the intervention group which led to a high risk of performance bias. For methodological quality via the Downs and Black checklist, 4 studies were of excellent quality [18, 25, 28, 31], 6 were of good quality [16, 17, 20, 26, 29, 30], and 1 was of poor quality [27]. Of all the RCTs, 1 study had a high or unknown risk of bias across all domains and poor methodological quality [27]. As quasi-experimental studies have higher inherent levels of bias and poorer methodological qualities than RCTs, no further quality analysis was completed.
Study characteristics
The characteristics for all 11 RCTs are presented in Table 1. Amongst all RCTs, the study length varied from 0.5 to 12 months with a mode of 1 month. Patient sample size ranged from 16 to 277 with 9 studies having less than 100 patients. The average age for the interventional group was 58.7 ± 11.9 years old and for the control group was 60.1 ± 12.7 years old (Additional file 1: Fig. S4). Between the two groups, the patient ages ranged from 39 to 73 years old. Time since the stroke was between 14 days to approximately 21 months. Three studies did not report the time since stroke [27,28,29] and 2 studies reported having a control group but did not describe it [16, 27].
The characteristics for all 18 quasi-experimental studies are presented in Table 2. Thirteen quasi-experimental studies used a pre-test post-test design [32,33,34, 36, 37, 40,41,42,43, 45,46,47,48,49], 3 were a nonrandomized clinical trial [35, 38, 39, 45], and 1 was a crossover design study [43].
Stroke impairments
Results extrapolated from 7 RCTs studies and 12 quasi-experimental studies examined a range of stroke impairments including motor paresis, aphasia, and neglect.
Motor paresis
Six RCTs (Table 3) and 5 quasi-experimental studies (Table 2) explored the effect of mobile apps on various motor paresis metrics (gait/ambulation, standing balance, facial movement, upper extremity or lower extremity range of motion, hand dexterity). Amongst these studies, 4 mobile app types were identified: therapy apps, rehab videos, reminders, and rehab videos with reminders. Therapy apps were used in 3 RCTs [16, 17, 20] and 3 quasi-experimental studies [32, 33, 49]. Each therapy app was designed to promote repetitive motion, often in the form of a game [16, 32, 33, 49]. For therapy apps, all RCTs showed a statistical benefit in a motor paresis metric compared to a control group whereas all quasi-experimental studies demonstrated improvement in a motor paresis metric compared to a pre-test baseline or control group. Rehab videos were used in 2 RCTs [18, 30] and 1 quasi-experimental study [43]. A statistical benefit in upper and lower extremity mobility was found in 1 RCT and 1 quasi-experimental study [18, 43] but 1 RCT [30] showed no statistical improvement of orofacial motor paresis compared to a control group using the standard exercise booklet guide. Reminders to exercise were used in 1 quasi-experimental study [38] and found no positive impact on ambulation (10 m walk test). A single RCT [25] used rehab videos with reminders for 1 month and found no impact on upper extremity function via the Wolf Motor Function Test.
Aphasia
Six quasi-experimental studies (Table 2) used therapy apps to study aphasia, and each showed improvement in aphasia recovery. The mobile app designs focused on expressive and receptive communication by creating visual associations with pictures [40, 45, 46] or using voice recognition software to guide tasks [34, 36, 44]. One study also used a spatial awareness game, Bejeweled, to target chronic (> 1 year) expressive aphasia but it had no impact on recovery [44].
Neglect
One RCT (Table 4) and 1 experimental study (Table 2) used a therapy app mimicking a “wack-a-mole” game, where users hit a moving target on the screen, in patients with neglect secondary to a stroke. In the quasi-experimental study [37], the wack-a-mole game also included positive and negative auditory feedback in the form of a ring. The game started with a base ring and each successful “hit” of a mole resulted in a higher pitched sound (positive feedback). When a mole was missed, the pitch restarted back to the base ring (negative feedback). The RCT [27] (with no auditory feedback) had no significant effect on any measure of neglect. The quasi-experimental study found that an added auditory feedback significantly improved reaction time but did not correlate reaction time to neglect outcomes.
Functional outcomes
Results extrapolated from 8 RCTs studies and 10 quasi-experimental studies examined a range of functional outcomes including adherence to exercise, ADLs, quality of life, secondary stroke prevention, and depression and anxiety.
Adherence to exercise
Three RCTs (Table 5) and 4 quasi-experimental studies (Table 2) assessed adherence to exercise using therapy apps, rehab videos, reminders, or a combination of rehab videos with reminders. One RCT [20] and 2 quasi-experimental studies [36, 47] used therapy apps and measured exercise adherence. The RCT [20] showed an improvement in adherence to ambulation and the 2 quasi-experimental studies [36, 47] found that therapy apps did not improve adherence to exercise. Rehab videos were used in 1 RCT [18] and 1 quasi-experimental study [43] to measure exercise adherence. The RCT by Chung et al. found that rehab videos significantly improved exercise adherence after 3 months but not after 1 month. The quasi-experimental study [43] using rehab videos demonstrated a correlation between improved exercise adherence and higher scores on the Montreal Cognitive Assessment test. Reminders used in 1 quasi-experimental study [38] found an improvement in exercise adherence. In 1 RCT [25], a combination of rehab videos with reminders was found to have no impact on exercise adherence.
Activities of daily living (ADLs)
Five RCTs (Table 6) and 5 quasi-experimental studies (Table 2) tracked ADL independence after patients used a therapy app, education app, rehab video, reminders, or rehab videos with reminders. Three RCTs [20, 26, 27] and 1 quasi-experimental study [32] used a therapy app. Of the RCTs, one study [20] showed a statistically significant benefit in ADLs as per the Barthel Index whereas the other 2 did not [26, 27]. The 1 quasi-experimental study [32] found its therapy app improved performing ADLs. An education app providing information on stroke and post-stroke management was used in 1 quasi-experimental study [42] and showed improvement in ADLs. Rehab videos used in 1 RCT [18] showed no benefit in ADLs while 1 quasi-experimental study [43] found rehab videos improved ADL independence. Reminders used in 2 quasi-experimental studies [38, 39] had no effect on ADLs. One RCT [28] used a combination of rehab videos with reminders and found no significant benefit in ADL independence after 1 year.
Quality of life
Three RCTs (Additional file 1: Table S3) and 2 quasi-experimental studies (Table 2) examined the impact on quality of life after using either a therapy app, education app, or reminders. The effect of different therapy apps on quality of life was studied in 2 RCTs [20, 26] and 1 quasi-experimental study [41]. One RCT [20] and the quasi-experimental study [41] found therapy apps improved patient perceived quality of life whereas the other RCT [26] did not. One RCT [29] using an education app with information on stroke risk factors found no significant benefit in quality of life. One quasi-experimental study [38] used reminders through a self and group monitoring app and found it had no impact on quality of life.
Secondary stroke prevention
Three RCTs (Additional file 1: Table S4) and 3 quasi-experimental studies (Table 2) used education apps, reminders, or rehab videos with reminders to measure impact on secondary stroke prevention. One RCT [29] used an education app to teach users about stroke risk factors. The post-study questionnaire results showed a non-significant increase in stroke risk factor knowledge. One RCT [31] and 3 quasi-experimental studies [35, 38, 48] used reminders to improve vascular risk factors. One RCT [31] and 1 quasi-experimental study [38] found that reminders made no significant impact on blood pressure. However, the other 2 quasi-experimental studies [35, 48] showed significant improvement in controlling several vascular risk factors such as blood pressure, glycemic control, lipid levels, and BMI. One RCT used rehab videos (Movies4Stroke) with reminders and had no significant change in control of hypertension, LDL, or HbA1.
Depression and anxiety
Depression and anxiety were studied in 1 RCT (Additional file 1: Table S5) using a therapy app and 1 quasi-experimental study (Table 2) using reminders. The RCT’s therapy app used therapy-like games with corrective help features to train cognition [26]. Neither the RCT [26] using its therapy app nor the quasi-experimental study [38] using reminders had a benefit on depression and anxiety.
Discussion
The purpose of this systematic review was to explore mobile apps for stroke rehabilitation and the stroke impairments and functional outcomes for which they have shown to be effective. Specifically, we delved into the impact of varying mobile app types on stroke rehabilitation. There was wide variation in the effectiveness of apps across several studies. This perhaps is not surprising given the variability in apps and stroke impairments described in the different studies.
Technology, as a method of delivery (for therapy, education, or reminders), should mirror what is found to be effective in non-technological methods of delivery. For example, in education there have been numerous systematic reviews and meta-analysis showing that online provision of education results in similar outcomes to face-to-face teaching [50,51,52]. However, the concepts which do improve outcomes (interactivity, feedback, repetition, practice exercises, social learning) are what makes learning effective in either online and face-to-face delivery of education [53]. Similarly, it is also important to consider beneficial concepts of effective face-to-face stroke rehabilitation therapy (massed practice, task-specific practice, goal-oriented practice, multisensory stimulation, rhythmic cueing, feedback, social interaction, and constraint-induced therapy) when evaluating mobile applications [54,55,56]. However, we need to be mindful of the benefits and challenges of technology enhanced delivery methods to find the right approach for the right outcome. There is also mounting evidence, including recent systematic reviews and meta-analyses, that integrating online and face-to-face delivery methods together (known as blended learning) produces improved outcomes over either alone [53, 54]. Although there is a paucity of evidence to determine if face-to-face therapy combined with mobile apps is better than either intervention alone, this would be an interesting area for future research.
Mobile apps, in combination with face-to-face delivery of stroke rehabilitation, may afford us with benefits such as augmenting therapy (types and time) for stroke deficits, providing stroke education, delivering rehab videos, and sending reminders, but further research is needed into how we can best use them to support stroke recovery.
Through this review, 3 stroke impairments were identified that may benefit from app usage: motor paresis (upper and lower extremity dexterity and coordination, gait training, orofacial paresis), aphasia, and neglect.
After a stroke, more than 70% of people will suffer from motor impairments including upper or lower extremity paresis [57]. Given this high prevalence, it is not surprising that motor paresis was the most studied deficit in this review. Eight of these studies conferred a positive impact on motor paresis by mobile apps during stroke rehabilitation, including 4 RCTs [16,17,18, 20]. Of the 2 RCTs [25, 30] that showed no impact, the study by Moon et al. had the smallest sample size (n = 16) amongst all RCTs and no power analysis, raising concerns that it may be underpowered. As well, it focused on post-stroke dysphagia, which may be harder to facilitate through videos than more obvious limb motions. There were 4 types of mobile apps that were studied (therapy apps, rehab videos, reminders, and a combination of rehab videos with reminders), with therapy apps being the most studied type. The main principle of each therapy app was to guide repetitive movement in the affected extremity, sometimes in the form of games [33, 49]. This aligns with face-to-face research that shows repetition of meaningful movements, such as with ADLs, resulted in better functional outcomes [58, 59]. Apps may also allow serious games and gamification principles to motivate patients to persist with exercise repetitions [60]. Overall, there is strong evidence indicating that therapy apps targeted for various motor paresis metrics are highly effective in stroke rehabilitation. Rehab videos also had potential as 1 RCT [18] and 1 quasi-experimental study [43] showed benefit. As for the use of reminders with or without rehab videos, there were only 2 studies [25, 38] which explored these types, none of which conferred any motor benefits.
Aphasia is also a common consequence of stroke, affecting 35% of patients [61]. In a 2020 systematic review and meta-analysis, there was evidence supporting the use of face-to-face constraint-induced aphasia therapy which focuses on forcing patients to use verbal language with massed practice [62]. In this review, 6 quasi-experimental studies [34, 36, 40, 44,45,46] explored the impact on aphasia using mobile apps built on similar principles to constraint-induced aphasia. In all the studies, aphasia scores were found to improve after each study period. All 6 studies used a therapy type of app. Most commonly, the therapy apps used repetitive language exercises in combination with a stimulus (pictures, written words, voice-guided tasks) to develop expressive and receptive communication skills. Only one study used a task based therapy app and Bejeweled, a spatial awareness and decision making game, to rehabilitate chronic aphasia [44]. In this study, the task-based therapy app improved domains of aphasia whereas the game Bejeweled did not. This is suggestive that therapy apps which focus on repetitive communication through various language exercises may assist in the rehabilitation of post-stroke aphasia.
For neglect, the evidence for stroke rehabilitation apps is mixed, similar to the literature on the impact of face-to-face visual scanning on post-stroke neglect [63, 64]. In this review, a single RCT [27] showed its therapy app had no impact on neglect but the study methodology scored poorly on the Downs and Black checklist and there were multiple bias concerns as per the Cochrane Risk of Bias tool. In 2017, the same authors published a quasi-experimental study exploring the same app but with a feedback system and this led to improved user reaction time, however no measure of neglect was used [37]. The improvement in reaction time may be due to dual channel assumption (using both auditory and visual channels to encode information) which is one aspect of the cognitive theory of multimedia learning and has been proven to be superior to using only one channel [65,66,67]. This may have further implications and should be considered in future research in stroke apps. Lastly, the small screen size of apps may have a negative outcome with respect to neglect or visuoperceptual therapy.
Within this review, 5 functional outcomes were identified after a review of the literature: adherence to exercise, ADLs, quality of life, secondary stroke prevention, and depression and anxiety. For each of these outcomes, there was mixed evidence for effectiveness.
Of these outcomes, adherence to exercise had the most positive impact from mobile apps use, with 2 out of 3 RCTs and 2 out of 4 quasi-experimental studies showing improvement. The 2 RCTs [18, 20] that showed an increase in exercise adherence had a study length of 3 months and an average time since stroke of less than 40 days. In comparison, the RCT [25] that showed mobile apps had no impact on exercise adherence had a short study length (1 month) and longer average time since stroke (120 days). Assuming increased exercise adherence leads to increased therapy time, mobile apps that promote adherence may have the potential to improve functional gain [10] since early rehabilitation results in better recovery up to 6 months since onset [68, 69]. Therefore, apps that promote exercise adherence earlier in stroke recovery and for longer duration may be more beneficial for increasing exercise time and possibly stroke outcomes, but further research is needed.
Exercise adherence showed the most improvement with rehab videos, having 1 RCT [18] and 1 quasi-experimental study [43] in support of this and no studies against. This again may point to the importance of dual channel learning and the impact that videos may have through the ability to revisit information and utilizing educational principles such as spaced repetition and distributed practice [65, 70]. Other than rehab videos, the remaining app types (therapy apps, reminders, reminders with rehab videos) used to study exercise adherence showed limited or mixed results. In two of the studies demonstrating increased adherence [20, 38], the mobile apps allowed for user interactions via a chat messenger or group monitoring, so that fellow stroke users could encourage one another. This built a social support network, which has known benefits on improving therapy adherence [71].
For ADLs, quality of life, secondary prevention control, and depression and anxiety this review found that the effect of mobile apps is inconclusive, regardless of the type. This suggests that further research is required in these specific areas.
A summary of the effects of mobile applications for each stroke impairment and functional outcome along with the relevant principles of effective education and face-to-face stroke rehab therapy are placed into Table 7.
There are several limitations within this review. First is the lack of high-quality studies in the body of literature on mobile apps for stroke rehabilitation as 18 of the eligible 29 studies were quasi-experimental studies, which carry a high risk of bias due to its methodology. For this reason, the most important recommendation for improving study quality is to have randomization to reduce bias in all other aspects of the study. As well, blinding where possible is important as blinding patients is challenging with technology, hence leading to a high risk of patient bias. One potential method to overcome the challenge of blinding patients with technology is to create a ‘control’ mobile application that does not directly relate to the outcome. For example, for a study exploring the effects of a stroke rehab mobile application on motor paresis, a ‘control’ mobile application could be designed to provide education on stroke prevention. Additionally, amongst the 11 RCTs examined in this review, there were several limitations noted in addition to those previously mentioned. Most of the RCTs generally had small sample sizes and only 4 trials [20, 25, 29, 31] showed a power analysis. This raises concerns of underpowered studies and thus, minimizes its clinical implications. The study follow-up times are also important to consider. Study lengths varied from 0.5 to 12 months with a mode of 1 month. One of the challenges of conducting clinical trials in the early and late sub-acute periods is that an intervention during this time period has the potential to have longer lasting impacts on stroke recovery out to 6 months or even longer [72, 73]. Consideration needs to be given to longer follow-up periods for these studies, even if the intervention itself is briefer (e.g., 4–6 weeks). Across the literature, many in-person interventions require several weeks to months to lead to a positive change [74,75,76]. The dose and length of time an intervention needs to be administered may be dependent on the specific problem being treated. Future research would benefit from having higher quality studies by using a ‘control’ mobile app, randomization, having larger sample sizes, and longer follow-up periods. For future mobile applications for stroke rehabilitation, we suggest incorporating features that we already have evidence for in face-to-face education and stroke rehabilitation therapy such as inter-user interactions to develop social feedback and encouragement, practice exercises with recommended number of structured repetitions, task-specific and goal-oriented practices, constraint-induced therapy, and direct user-interactivity with the mobile application. Of note, we recognize that several national agencies are looking to regulate “software as a medical device” and apps will fall under these regulations. However, at this time, we would not recommend a regulatory body for standardization given the infancy of this field and that regulations can limit the creativity of mobile application development for stroke rehab.
As the demand for limited healthcare resources continues to rise due to multiple factors such as the COVID19 pandemic and aging population [77,78,79], technology has developed a larger role in patient care. COVID-19 has also necessitated increased technology usage leading to improved comfort level by clinicians, patients and caregivers [80, 81]. However, the integration of new technology continues to be limited due to multiple challenges such as clinical acceptance, user learning curve, privacy and security, and the lack of funding models that support the use of technology to augment therapy [82,83,84,85,86]. Future studies should also examine real world use of mobile apps to examine barriers of implementation such as mobile app feasibility and privacy, organizational resource and time use, and motivating factors for patients and/or healthcare providers use [87]. Despite these barriers, mobile applications continue to be an area of growing interest in stroke rehabilitation based on the rising numbers of new studies. With the advantages described in this review and the rapid evolution and acceptance of technology, mobile applications for stroke rehabilitation appear to be a potentially exciting field for research expansion.
Conclusions
This systematic review provides evidence that mobile apps can be used to improve stroke rehabilitation, particularly in combination with face-to-face therapy for motor paresis, aphasia, and adherence to exercise. When mapping out app types, there were several studies in support of using therapy apps for motor paresis and aphasia, rehab videos for exercise adherence, and reminders for exercise adherence. Since stroke rehabilitation inpatients spend much of their time sedentary, providing cost effective mobile apps for therapy may bring patients closer to, or even exceed, the standards set by the Canadian Stroke Best Practices and American Heart Association/American Stroke Association [8, 9, 11,12,13], potentially improving stroke recovery. With the ubiquitous presence of smartphones, there has been a growing accessibility to devices and comfort with technology utilization which also paves a path for increased uptake [88]. Technology acceptance has also been accelerated with necessitated use since the COVID-19 pandemic [80]. Although changing the way we provide therapy may be met with resistance and challenges, it is imperative that we continue to strive to provide the best evidence-based stroke rehabilitation possible, examining the advantages, disadvantages and opportunities associated with technology enhanced therapy provision. With this potential, there is a need for further research to better understand the impact of mobile apps on varying types on stroke deficits and functional outcomes, both alone and in combination with face-to-face stroke rehabilitation. Future studies would benefit in having higher quality RCTs with less reporting and attrition bias (especially for aphasia), larger sample sizes with power analysis, increased study duration of at least 6 months, studies focused on mobile applications with characteristics of face-to-face therapy, clustering patient populations to stoke lesion and acuity, and usability studies to improve user experiences.
Availability of data and materials
Not applicable.
Abbreviations
- CAD:
-
Canadian dollar
- USD:
-
United States dollar
- SD:
-
Standard deviation
- RCT:
-
Randomized control trial
- ADLs:
-
Activities of daily living
- IG:
-
Intervention group
- CG:
-
Control group
- QR code:
-
Quick response code
- SHEMA:
-
Stroke health education mobile app
- HEP:
-
Home exercise program
- PT:
-
Physiotherapy
- OT:
-
Occupational therapy
- SLP:
-
Speech language pathology
- WAM:
-
Wack-a-mole
- SMS:
-
Short message service
- PROM:
-
Passive range of movement
- AROM:
-
Active range of movement
- K-WAB:
-
Korean version of the Western Aphasia Battery
- ASR:
-
Automatic speech recognition
- MoCA:
-
Montreal cognitive assessment
- HP:
-
Home-practice
- KUHMS2 :
-
Korea University Health Monitoring System for Stroke
- BP:
-
Blood pressure
- LDL:
-
Low-density lipoproteins
- ICAP:
-
Intensive, comprehensive aphasia program
- CILT:
-
Constraint induced language treatment
- SIS:
-
Stroke impact scale
- ASHA FACS:
-
American Speech-Language-hearing Association Functional Assessment of Communication Skills for Adults
- 10MWT comfort:
-
10 M walk test at a comfortable speed
- 10MWT fast:
-
10 M walk test a maximum speed
- 6MWT:
-
6 Minute walk test
- TUG:
-
Timed up and go test
- R-HBGS:
-
Regional house-Brackman grading system
- MMT:
-
Manual muscle test
- WE:
-
Wrist extension
- WF:
-
Wrist flexion
- FE:
-
Finger extension
- FF:
-
Finger flexion
- PPT:
-
Purdue pegboard test
- MFAC:
-
Modified functional ambulatory category
- FDS:
-
Functional dysphagia scale
- PAS:
-
Penetration-aspiration scale
- WMFT:
-
Wolf motor function test
- SDMT:
-
Symbol digit modality test
- CBS:
-
Catherine Bergego scale
- VAS:
-
Visual analog scale
- MMAS:
-
Morisky medication adherence scale
- BI:
-
Barthel Index
- mBI:
-
Modified Barthel index
- FIM:
-
Functional independence measure
- SS-QOL-2:
-
Stroke specific quality of life scale
- EQ-5D-5L:
-
EuroQoL 5-dimension 5-level instrument
- HADS:
-
Hospital anxiety and depression scale
References
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. 2020;76(25):2982–3021.
Mittmann N, Seung SJ, Hill MD, Phillips SJ, Hachinski V, Coté R, et al. Impact of disability status on ischemic stroke costs in canada in the first year. Can J Neurol Sci J Can Sci Neurol. 2012;39(6):793–800.
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019. https://doi.org/10.1161/CIR.0000000000000659.
Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2013. https://doi.org/10.1002/14651858.CD000197.pub3.
Gilligan AK, Thrift AG, Sturm JW, Dewey HM, Macdonell RAL, Donnan GA. Stroke units, tissue plasminogen activator, aspirin and neuroprotection: which stroke intervention could provide the greatest community benefit? Cerebrovasc Dis. 2005;20(4):239–44.
Hankey GJ, Warlow CP. Treatment and secondary prevention of stroke: evidence, costs, and effects on individuals and populations. Lancet. 1999;354(9188):1457–63.
Launois R, Giroud M, Mégnigbêto AC, Le Lay K, Présenté G, Mahagne MH, et al. Estimating the cost-effectiveness of stroke units in France compared with conventional care. Stroke. 2004;35(3):770–5.
Teasell R, Salbach NM, Foley N, Mountain A, Cameron JI, de Jong A, et al. Canadian stroke best practice recommendations: rehabilitation, recovery, and community participation following stroke. Part one: rehabilitation and recovery following stroke; 6th edition update 2019. Int J Stroke. 2020;15(7):763–88.
Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016. https://doi.org/10.1161/STR.0000000000000098.
Wang H, Camicia M, Terdiman J, Mannava MK, Sidney S, Sandel ME. Daily treatment time and functional gains of stroke patients during inpatient rehabilitation. PM&R. 2013;5(2):122–8.
Barrett M, Snow JC, Kirkland MC, Kelly LP, Gehue M, Downer MB, et al. Excessive sedentary time during in-patient stroke rehabilitation. Top Stroke Rehabil. 2018. https://doi.org/10.1080/10749357.2018.1458461.
Sjöholm A, Skarin M, Churilov L, Nilsson M, Bernhardt J, Lindén T. Sedentary behaviour and physical activity of people with stroke in rehabilitation hospitals. Stroke Res Treat. 2014;2014:1–7.
Putman K, de Wit L, Schupp W, Ilse B, Berman P, Connell L, et al. Use of time by physiotherapists and occupational therapists in a stroke rehabilitation unit: a comparison between four European rehabilitation centres. Disabil Rehabil. 2006;28(22):1417–24.
Foley N, McClure JA, Meyer M, Salter K, Bureau Y, Teasell R. Inpatient rehabilitation following stroke: amount of therapy received and associations with functional recovery. Disabil Rehabil. 2012;34(25):2132–8.
Bayley MT, Hurdowar A, Richards CL, Korner-Bitensky N, Wood-Dauphinee S, Eng JJ, et al. Barriers to implementation of stroke rehabilitation evidence: findings from a multi-site pilot project. Disabil Rehabil. 2012;34(19):1633–8.
Jang SH, Jang WH. The effect of a finger training application using a tablet PC in chronic hemiparetic stroke patients. Somatosens Mot Res. 2016;33(2):124–9.
Kang JA, Chun MH, Choi SJ, Chang MC, Yi YG. Effects of mirror therapy using a tablet PC on central facial paresis in stroke patients. Ann Rehabil Med. 2017;41(3):347.
Chung BPH, Chiang WKH, Lau H, Lau TFO, Lai CWK, Sit CSY, et al. Pilot study on comparisons between the effectiveness of mobile video-guided and paper-based home exercise programs on improving exercise adherence, self-efficacy for exercise and functional outcomes of patients with stroke with 3-month follow-up: a single-blind randomized controlled trial. Hong Kong Physiother J. 2020;40(01):63–73.
Zhou X, Du M, Zhou L. Use of mobile applications in post-stroke rehabilitation: a systematic review. Top Stroke Rehabil. 2018;25(7):489–99.
Grau-Pellicer M, Lalanza J, Jovell-Fernández E, Capdevila L. Impact of mHealth technology on adherence to healthy PA after stroke: a randomized study. Top Stroke Rehabil. 2020;27(5):354–68.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928–d5928.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84.
Nascimento DC, Petriz B, Oliveira SC, Vieira DCL, Funghetto SS, Silva AO, et al. Effects of blood flow restriction exercise on hemostasis: a systematic review of randomized and non-randomized trials. Int J Gen Med. 2019;12:91–100.
Emmerson KB, Harding KE, Taylor NF. Home exercise programmes supported by video and automated reminders compared with standard paper-based home exercise programmes in patients with stroke: a randomized controlled trial. Clin Rehabil. 2017;31(8):1068–77.
Prokopenko SV, Mozheyko EY, Petrova MM, Koryagina TD, Kaskaeva DS, Chernykh TV, et al. Correction of post-stroke cognitive impairments using computer programs. J Neurol Sci. 2013;325(1–2):148–53.
Knoche H, Hald K, Richter D, Rovsing Møller Jørgensen H. Playing to (self-)rehabilitate: a month-long randomized control trial with brain lesion patients and a tablet game. In: proceedings of the 10th EAI international conference on pervasive computing technologies for healthcare. Cancun, Mexico: ACM; 2016. https://doi.org/10.4108/eai.16-5-2016.2263289.
Kamal A, Khoja A, Usmani B, Magsi S, Malani A, Peera Z, et al. Effect of 5-minute movies shown via a mobile phone app on risk factors and mortality after stroke in a low- to middle-income country: randomized controlled trial for the stroke caregiver dyad education intervention (Movies4Stroke). JMIR. 2020;8(1): e12113.
Kang YN, Shen HN, Lin CY, Elwyn G, Huang SC, Wu TF, et al. Does a mobile app improve patients’ knowledge of stroke risk factors and health-related quality of life in patients with stroke? A randomized controlled trial. BMC Med Inform Decis Mak. 2019;19(1):282.
Moon JH, Heo SJ, Jung JH. Effects of orofacial muscles exercise program on swallowing function and satisfaction in sub-acute stroke patients with dysphagia. Medico-Leg Update. 2019;19(1):623.
Kamal AK, Shaikh Q, Pasha O, Azam I, Islam M, Memon AA, et al. A randomized controlled behavioral intervention trial to improve medication adherence in adult stroke patients with prescription tailored short messaging service (SMS)-SMS4Stroke study. BMC Neurol. 2015;15(1):212.
Lawson S, Tang Z, Feng J. Supporting stroke motor recovery through a mobile application: a pilot study. Am J Occup Ther. 2017;71(3):7103350010p1-5.
Carabeo CGG, Dalida CMM, Padilla EMZ, Rodrigo MMT. Stroke patient rehabilitation: a pilot study of an android-based game. Simul Gaming. 2014;45(2):151–66.
Choi YH, Park HK, Paik NJ. A telerehabilitation approach for chronic aphasia following stroke. Telemed e-Health. 2016;22(5):434–40.
Requena M, Montiel E, Baladas M, Muchada M, Boned S, López R, et al. Farmalarm: application for mobile devices improves risk factor control after stroke. Stroke. 2019;50(7):1819–24.
Ballard KJ, Etter NM, Shen S, Monroe P, Tien TC. Feasibility of automatic speech recognition for providing feedback during tablet-based treatment for apraxia of speech plus aphasia. Am J Speech Lang Pathol. 2019;28(2S):818–34.
Hald K, Knoche H. Ring a bell adaptive auditory game feedback to sustain performance in stroke rehabilitation. In: Giokas K, Bokor L, Hopfgartner F, editors. eHealth 360°, vol. 181. Cham: Springer; 2017. p. 92–9. https://doi.org/10.1007/978-3-319-49655-9_13.
Paul L, Wyke S, Brewster S, Sattar N, Gill JMR, Alexander G, et al. Increasing physical activity in stroke survivors using STARFISH, an interactive mobile phone application: a pilot study. Top Stroke Rehabil. 2016;23(3):170–7.
Kamwesiga JT, Eriksson GM, Tham K, Fors U, Ndiwalana A, von Koch L, et al. A feasibility study of a mobile phone supported family-centred ADL intervention, F@ce™, after stroke in Uganda. Glob Health. 2018;14(1):82.
Kerry SJ, Aguilar OM, Penny W, Crinion JT, Leff AP, Woodhead ZVJ. How does iReadMore therapy change the reading network of patients with central alexia? J Neurosci. 2019;39(29):5719–27.
Hoover E, Carney A. Integrating the iPad into an intensive, comprehensive aphasia program. Semin Speech Lang. 2014;35(01):025–37.
Sureshkumar K, Murthy G, Natarajan S, Naveen C, Goenka S, Kuper H. Evaluation of the feasibility and acceptability of the ‘care for stroke’ intervention in India, a smartphone-enabled, carer-supported, educational intervention for management of disability following stroke. BMJ Open. 2016;6(2): e009243.
Sarfo FS, Adusei N, Ampofo M, Kpeme FK, Ovbiagele B. Pilot trial of a tele-rehab intervention to improve outcomes after stroke in Ghana: a feasibility and user satisfaction study. J Neurol Sci. 2018;387:94–7.
Stark BC, Warburton EA. Improved language in chronic aphasia after self-delivered iPad speech therapy. Neuropsychol Rehabil. 2018;28(5):818–31.
Kurland J, Wilkins A, Stokes P. iPractice: piloting the effectiveness of a tablet-based home practice program in aphasia treatment. Semin Speech Lang. 2014;35(01):051–64.
Kurland J, Liu A, Stokes P. Effects of a tablet-based home practice program with telepractice on treatment outcomes in chronic aphasia. J Speech Lang Hear Res. 2018;61(5):1140–56.
Pugliese M, Ramsay T, Shamloul R, Mallet K, Zakutney L, Corbett D, et al. RecoverNow: a mobile tablet-based therapy platform for early stroke rehabilitation. PLoS ONE. 2019;14(1): e0210725.
Seo WK, Kang J, Jeon M, Lee K, Lee S, Kim JH, et al. Feasibility of using a mobile application for the monitoring and management of stroke-associated risk factors. J Clin Neurol. 2015;11(2):142.
Kizony R, Zeilig G, Dudkiewicz I, Schejter-Margalit T, Rand D. Tablet apps and dexterity: comparison between 3 age groups and proof of concept for stroke rehabilitation. J Neurol Phys Ther. 2016;40(1):31–9.
Cook DA, Levinson AJ, Garside S, Dupras DM, Erwin PJ, Montori VM. Internet-based learning in the health professions: a meta-analysis. JAMA. 2008;300(10):1181–96.
Means B, Toyama Y, Murphy R, Bakia M, Jones K. Evaluation of evidence-based practices in online learning: a meta-analysis and review of online learning studies. US Department of Education. 2009. https://eric.ed.gov/?id=ED505824. Accessed 22 Feb 2022.
No significant difference. DETA Center | National Research Center for Distance Education and Technological Advancements (DETA). https://detaresearch.org/research-support/no-significant-difference/. Accessed 5 Oct 2022.
Cook DA, Levinson AJ, Garside S, Dupras DM, Erwin PJ, Montori VM. Instructional design variations in internet-based learning for health professions education: a systematic review and meta-analysis. Acad Med. 2010;85(5):909–22.
Maier M, Ballester BR, Verschure PFMJ. Principles of neurorehabilitation after stroke based on motor learning and brain plasticity mechanisms. Front Syst Neurosci. 2019;13:74.
Kwakkel G, Veerbeek JM, van Wegen EEH, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015;14(2):224–34.
Zhang J, Yu J, Bao Y, Xie Q, Xu Y, Zhang J, et al. Constraint-induced aphasia therapy in post-stroke aphasia rehabilitation: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2017;12(8): e0183349.
Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001;32(6):1279–84.
Scrivener K, Sherrington C, Schurr K. Exercise dose and mobility outcome in a comprehensive stroke unit: description and prediction from a prospective cohort study. J Rehabil Med. 2012;44(10):824–9.
Harris JE, Eng JJ, Miller WC, Dawson AS. A self-administered graded repetitive arm supplementary program (GRASP) improves arm function during inpatient stroke rehabilitation. Stroke. 2009;40(6):2123–8.
Boyle EA, Hainey T, Connolly TM, Gray G, Earp J, Ott M, et al. An update to the systematic literature review of empirical evidence of the impacts and outcomes of computer games and serious games. Comput Educ. 2016;94:178–92.
Dickey L, Kagan A, Lindsay MP, Fang J, Rowland A, Black S. Incidence and profile of inpatient stroke-induced aphasia in Ontario, Canada. Arch Phys Med Rehabil. 2010;91(2):196–202.
Wang G, Ge L, Zheng Q, Huang P, Xiang J. Constraint-induced aphasia therapy for patients with aphasia: a systematic review. Int J Nurs Sci. 2020;7(3):349–58.
Chan DYW, Man WK. Unilateral neglect in stroke: a comparative study. Top Geriatr Rehabil. 2013;29(2):126–34.
Pereira Ferreira H, Alvim Leite Lopes M, Raggio Luiz R, Cardoso L, André C. Is visual scanning better than mental practice in hemispatial neglect? Results from a pilot study. Top Stroke Rehabil. 2011;18(2):155–61.
Mayer RE. Multimedia learning. 2nd ed. Cambridge: Cambridge University Press; 2009.
Pusic MV, Ching K, Yin HS, Kessler D. Seven practical principles for improving patient education: evidence-based ideas from cognition science. Paediatr Child Health. 2014;19(3):119–22.
Young J, Merriënboer JJG van, Durning S, Cate O ten. Cognitive load theory: implications for medical education: AMEE guide No. 86. Med Teach. 2014. https://www.scinapse.io. Accessed 5 Oct 2022.
Ferrucci L, Bandinelli S, Guralnik JM, Lamponi M, Bertini C, Falchini M, et al. Recovery of functional status after stroke. A postrehabilitation follow-up study. Stroke. 1993;24(2):200–5.
Duncan PW, Goldstein LB, Matchar D, Divine GW, Feussner J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke. 1992;23(8):1084–9.
Van Hoof TJ, Sumeracki MA, Madan CR. Science of learning strategy series: article 1, distributed practice. J Contin Educ Health Prof. 2021;41(1):59–62.
Jack K, McLean SM, Moffett JK, Gardiner E. Barriers to treatment adherence in physiotherapy outpatient clinics: a systematic review. Man Ther. 2010;15(3):220–8.
Horgan NF, O’Regan M, Cunningham CJ, Finn AM. Recovery after stroke: a 1-year profile. Disabil Rehabil. 2009;31(10):831–9.
Lee KB, Lim SH, Kim KH, Kim KJ, Kim YR, Chang WN, et al. Six-month functional recovery of stroke patients: a multi-time-point study. Int J Rehabil Res. 2015;38(2):173–80.
Rose DK, Nadeau SE, Wu SS, Tilson JK, Dobkin BH, Pei Q, et al. Locomotor training and strength and balance exercises for walking recovery after stroke: response to number of training sessions. Phys Ther. 2017;97(11):1066–74.
Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364(21):2026–36.
Dawson J, Liu CY, Francisco GE, Cramer SC, Wolf SL, Dixit A, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet. 2021;397(10284):1545–53.
Bartsch SM, Ferguson MC, McKinnell JA, O’Shea KJ, Wedlock PT, Siegmund SS, et al. The potential health care costs and resource use associated with COVID-19 in the United States. Health Aff. 2020;39(6):927–35.
Ellison D, White D, Farrar FC. Aging population. Nurs Clin North Am. 2015;50(1):185–213.
McMaughan DJ, Oloruntoba O, Smith ML. Socioeconomic status and access to healthcare: interrelated drivers for healthy aging. Front Public Health. 2020;8:231.
Golinelli D, Boetto E, Carullo G, Nuzzolese AG, Landini MP, Fantini MP. Adoption of digital technologies in health care during the COVID-19 pandemic: systematic review of early scientific literature. J Med Internet Res. 2020;22(11): e22280.
Fiani B, Siddiqi I, Lee SC, Dhillon L. Telerehabilitation: development, application, and need for increased usage in the COVID-19 era for patients with spinal pathology. Cureus. 2020;12(9): e10563.
Nadal C, Sas C, Doherty G. Technology acceptance in mobile health: scoping review of definitions, models, and measurement. J Med Internet Res. 2020;22(7): e17256.
Attaran S, Thourani VH. With every new technology comes a learning curve. Semin Thorac Cardiovasc Surg. 2018;30(2):158–9.
Duwelius PJ, Parvizi J, Matsen KL. New technology: safety, efficacy, and learning curves. Clin Orthop. 2014;472(4):1080–5.
Dinh-Le C, Chuang R, Chokshi S, Mann D. Wearable health technology and electronic health record integration: scoping review and future directions. JMIR. 2019;7(9): e12861.
Govil A, Hao SC. Integration of new technology into clinical practice after FDA approval. J Interv Card Electrophysiol. 2016;47(1):19–27.
Brouns B, Meesters JJL, Wentink MM, de Kloet AJ, Arwert HJ, Vliet Vlieland TPM, et al. Why the uptake of eRehabilitation programs in stroke care is so difficult—a focus group study in the Netherlands. Implement Sci. 2018;13(1):133.
Miralles I, Granell C, Díaz-Sanahuja L, Van Woensel W, Bretón-López J, Mira A, et al. Smartphone apps for the treatment of mental disorders: systematic review. JMIR. 2020;8(4): e14897.
Acknowledgements
Not applicable.
Funding
Physiatry Resident Research Grant provided by the Division of Physical Medicine and Rehabilitation, University of Toronto.
Author information
Authors and Affiliations
Contributions
SGS and HW collected data and performed analysis and interpretation. MA aided in data interpretation. SGS wrote the manuscript. HW, MA, SD, and HM assisted in revising the manuscript. SGS and HW designed the study with guidance from SD and HM. SD and HM supervised data collection and analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional materials, additional Figures S1–S4, additional Tables S1–S5.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Szeto, S.G., Wan, H., Alavinia, M. et al. Effect of mobile application types on stroke rehabilitation: a systematic review. J NeuroEngineering Rehabil 20, 12 (2023). https://doi.org/10.1186/s12984-023-01124-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12984-023-01124-9