Abstract
Objectives
The objective of this review was to summarize and appraise evidence on functional electrical stimulation (FES) cycling exercise after spinal cord injury (SCI), in order to inform the development of evidence-based clinical practice guidelines.
Methods
PubMed, the Cochrane Central Register of Controlled Trials, EMBASE, SPORTDiscus, and CINAHL were searched up to April 2021 to identify FES cycling exercise intervention studies including adults with SCI. In order to capture the widest array of evidence available, any outcome measure employed in such studies was considered eligible. Two independent reviewers conducted study eligibility screening, data extraction, and quality appraisal using Cochranes’ Risk of Bias or Downs and Black tools. Each study was designated as a Level 1, 2, 3 or 4 study, dependent on study design and quality appraisal scores. The certainty of the evidence for each outcome was assessed using GRADE ratings (‘High’, ‘Moderate’, ‘Low’, or ‘Very low’).
Results
Ninety-two studies met the eligibility criteria, comprising 999 adults with SCI representing all age, sex, time since injury, lesion level and lesion completeness strata. For muscle health (e.g., muscle mass, fiber type composition), significant improvements were found in 3 out of 4 Level 1–2 studies, and 27 out of 32 Level 3–4 studies (GRADE rating: ‘High’). Although lacking Level 1–2 studies, significant improvements were also found in nearly all of 35 Level 3–4 studies on power output and aerobic fitness (e.g., peak power and oxygen uptake during an FES cycling test) (GRADE ratings: ‘Low’).
Conclusion
Current evidence indicates that FES cycling exercise improves lower-body muscle health of adults with SCI, and may increase power output and aerobic fitness. The evidence summarized and appraised in this review can inform the development of the first international, evidence-based clinical practice guidelines for the use of FES cycling exercise in clinical and community settings of adults with SCI.
Registration review protocol: CRD42018108940 (PROSPERO)
Similar content being viewed by others
Background
Functional electrical stimulation (FES) applies low-level electrical pulses to paretic or paralyzed muscles to restore or improve their functional capacity. It is a neuroprosthetic, therapeutic or exercise modality for individuals with a nervous system injury to reactivate the peripheral nervous system without significant lower motor neuron damage [1]. In clinical and community settings, one of the most commonly available and researched FES exercise modalities is FES-evoked cycling [2,3,4]. FES cycling allows people with little or no voluntary leg movement to pedal an exercise bicycle, usually indoors on a stationary system. Computer generated, low-level electrical pulses are transmitted through transcutaneous electrodes to the leg muscles. This evokes coordinated contractions and a pedaling motion that mimics voluntary exercise training. Potential or anecdotal benefits include improvements in muscle, bone and cardiovascular health, fitness, feelings of well-being, and motor function of people with neurological conditions such as stroke, multiple sclerosis and spinal cord injury (SCI) [1, 5,6,7,8].
Despite the potential and its availability, FES cycling is currently not consistently deployed as a component of the lifelong rehabilitation care plan for all eligible individuals with SCI who are responsive to FES. More evidence-based exercise and rehabilitation options would be of particular benefit to the SCI community [9], given their high risk of secondary health complications [10], and barriers to participate in exercise [11]. The availability of evidence-based clinical practice guidelines can enhance the use of therapeutic exercise and rehabilitation options [12,13,14]. Essential to the development of such guidelines is a systematic literature review in accordance with Grading of Recommendations Assessment, Development and Evaluation (GRADE) [13, 15, 16]. Although recent systematic reviews have provided helpful insight into specific outcomes [17,18,19], a comprehensive systematic review including GRADE assessments is currently not available for FES cycling research in SCI.
Accordingly, this review sought to summarize and appraise evidence of randomized controlled trials (RCTs), non-RCTs, pre-post studies, case series, case studies and cross-sectional controlled studies evaluating the effects of FES cycling exercise among adults with SCI. Any health or fitness-related outcome measures used in those studies were considered eligible for inclusion, to ensure a complete overview of what outcomes have been used in FES cycling exercise research for the SCI population. Although not a primary objective, the review also sought to provide an overview of adverse events reported in the included studies.
Methods
We designed the review’s protocol in accordance with international reporting standards [20, 21], and in consideration for the future development of practice guidelines for clinical and community settings [14]. The review was registered in PROSPERO (CRD42018108940). Information required for compliance with the reporting standards that has not been provided in this paper can be found in an online data repository at https://osf.io/u9mvx/, including the reference list of eligible studies, a ‘grey’ literature search, data extractions and risk of bias (quality appraisal) scoring.
Search strategy
PubMed, the Cochrane Central Register of Controlled Trials, EMBASE (OVID), SPORTDiscus (EBSCOhost), and CINAHL (EBSCOhost) were searched from the earliest record until April 1st, 2021. To coincide with two guideline development meetings, these databases were first searched to June 2018, and then updated to May 2019 (Fig. 1). An updated search was also conducted in April 2021 (Fig. 1). An independent librarian contributed to the search strategy. Keywords were a combination of terms representing SCI (e.g., paraplegia, tetraplegia) [2], FES (e.g., functional electric stimulation, electrotherapy) and cycling (e.g., cycle, pedalling), including database-specific indexing terms (e.g., Emtree for EMBASE). The online repository (https://osf.io/u9mvx/) provides the tailored search strings for each database. To identify other relevant studies, we consulted content experts and searched the reference lists of previous reviews (Fig. 1). To identify potential publication bias, the World Health Organization trial registry was searched for unpublished RCTs or non-RCTs matching the study eligibility criteria (i.e., ‘grey’ literature search). Language familiarity of the review team limited the search to peer-reviewed articles written in English, which we anticipated to have limited effect on our conclusions [22].
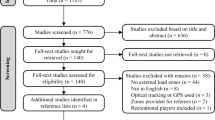
Flow chart of the literature search and selection of eligible articles. Note The reference list of the 97 included articles with the 92 unique datasets is provided in the online repository (https://osf.io/u9mvx/). FES functional electrical stimulation; RCT randomized controlled trial; SCI spinal cord injury; WHO World Health Organization
Study eligibility criteria
As part of the guideline development process, international stakeholder meetings with FES users, researchers, clinicians and other practitioners were conducted in 2018 and 2019 (Edmonton, Canada; Loughborough, UK; manuscript in preparation). These meetings were informed by a 2016 overview of SCI exercise evidence that included FES studies [2], and an additional scoping review on FES exercise RCTs and non-RCTs. The preliminary available evidence and stakeholders discussions informed the decision to focus the guideline development process (including the current review) on FES cycling, given that it is one of the most commonly used and accessible FES modalities with the largest body of high-quality evidence supporting it. Informed by these stakeholder meetings, the following selection of study eligibility criteria was established:
-
Participants: Studies that included a sample of at least 50% with adults (≥ 16 years) with traumatic or non-traumatic SCI (any time post-onset SCI) who were eligible and responsive to FES cycling. Excluded were those with a congenital condition (e.g., spina bifida), or a progressive disease (e.g., multiple sclerosis with spinal cord involvement).
-
Interventions: Studies that employed an FES lower-body cycling exercise intervention and describing exercise prescription parameters such as intervention period (e.g., 12 weeks), exercise frequency (e.g., three times per week), and/or exercise duration (e.g., 30 min per session). FES cycling was defined as a modality whereby transcutaneous electrical currents are applied to paralyzed or paretic muscles, with the necessary stimulation characteristics provided to evoke muscle contractions for lower-body cycling movements. “Exercise” was defined as planned, structured, and repetitive physical activity that is performed to improve or maintain physical fitness component(s) [23]. Excluded were interventions shorter than two weeks [2], and interventions that did not allow inferences about the specific contributions of FES cycling, e.g. activity-based restorative therapy [24, 25].
-
Comparator/Control: Studies were eligible as a controlled study if the comparator for the exercise intervention was a control group not receiving an FES cycling exercise intervention. Receiving usual care (e.g., during the inpatient rehabilitation period) was also accepted as a control condition when the exercise group also received this usual care in addition to the exercise intervention [2]. Studies comparing two FES cycling interventions (e.g., low-cadence vs high-cadence cycling) were included and appraised as pre-post studies.
-
Outcome measures: Rather than focusing on a fixed set of outcomes, studies employing any type of health or fitness-related outcome measure were included, so long as they were measured in response to FES cycling exercise in participants with SCI. This wide array was chosen to ensure a complete overview of what outcomes have been used in FES cycling intervention research for the SCI population.
-
Study designs: RCTs, non-RCTs, pre-post, case series, case report, and cross-sectional controlled studies, in order to capture all available evidence beyond the limited available exercise RCTs in SCI [2]. Only cross-sectional studies without a control group were excluded, given the impossibility to make any assumptions about causality.
Study eligibility screening
Co-author SEV and a review team with content expertise (see ‘Acknowledgements’) conducted the study selection, supervised by primary author JWvdS. Two reviewers screened the titles and abstracts independently after duplication removal. Full-text articles were retrieved if one or both reviewers considered a study potentially eligible for inclusion. Two reviewers independently reviewed the full-text articles for eligibility, while recording all reasons for exclusion. Any disagreements during this process were discussed between the reviewers. If no consensus was reached, JWvdS adjudicated the inclusion/exclusion of an article. Reviewers were not blinded to authors or journals.
Data extraction
The data extraction sheets are provided in https://osf.io/u9mvx/. Data extracted included details on: study design, demographics, spinal cord lesion characteristics, training status at baseline, participant exclusion criteria, intervention location and environment, exercise prescription, neuromuscular stimulation characteristics, outcome measures, confidence intervals, statistical power, and adverse events. JWvdS and SEV pilot tested preliminary data extractions sheets and developed the final data extraction sheets with the other authors. Using these, two reviewers independently extracted data from a sample of eligible studies (10%) and achieved good agreement (at least 80% concordance), with the remainder extracted by one reviewer. JWvdS verified all data extractions.
Risk of bias in individual studies
Two reviewers independently appraised the included RCTs using Cochrane’s RoB 2.0 [26], non-RCTs using ROBINS-I [27], and used a modified Downs and Black tool [28, 29] for the other study designs (see https://osf.io/u9mvx/. The reviewers discussed differences until full consensus was reached, if necessary adjudicated by JWvdS. One of 4 Levels of evidence was established for each study (Table 1), based on the strength of the study design and cut-off scores from the quality appraisal tools, similar to previous approaches [2, 29]. A Level 1 study indicated a study with the least risk of bias, and a Level 4 study highest risk of bias.
Evidence summary
The outcome measures that were identified during data extraction were categorized, in accordance with a previously published systematic review on cardiorespiratory fitness, power output, muscle strength, cardiometabolic health and bone health [2]. We then expanded the outcome categories to fit the wider scope of outcomes covered by this review (e.g., subjective well-being [30]) and this review’s specific focus on FES cycling exercise. The proposed categorisation of outcome measures was validated and confirmed by FES content experts as part of the international expert panel meetings to develop FES cycling guidelines. The following outcome categories were defined:
-
Muscle health: Including measures representing muscle volume, circumference and fiber type composition (e.g., cross-sectional leg muscle area, mid-thigh muscle volume, % type IIa vs type IIb fibers)
-
Power output: Including measures representing lower-body power output (e.g., peak power output during an incremental FES cycle test, or average power output during training)
-
Aerobic fitness: Including measures representing peak oxygen uptake and respiratory capacity (e.g., peak oxygen uptake during an incremental FES cycle test, tidal volume)
-
Muscle strength: Including measures representing isometric or isokinetic muscle force and torque (e.g., electrically stimulated peak leg extension torque, isometric knee extension force)
-
Fat mass: Including measures representing adipose tissue (e.g., abdominal ectopic fat, cross-sectional leg fat area)
-
Cardiovascular and metabolic factors: Including measures representing cardiac, arterial and metabolic structure and function (e.g., arterial pulse wave velocity, insulin sensitivity, cytokine profiles)
-
Bone health: Including measures representing bone mineral density (BMD), bone turnover markers and histomorphometry (e.g., whole-body BMD, bone-specific alkaline phosphatase, N-telopeptides)
-
Subjective well-being: Including measures representing anxiety and depression, life satisfaction, perceived stress (e.g., Hospital Anxiety and Depression Scale, World Health Organization Quality of Life Scale, Perceived Stress Scale)
-
Functional and neurological outcomes: Including measures representing functional independence or neurological recovery (e.g., motor and sensory function, 6-min walking test, Functional Independence Measure, Spinal Cord Independence Measure)
-
Other secondary health conditions: Including measures representing SCI-specific secondary conditions such as spasticity, bowel function or oedema (e.g., Modified Ashworth Scale, Neurogenic Bowel Dysfunction Score).
Following, the review team designated for each study whether the intervention showed an improvement in an outcome category or not, similar to a previous review [2]. Given the lack of benchmarks for clinically meaningful improvements [31], and the anticipated large variety of outcome measures [2], “improvement” was defined as a statistically significant positive change following the intervention in at least one of the outcome measures within an outcome category [2]. For studies in which statistics were not applied, for example in a case series study, when all participants improved in an outcome, this was classified as an improvement. A study’s intervention could also be designated to provide an “inconclusive” result, for example when one subgroup improved in contrast to another, when one measure indicated an improvement and another measure of that same outcome category indicated worsening, or when no statistics were provided in a pre-post study. JWvdS verified all designations.
Studies showing an improvement or not were summarized separately for Level 1, 2, 3 and 4 studies across each outcome category, to enable the evidence appraisal using GRADE (see below). Given the variety of study designs, interventions and reported outcome measures, we did not consider it feasible or valid to synthesise the results quantitatively using meta-analyses or forest plots. Combining data on these measures for the purpose of meta-analysis could be misleading if the magnitude of effects differed across outcomes and study designs. The potential for meta-analyses and forest plots was also limited by the low reporting quality in many studies. For example, some studies failed to provide group descriptive statistics, while many studies did not report effect sizes or relative differences within and between groups.
Evidence appraisal using GRADE
GRADE methodology was used to assess certainty of the evidence for each outcome category [13, 15]. The GRADE method prescribes assessing the body of evidence (i.e., all studies taken together) for the following criteria: very serious risk of bias, serious risk of bias, inconsistency, imprecision, indirectness, and publication bias (Table 2) [13, 15]. If one or more of those issues appear, GRADE certainty in the evidence is to be downgraded from ‘High’ to ‘Moderate’, ‘Low’ or ‘Very Low’ [13, 15]. Conversely, the GRADE method prescribed that certainty in the evidence can be upgraded if there are indications of a dose–response gradient, plausible bias or large magnitude of effects in lower-quality studies [13, 15]. The higher the certainty, the more confidence one can have that the measured effect aligns with the true effect [16]. ‘Low’ or ‘Very Low’ certainty in the evidence does not imply an intervention does not work; it merely indicates that confidence is limited about the measured effect aligning with the true effect [16].
For the purpose of this review, we developed benchmarks for each GRADE criterion (Table 2) in accordance with previously developed criteria [2]. GRADE certainty in the evidence was downgraded by two levels (e.g., from ‘High’ to ‘Low’) if there was very serious risk of bias. It was downgraded by one level (e.g., from ‘High’ to ‘Moderate’) when serious risk of bias, inconsistency, imprecision, indirectness or publication bias was present. Certainty of the evidence was upgraded by one level if we observed consistent effects, plausible bias and/or a dose–response gradient across the Level 2, 3 and 4 studies.
Adverse events
Although not a primary objective of this review, the included studies were summarized for their descriptions of suspected adverse reactions. These were defined in accordance with the US FDA as adverse events for which there was a reasonable possibility that the FES intervention caused the adverse event [32]. For the studies that described adverse events, the summaries included the total number of participants reporting serious suspected adverse reactions (e.g., life-threatening event, event that required prolonged hospitalization), or other suspected adverse reactions [32].
Results
The search strategy and eligibility screening led to the inclusion of 97 articles that comprised 92 studies without identical data/samples [33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129] (Fig. 1). The online repository (https://osf.io/u9mvx/) provides the reference list of the 97 articles, data extractions for each of the 92 studies, and details of the literature search in the trial registers. Tables 3, 4 and 5 provide an overview of extracted characteristics of participants, interventions and outcome measures.
Risk of bias in individual studies
Each of the 92 studies was classified for its individual Level of evidence in accordance with Table 1. Two were classified as Level 1 studies, 7 as Level 2 studies, 65 as Level 3 studies, and 18 as Level 4 studies. An RCT design was used in five studies, with RoB 2.0 scores ranging from Low to Serious risk of bias. A non-RCT design was used in four studies, with ROBINS-I scores ranging from Low to Moderate risk of bias. Downs and Black scores ranged from 4 to 22 (mean ± SD: 12 ± 4) across the studies with pre-post, case series, case report and cross-sectional designs. Detailed risk of bias scores for each checklist item of the studies are available at https://osf.io/u9mvx/.
Participant characteristics
Overall, the evidence included 999 participants representing all demographic and spinal cord lesion characteristic strata (Table 3). Underrepresented in the evidence were women, adults > 65 years, participants with motor incomplete injuries and those with high cervical or lumbar lesions. Most participants were untrained in FES cycling exercise at baseline, although some received FES strength training before starting the intervention. They were free of bone fractures, pressure injuries or other common reasons for exclusion from participating in FES exercise.
Intervention and control characteristics
The average intervention period across all studies was 16 weeks, mostly cycling three times per week for 30 min at 35–50 revolutions per minute, using a neuromuscular stimulation amplitude up to 140 mA, a pulse width of 300 µs, and a pulse frequency of 35 Hz (Table 4). If a form of progression was used and reported in the studies, it consisted of increasing absolute resistance or torque levels within or across sessions, based on participants’ cycling frequency, fatigue, and/or personal tolerability. None of the studies reported gauging exercise intensity using physiological criteria such as percent peak oxygen uptake or heart rate, except for peak power output.
The majority of interventions took place in research and/or clinical environments, while 19 studies employed home-based environments (Table 4). In 19 studies, FES cycling was preceded or complemented by other lower-body strength exercise, such as a number of weeks of FES quadriceps strengthening preceding subsequent weeks of FES cycling. Almost half of the studies (44 out of 92) were conducted in the USA, while 4–8 studies took place in Australia, Canada, Denmark, the Netherlands, Switzerland, or the UK (Table 4). The remainder of the studies took place in other countries across Asia, Australia, Europe, the Middle East and South America. Control groups followed usual in-patient rehabilitation care, conducted passive cycling or upper-body exercise, or did not participate in any exercise intervention.
Outcomes
As summarized in Table 5, the most frequently employed outcome measures were indices of muscle health (e.g., muscle cross-sectional area, ratio between muscle fiber types), power output (e.g., peak power output on an incremental FES cycling test, average power output during training), or aerobic fitness (e.g., peak oxygen uptake on an incremental FES cycling test, tidal volume). For muscle health (36 studies), the one Level 1 study reported non-significant findings, while the four Level 2 studies and over 80% of Level 3 or 4 studies demonstrated significant improvements. For power output and aerobic fitness, Level 1 or 2 studies were lacking, but over 35 Level 3 and 4 studies were available. Nearly all of these studies showed significant improvements, for example in 29 out of 30 Level 3 studies on power output and 17 out of 21 Level 3 studies on aerobic fitness. Lower consistency or less evidence was available for the other outcomes (Table 5). For example, less than half of the studies on bone health (11 out of 23 studies) found significant improvements after 8–26 weeks of FES cycling exercise in measures such as bone mineral density and bone turnover markers. Studies on functional and neurological outcomes (e.g., independence measures, ISNCSCI motor scores) were limited to five Level 3 studies, of which three demonstrated significant improvements.
Evidence appraisal using GRADE
For muscle health, the GRADE assessment identified potential imprecision (Table 2), due to limited or no information on statistical power or confidence intervals around effect estimates. The GRADE assessment also revealed indirectness (i.e., limited generalizability), but only for older adults > 65 years. The evidence on muscle health included participants with paraplegia or tetraplegia (C1 to L1, AIS A, B, C or D), 0.04–53 years post-injury (mean: 10 years), aged 16–67 years (mean: 36 years). We upgraded certainty in the evidence for muscle health by one level due to the consistent effects found across the large number of Level 2, 3 and 4 studies (Table 5). This led to ‘Moderate’ certainty in the evidence for any adult with SCI, and ‘High’ certainty in the evidence for young to middle-aged adults with SCI.
For power output, the GRADE assessment revealed very serious risk of bias due to the absence of Level 1 or 2 studies, and potential imprecision due to lack of information about statistical power and confidence intervals. The evidence on power output included participants with paraplegia or tetraplegia (C3 to L1, AIS A, B, C or D), 0.16–53 years post-injury (mean: 10 years), aged 17–80 years (mean: 38 years). We upgraded certainty in the evidence by one level due to the highly consistent effects found across the large number of Level 3 studies (Table 5). Therefore, GRADE certainty in the evidence for augmented power output was ‘Low’ for any adult with SCI.
The GRADE assessment for aerobic fitness was similar to that of power output; very serious risk of bias and potential imprecision, and strengthening of confidence in the evidence by the consistent effects across the Level 3 studies (Table 5). The evidence on power output included participants with paraplegia or tetraplegia (C3 to L2, AIS A, B, C or D), 0.08–33 years post-injury (mean: 9 years), aged 16–70 years (mean: 35 years). Accordingly, the GRADE assessment established ‘Low’ certainty in the evidence for improved aerobic fitness after FES cycling exercise.
The GRADE assessments led to ‘Very Low’ certainty in the evidence for the other outcomes shown in Table 5, due to an absence of Level 1 or 2 studies, effects being inconsistent across the studies, imprecision, and/or indirectness.
Adverse events
None of the studies had adverse events as its primary outcome. Adverse events were described in 21 studies comprising 203 participants, as detailed in the data extraction table (https://osf.io/u9mvx/). Of these, 18 participants experienced suspected adverse reactions to FES cycling. One out of these 18 participants experienced a serious suspected adverse reaction; the participant was reported to be withdrawn from an FES-cycling intervention related to haemotoma development in the ischial region, which may or may not have been associated with the intervention. Seventeen participants experienced other suspected adverse reactions such as temporary post-exercise hypotension (n = 4), increased spasticity (n = 4), light-headedness (n = 2), skin redness (n = 2), bowl accident (n = 1), autonomic dysreflexia caused by stimulation (n = 2), increased leg swelling (n = 1), and a small quadriceps haemotoma that was resolved within 2 weeks (n = 1). Two of these could not finish the FES intervention due to increased spasticity.
Discussion
This review has provided the first summary and appraisal of evidence for the effects of FES cycling exercise interventions on health and fitness-related outcomes measured after SCI. The GRADE assessments revealed ‘High’ certainty in the evidence for significant improvements in lower-body muscle health (e.g., larger muscle volume, shift to more fatigue-resistant fiber types), and ‘Low’ certainty in the evidence for significant improvements in power output and aerobic fitness (e.g., peak power output and oxygen uptake during an incremental FES cycling test) of adults with SCI. This review also highlighted that future high-quality research is necessary to validate conclusions about other potential benefits, such as improved cardiovascular health, and functional or neurological adaptations. The limited available evidence on adverse events suggested that harmful reactions are unlikely to occur when adults with SCI engage in FES cycling.
All but one RCT and a large number of Level 3–4 studies found significant improvements in outcomes for muscle health. The one RCT without significant improvements may be explained by a relatively short intervention duration (i.e., < 3 months), and insensitivity of its outcome measure related to the location of measurement of cross-sectional area [71, 130]. Overall, the evidence indicated that FES cycling could help counteract the vast loss of muscle mass after SCI, which can be as high as 80% when compared to able-bodied controls [131, 132]. This might reduce risk of pressure injuries [133], increase the low resting metabolic rates that can contribute to obesity [134], and enhance satisfaction with body appearance [135]. The changes in fiber type composition shown by the evidence (e.g., shift from type IIb and IIx fibers to type IIa fibers) indicate that FES cycling can help reverse the loss of oxidative capacity of paralyzed muscles [136]. This may aid beneficial vascular adaptations [137], improve aerobic metabolism [5], and reduce the onset of fatigue during further FES training [138].
A large number of Level 3 and 4 studies provided consistent evidence that FES cycling exercise could improve lower-body power output and aerobic fitness. If these improvements relate at least to some extent to the cardiovascular and cognitive health benefits found in lower-body exercise in the able-bodied population [139, 140], then FES cycling has great potential for reducing the high risk of cardiovascular and cerebrovascular conditions after SCI [141,142,143,144].
Strengths and limitations of this review
One of the strengths of this review was the transparent use of GRADE to appraise the body of evidence for each outcome, in accordance with international standards [13, 16]. However, we also acknowledge that a sole focus on GRADE criteria may not provide recommendations that clinicians can easily utilize [145]. For example, the quality of SCI evidence about exercise will always be prone to downgrading using the GRADE criteria due to imprecision and indirectness, considering the inherent challenges in undertaking high-quality exercise research in this population [146]. These include the small potential participant pools, an inherent age and sex distribution in the SCI population traditionally representing relatively fewer women and older adults, neurological heterogeneity common in SCI samples, and the complexity of spinal cord lesion characteristics influencing outcomes. Notwithstanding, evidence-based guidelines can still be developed even when the GRADE assessment reveals ‘Low’ certainty in the evidence, by weighing in the views, preferences and experiences of stakeholders [147]. We involved a large number of clinical and community stakeholders in designing this review and developing evidence-based FES cycling clinical practice guidelines (manuscript in preparation). This process demonstrated that many people with SCI and their health-care providers encourage the cautious use of evidence beyond gold-standard RCTs, given the importance they see in deploying FES cycling in clinical and community environments.
A limitation of this review is the use of counting the number of studies showing statistically significant improvements [2, 148]. However, lack of established benchmarks for clinically meaningful improvements in SCI [31], and mere absence of reporting mean differences, effect sizes, 95% confidence intervals, or individual data, rendered this the best possible approach towards synthesizing the evidence [2]. Although this approach increased the risk of type II errors and family-wise error rates [148], it is unlikely that such errors influenced the primary findings of this review, as significant improvements were found in nearly all studies and outcome measures related to muscle health, power output and aerobic fitness.
Implications for future research: gaps identified in this review
The gaps in the evidence identified in this review can inform the prioritization and direction of future research. It was encouraging to observe that the research base for FES cycling after SCI has steadily increased since the 1980s, with many new studies conducted over the last decade (e.g., almost half of all included studies in this review were published between 2010 and 2020). Important evidence gaps remain however, and clinical practice and policy development would be served by addressing these.
One key gap is the current lack of high-quality evidence on potential functional or neurological benefits of FES cycling. The few Level 3 pre-post studies identified in this review showed some improvements in adults with chronic SCI. Animal studies have suggested that initiating exercise during a critical early period may enhance functional recovery [149]. However, lacking are high-quality FES cycling controlled trials taking place within the first 3–6 months after SCI when recovery is most likely [150], while focusing on underlying mechanisms, and functional and neurological outcomes sensitive to change. Such trials can also inform the ongoing debate about the potential of FES cycling for neurorecovery [24].
Other key research gaps identified by this review relate to potential effects of FES cycling on the risk of cardiometabolic disease [141, 151], reduction of debilitating secondary health conditions such as pressure injuries, chronic pain, and urinary tract infections [152,153,154], and enhancement of subjective well-being [155, 156]. For these outcomes, the review highlighted a lack of high-quality research employing instruments sensitive to exercise-induced changes in adults with SCI that can provide insight into the magnitude of potential improvement of these outcomes following SCI. Such research should be aligned and combined with what SCI users of FES cycling often report anecdotally, such as functional and neurological improvements and psychological benefits.
Changes in many of these outcomes may require intervention periods over a longer period (e.g., 1–2 years) than what most FES cycling studies have used so far (on average 16 weeks, see Table 3). For example, structural cardiac and vascular improvements may occur secondary to adaptations in muscle health and aerobic fitness, but might not be visible in the first months of a person with SCI engaging in FES or other forms of exercise [157]. If they occur, changes in bone health may require at least one year of FES cycling exercise [87, 158, 159].
RCTs over such long periods are costly, likely face ethical challenges, and are often not feasible due to small potential participant pools [146]. A solution is the use of longitudinal designs taking place in clinical and community centres where FES cycling is used daily as part of ongoing rehabilitation and exercise programs. The statistical power and high external validity of such a design, combined with high-quality reporting of the intervention details and environment, could provide a wealth of information about a range of outcomes on which future clinical practice guidelines can be build. An additional or alternative successful approach could be home-based FES cycling [58], in particular when combined with better user education and establishment of user-specific goals between a practitioner and a person with SCI [3].
The intervention studies identified by this review did not analyse or provide sufficient information to draw conclusions about the minimum or optimal dose of FES cycling exercise and which neuromuscular stimulation characteristics would be required for that. This highlights a need for more robust comparisons of FES exercise prescriptions and approaches to selecting neuromuscular stimulation characteristics, how to keep providing progressive overload for continued improvements, and how to best deal with the “fatigue” problem due to reverse-order muscle fibre recruitment [160,161,162]. This for example requires novel comparative studies on dose–response and stimulation strategies tailored towards informing clinical practice guideline development.
Finally, current limitations of the evidence base, which prohibited meaningful synthesis of the evidence using forest plots and meta-analysis, could be overcome by improving reporting quality and establishing standardized outcome measures for each outcome category. The relatively poor scores on the risk of bias assessments highlight the need for better description of randomization procedures, intervention protocols, control conditions, dropout rates, sample size calculations, effect sizes, confidence intervals, and incidence of adverse events, in accordance with international reporting standards [163,164,165]. Using a set of standardized outcome measures would enlarge the potential for a clinically relevant meta-analysis. Provision of data specific for subgroups with different levels of injury and impairment scales could help determine potential differences in effects among various groups of people with SCI.
Conclusion
The current evidence indicates that FES cycling exercise improves lower-body muscle health (e.g., muscle mass, fiber type composition) of adults with SCI, and may increase power output and aerobic fitness (e.g., peak power and oxygen uptake during an FES cycling test). The evidence summarized and appraised in this review can inform the development of the first international, evidence-based clinical practice guidelines for the use of FES cycling exercise in clinical and community settings of adults with SCI. Ultimately, these clinical practice guidelines help to shape lifelong rehabilitation care plans for the SCI population that fit national and local care contexts and resources.
Availability of data and materials
All data generated or analyzed during this study are included in this paper or in the online repository at https://osf.io/u9mvx/ including details on data extraction, risk of bias, grey literature search, search strings, and the full reference list of the 97 eligible articles.
Abbreviations
- AIS:
-
American Spinal Injury Association Impairment Scale
- BMD:
-
Bone mineral density
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- FES:
-
Functional electrical stimulation
- RCTs:
-
Randomized controlled trials
- SCI:
-
Spinal cord injury
References
Ho CH, Triolo RJ, Elias AL, Kilgore KL, DiMarco AF, Bogie K, et al. Functional electrical stimulation and spinal cord injury. Phys Med Rehabil Clin N Am. 2014;25(3):631–54.
van der Scheer JW, Martin Ginis KA, Ditor DS, Goosey-Tolfrey VL, Hicks AL, West CR, et al. Effects of exercise on fitness and health of adults with spinal cord injury: a systematic review. Neurology. 2017;89(7):736–45.
Kressler J, Ghersin H, Nash MS. Use of functional electrical stimulation cycle ergometers by individuals with spinal cord injury. Top Spinal Cord Inj Rehabil. 2014;20(2):123–6.
Ibitoye MO, Hamzaid NA, Hasnan N, Abdul Wahab AK, Davis GM. Strategies for rapid muscle fatigue reduction during FES exercise in individuals with spinal cord injury: a systematic review. PLoS ONE. 2016;11(2):e0149024.
Davis GM, Hamzaid NA, Fornusek C. Cardiorespiratory, metabolic, and biomechanical responses during functional electrical stimulation leg exercise: health and fitness benefits. Artif Organs. 2008;32(8):625–9.
Shariat A, Najafabadi MG, Ansari NN, Cleland JA, Singh MAF, Memari AH, et al. The effects of cycling with and without functional electrical stimulation on lower limb dysfunction in patients post-stroke: a systematic review with meta-analysis. NeuroRehabilitation. 2019;44(3):389–412.
Scally JB, Baker JS, Rankin J, Renfrew L, Sculthorpe N. Evaluating functional electrical stimulation (FES) cycling on cardiovascular, musculoskeletal and functional outcomes in adults with multiple sclerosis and mobility impairment: a systematic review. Mult Scler Relat Disord. 2019;37:101485.
Bekhet AH, Bochkezanian V, Saab IM, Gorgey AS. The effects of electrical stimulation parameters in managing spasticity after spinal cord injury: a systematic review. Am J Phys Med Rehabil. 2019;98(6):484–99.
Martin Ginis KA, van der Scheer JW, Latimer-Cheung AE, Barrow A, Bourne C, Carruthers P, et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord. 2018;56(4):308–21.
Jensen MP, Truitt AR, Schomer KG, Yorkston KM, Baylor C, Molton IR. Frequency and age effects of secondary health conditions in individuals with spinal cord injury: a scoping review. Spinal Cord. 2013;51(12):882–92.
Martin Ginis KA, Ma JK, Latimer-Cheung AE, Rimmer JH. A systematic review of review articles addressing factors related to physical activity participation among children and adults with physical disabilities. Health Psychol Rev. 2016;10(4):478–94.
Tremblay MS, Shephard RJ, Brawley LR. Research that informs Canada’s physical activity guides: an introduction. Can J Public Health. 2007;98(Suppl 2):S1-8.
World Health Organization. WHO handbook for guideline development, 2nd ed. Available at: https://apps.who.int/iris/handle/10665/145714. Accessed 30 Apr 2021. 2014.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–42.
Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6.
Qaseem A, Kansagara D, Lin JS, Mustafa RA, Wilt TJ. The development of clinical guidelines and guidance statements by the clinical guidelines committee of the American College of Physicians: update of methods. Ann Intern Med. 2019;170(12):863–70.
Alashram AR, Annino G, Mercuri NB. Changes in spasticity following functional electrical stimulation cycling in patients with spinal cord injury: a systematic review. J Spinal Cord Med. 2020. https://doi.org/10.1080/10790268.2020.1763713.
Farrow M, Nightingale TE, Maher J, McKay CD, Thompson D, Bilzon JLJ. Effect of exercise on cardiometabolic risk factors in adults with chronic spinal cord injury: a systematic review. Arch Phys Med Rehabil. 2020;101(12):2177–205.
Figoni SF, Dolbow DR, Crawford EC, White ML, Pattanaik S. Does aerobic exercise benefit persons with tetraplegia from spinal cord injury? A systematic review. J Spinal Cord Med. 2020. https://doi.org/10.1080/10790268.2020.1722935.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28(2):138–44.
Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31.
Quel de Oliveira C, Refshauge K, Middleton J, de Jong L, Davis GM. Effects of activity-based therapy interventions on mobility, independence, and quality of life for people with spinal cord injuries: a systematic review and meta-analysis. J Neurotrauma. 2017;34(9):1726–43.
Sadowsky CL, McDonald JW. Activity-based restorative therapies: concepts and applications in spinal cord injury-related neurorehabilitation. Dev Disabil Res Rev. 2009;15(2):112–6.
Higgins J, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, et al. A revised tool for assessing risk of bias in randomized trials In: Chandler J, McKenzie J, Boutron I, Welch V, editors. Cochrane Methods. Cochrane Database of Systematic Reviews. 2016. https://doi.org/10.1002/14651858.CD201601.
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–84.
Eng JJ, Teasell R, Miller WC, Wolfe DL, Townson AF, Aubut JA, et al. Spinal cord injury rehabilitation evidence: methods of the SCIRE systematic review. Top Spinal Cord Inj Rehabil. 2007;13(1):1–10.
Diener E, Suh EM, Lucas RE, Smith HL. Subjective well-being: three decades of progress. Psychol Bull. 1999;125(2):276.
Harvey LA, Lin CW, Glinsky JV, De Wolf A. The effectiveness of physical interventions for people with spinal cord injuries: a systematic review. Spinal Cord. 2009;47(3):184–95.
FDA. Food and Drug Administration. Paragraph 312.32. IND safety reporting. Available at: https://www.ecfr.gov/cgi-bin/text-idx?SID=49f68109df276fb151827f397ed1290b&mc=true&node=pt21.5.312&rgn=div5#se21.5.312_132. Accessed 30 Apr 2021. 2019.
Allison DJ, Thomas A, Beaudry K, Ditor DS. Effects of a functional electrical stimulation-assisted cycling program on immune and cardiovascular health in persons with spinal cord injury. Top Spinal Cord Inj Rehabil. 2016;22(1):71–8.
Andersen JL, Mohr T, Biering-Srensen F, Galbo H, Kjaer M. Myosin heavy chain isoform transformation in single fibres from m. vastus lateralis in spinal cord injured individuals: effects of long-term functional electrical stimulation (FES). Eur J Appl Physiol. 1996;431(4):513–8.
Arnold PB, McVey PP, Farrell WJ, Deurloo TM, Grasso AR. Functional electric stimulation: its efficacy and safety in improving pulmonary function and musculoskeletal fitness. Arch Phys Med Rehabil. 1992;73(800):665–8.
Ashe MC, Eng JJ, Krassioukov AV, Warburton DER, Hung C, Tawashy A. Response to functional electrical stimulation cycling in women with spinal cord injuries using dual-energy X-ray absorptiometry and peripheral quantitative computed tomography: a case series. J Spinal Cord Med. 2010;33(1):68–72.
Baldi JC, Jackson RD, Moraille R, Mysiw WJ. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord. 1998;36(7):463–9.
Barstow TJ, Scremin AM, Mutton DL, Kunkel CF, Cagle TG, Whipp BJ. Changes in gas exchange kinetics with training in patients with spinal cord injury. Med Sci Sports Exerc. 1996;28(10):1221–8.
BeDell KK, Ame S, Perell KI, Kunkel CF. Effects of functional electrical stimulation-induced lower extremity cycling on bone density of spinal cord-injured patients. Am J Phys Med Rehabil. 1996;75(1):29–34.
Berry HR, Kakebeeke TH, Donaldson N, Perret C, Hunt KJ. Energetics of paraplegic cycling: adaptations to 12 months of high volume training. Technol Health Care. 2012;20(2):73–84.
Berry HR, Perret C, Saunders BA, Kakebeeke TH, Donaldson NDN, Allan DB, et al. Cardiorespiratory and power adaptations to stimulated cycle training in paraplegia. Med Sci Sports Exerc. 2008;40(9):1573–80.
Bloomfield SA, Mysiw WJ, Jackson RD. Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone. 1996;19(1):61–8.
Bremner LA, Sloan KE, Day RE, Scull ER, Ackland T. A clinical exercise system for paraplegics using functional electrical stimulation. Paraplegia. 1992;30(9):647–55.
Chen S-C, Lai C-H, Chan WP, Huang M-H, Tsai H-W. Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil Rehabil. 2005;27(22):1337–41.
Chilibeck PD, Bell G, Jeon J, Weiss CB, Murdoch G, Ryan E, et al. Functional electrical stimulation exercise increases GLUT1 and GLUT4 in paralyzed skeletal muscle. Metabolism. 1999;48(11):1409–13.
Chilibeck PD, Jeon J, Weiss C, Bell G, Burnham R. Histochemical changes in muscle of individuals with spinal cord injury following functional electrical stimulated exercise training. Spinal Cord. 1999;37(4):264–8.
Corbin GN, Weaver K, Dolbow DR, Credeur D, Pattanaik S, Stokic DS. Safety and preliminary efficacy of functional electrical stimulation cycling in an individual with cervical cord injury, autonomic dysreflexia, and a pacemaker: case report. J Spinal Cord Med. 2019. https://doi.org/10.1080/10790268.2019.1692180.
Crameri RM, Cooper P, Sinclair PJ, Bryant G, Weston A. Effect of load during electrical stimulation training in spinal cord injury. Muscle Nerve. 2004;29(1):104–11.
Crameri RM, Weston A, Climstein M, Davis GM, Sutton JR. Effects of electrical stimulation-induced leg training on skeletal muscle adaptability in spinal cord injury. Scand J Med Sci Sports. 2002;12(5):316–22.
Crosbie J, Tanhoffer AIP, Fornusek C. FES assisted standing in people with incomplete spinal cord injury: a single case design series. Spinal Cord. 2014;52:251–4.
Demchak TJ, Linderman JK, Mysiw WJ, Jackson R, Suun J, Devor ST. Effects of functional electric stimulation cycle ergometry training on lower limb musculature in acute sci individuals. J Sports Sci Med. 2005;4(3):263–71.
Dolbow DR, Credeur DP. Effects of resistance-guided high intensity interval functional electrical stimulation cycling on an individual with paraplegia: a case report. J Spinal Cord Med. 2018;41(2):248–52.
Dolbow DR, Credeur DP, Lemacks JL, Stokic DS, Pattanaik S, Corbin GN, et al. Electrically induced cycling and nutritional counseling for counteracting obesity after spinal cord injury: a pilot study. J Spinal Cord Med. 2020. https://doi.org/10.1080/10790268.2019.1710939.
Dolbow DR, Gorgey AS, Cifu DX, Moore JR, Gater DR. Feasibility of home-based functional electrical stimulation cycling: case report. Spinal Cord. 2012;50(2):170–1.
Dolbow DR, Gorgey AS, Dolbow JD, Gater DR. Seat pressure changes after eight weeks of functional electrical stimulation cycling: a pilot study. Top Spinal Cord Inj Rehabil. 2013;19(3):222–8.
Dolbow DR, Gorgey AS, Gater DR, Moore JR. Body composition changes after 12 months of FES cycling: case report of a 60-year-old female with paraplegia. Spinal Cord. 2014;52(Suppl 1):S3-4.
Dolbow DR, Gorgey AS, Ketchum JM, Gater DR. Home-based functional electrical stimulation cycling enhances quality of life in individuals with spinal cord injury. Top Spinal Cord Inj Rehabil. 2013;19(4):324–9.
Dolbow DR, Gorgey AS, Ketchum JM, Moore JR, Hackett LA, Gater DR. Exercise adherence during home-based functional electrical stimulation cycling by individuals with spinal cord injury. Am J Phys Med Rehabil. 2012;91(11):922–30.
Dolbow DR, Gorgey AS, Khalil RK, Gater DR. Effects of a fifty-six month electrical stimulation cycling program after tetraplegia: case report. J Spinal Cord Med. 2017;40(4):485–8.
Dolbow DR, Gorgey AS, Moore JR, Gater DR. Report of practicability of a 6-month home-based functional electrical stimulation cycling program in an individual with tetraplegia. J Spinal Cord Med. 2012;35(3):182–6.
Donaldson N, Perkins TA, Fitzwater R, Wood DE, Middleton F. FES cycling may promote recovery of leg function after incomplete spinal cord injury. Spinal Cord. 2000;38(11):680–2.
Duffell LD, de Donaldson NN, Perkins TA, Rushton DN, Hunt KJ, Kakebeeke TH, et al. Long-term intensive electrically stimulated cycling by spinal cord-injured people: effect on muscle properties and their relation to power output. Muscle Nerve. 2008;38(4):1304–11.
Duffell LD, Paddison S, Alahmary AF, Donaldson N, Burridge J. The effects of FES cycling combined with virtual reality racing biofeedback on voluntary function after incomplete SCI: a pilot study. J Neuroeng Rehabil. 2019;16(1):149.
Duffell LD, Rowlerson AM, Donaldson NDN, Harridge SDR, Newham DJ. Effects of endurance and strength-directed electrical stimulation training on the performance and histological properties of paralyzed human muscle: a pilot study. Muscle Nerve. 2010;42(5):756–63.
Eser P, De Bruin ED, Telley I, Lechner HE, Knecht H. Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord-injured patients. Eur J Clin Invest. 2003;33(5):412–9.
Faghri PD, Glaser RM, Figoni SF. Functional electrical stimulation leg cycle ergometer exercise: training effects on cardiorespiratory responses of spinal cord injured subjects at rest and during submaximal exercise. Arch Phys Med Rehabil. 1992;73(11):1085–93.
Fattal C, Sijobert B, Daubigney A, Fachin-Martins E, Lucas B, Casillas J, et al. Training with FES-assisted cycling in a subject with spinal cord injury: psychological, physical, and physiological considerations. J Spinal Cord Med. 2018;43(3):402–13.
Fornusek C, Davis GM, Russold MF. Pilot study of the effect of low-cadence functional electrical stimulation cycling after spinal cord injury on thigh girth and strength. Arch Phys Med Rehabil. 2013;94(5):990–3.
Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, de Donaldson NN, et al. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone. 2008;43:169–76.
Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, Eser P. Effect of detraining on bone and muscle tissue in subjects with chronic spinal cord injury after a period of electrically-stimulated cycling: a small cohort study. J Rehabil Med. 2009;41:282–5.
Galea MP, Panisset MG, El-Ansary G, Dunlop SA, Marshall R, Clark JM, et al. SCIPA switch-on: a randomized controlled trial investigating the efficacy and safety of functional electrical stimulation-assisted cycling and passive cycling initiated early after traumatic spinal cord injury. Neurorehabil Neural Repair. 2017;31(6):540–51.
Gerrits HL, de Haan A, Sargeant AJ, Dallmeijer A, Hopman MT. Altered contractile properties of the quadriceps muscle in people with spinal cord injury following functional electrical stimulated cycle training. Spinal Cord. 2000;38(4):214–23.
Gerrits HLHLL, de Haan A, Sargeant AJ, van Langen H, Hopman MT. Peripheral vascular changes after electrically stimulated cycle training in people with spinal cord injury. Arch Phys Med Rehabil. 2001;82(6):832–9.
Gill S, Adler J, Khalil RE, Gorgey AS. Attenuation of autonomic dysreflexia during functional electrical stimulation cycling by neuromuscular electrical stimulation training: case reports. Spinal Cord Ser Cases. 2020;6(1):12.
Gorgey AS, Graham ZA, Bauman WA, Cardozo C, Gater DR. Abundance in proteins expressed after functional electrical stimulation cycling or arm cycling ergometry training in persons with chronic spinal cord injury. J Spinal Cord Med. 2017;40(4):439–48.
Gorgey AS, Harnish CR, Daniels JA, Dolbow DR, Keeley A, Moore J, et al. A report of anticipated benefits of functional electrical stimulation after spinal cord injury. J Spinal Cord Med. 2012;35(2):107–12.
Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol. 2009;19(4):614–22.
Guimaraes JA, da Fonseca LO. Towards parameters and protocols to recommend FES-Cycling in cases of paraplegia: a preliminary report. Eur J Transl Myol. 2016;26(3):209–14.
Gurney AB, Robergs RA, Aisenbrey J, Cordova JC, McClanahan L. Detraining from total body exercise ergometry in individuals with spinal cord injury. Spinal Cord. 1998;36(11):782–9.
Hangartner TN, Rodgers MM, Glaser RM, Barre PS. Tibial bone density loss in spinal cord injured patients: effects of FES exercise. J Rehabil Res Dev. 1994;31(1):50–61.
Hjeltnes N, Aksnes AK, Birkeland KI, Johansen J, Lannem A, Wallberg-Henriksson H. Improved body composition after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol. 1997;273(3 Pt 2):R1072–9.
Hjeltnes N, Galuska D, Bjornholm M, Aksnes AK, Lannem A, Zierath JR, et al. Exercise-induced overexpression of key regulatory proteins involved in glucose uptake and metabolism in tetraplegic persons: molecular mechanism for improved glucose homeostasis. FASEB J. 1998;12(15):1701–12.
Hooker SP, Figoni SF, Rodgers MM, Glaser RM, Mathews T, Suryaprasad AG, et al. Physiologic effects of electrical stimulation leg cycle exercise training in spinal cord injured persons. Arch Phys Med Rehabil. 1992;73(5):470–6.
Hooker SP, Scremin AME, Mutton DL, Kunkel CF. Peak and submaximal physiologic responses following electrical stimulation leg cycle ergometer training. J Rehabil Res Dev. 1995;32(4):361–6.
Janssen TWJ, Pringle DD. Effects of modified electrical stimulation-induced leg cycle ergometer training for individuals with spinal cord injury. J Rehabil Res Dev. 2008;45(6):819–30.
Jeon JY, Weiss CB, Steadward RD, Ryan E, Burnham RS, Bell G, et al. Improved glucose tolerance and insulin sensitivity after electrical stimulation-assisted cycling in people with spinal cord injury. Spinal Cord. 2002;40(3):110–7.
Johnston TE, Marino RJ, Oleson CV, Schmidt-Read M, Leiby BE, Sendecki J, et al. Musculoskeletal effects of 2 functional electrical stimulation cycling paradigms conducted at different cadences for people with spinal cord injury: a pilot study. Arch Phys Med Rehabil. 2016;97(9):1413–22.
Johnston TE, Marino RJ, Oleson CV, Schmidt-Read M, Modlesky CM. Cycling with functional electrical stimulation before and after a distal femur fracture in a man with paraplegia. Top Spinal Cord Inj Rehabil. 2015;21(4):275–81.
Kahn NN, Feldman SP, Bauman WA. Lower-extremity functional electrical stimulation decreases platelet aggregation and blood coagulation in persons with chronic spinal cord injury: a pilot study. J Spinal Cord Med. 2010;33(2):150–8.
Kakebeeke TH, Hofer PJ, Frotzler A, Lechner HE, Hunt KJ, Perret C. Training and detraining of a tetraplegic subject: high-volume FES cycle training. Am J Phys Med Rehabil. 2008;87(1):56–64.
Kjær M, Mohr T, Biering-Srensen F, Bangsbo J. Muscle enzyme adaptation to training and tapering-off in spinal-cord-injured humans. Eur J Appl Physiol. 2001;84(5):482–6.
Krauss JC, Robergs RA, Depaepe JL, Kopriva LM, Aisenbury JA, Anderson MA, et al. Effects of electrical stimulation and upper body training after SCI. Med Sci Sports Exerc. 1993;25(9):1054–61.
Kuhn D, Leichtfried V, Schobersberger W. Four weeks of functional electrical stimulated cycling after spinal cord injury: a clinical cohort study. Int J Rehabil Res. 2014;37(3):243–50.
Lai C-H, Chang WH-S, Chan WP, Peng C-W, Shen L-K, Chen J-JJ, et al. Effects of functional electrical stimulation cycling exercise on bone mineral density loss in the early stages of spinal cord injury. J Rehabil Med. 2010;42(2):150–4.
Lammers G, van Duijnhoven NT, Hoenderop JG, Horstman AM, de Haan A, Janssen TW, et al. The identification of genetic pathways involved in vascular adaptations after physical deconditioning versus exercise training in humans. Exp Physiol. 2013;98(3):710–21.
Liu C-WW, Chen S-CC, Chen C-HH, Chen T-WW, Chen J-JJJ, Lin C-SS, et al. Effects of functional electrical stimulation on peak torque and body composition in patients with incomplete spinal cord injury. Kaohsiung J Med Sci. 2007;23(5):232–40.
Mazzoleni S, Battini E, Rustici A, Stampacchia G. An integrated gait rehabilitation training based on Functional Electrical Stimulation cycling and overground robotic exoskeleton in complete spinal cord injury patients: preliminary results. IEEE Int Conf Rehabil Robot. 2017;2017:289–93.
Mazzoleni S, Stampacchia G, Gerini A, Tombini T, Carrozza MC. FES-cycling training in spinal cord injured patients. Annu Int Conf IEEE Eng Med Biol Soc. 2013;2013:5339–41.
Mohr T, Andersen JL, Biering-Sorensen F, Galbo H, Bangsbo J, Wagner A, et al. Long-term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord. 1997;35(1):1–16.
Mohr T, Dela F, Handberg A, Bierling-Sorenson F, Galbo H, Kjaer M. Insulin action and long-term electrically induced training in individuals with spinal cord injuries. Med Sci Sports Exerc. 1999;33:1247–52.
Mohr T, Podenphant J, Biering-Sorensen F, Galbo H, Thamsborg G, Kjaer M. Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int. 1997;61(1):22–5.
Mutton DL, Scremin AM, Barstow TJ, Scott MD, Kunkel CF, Cagle TG. Physiologic responses during functional electrical stimulation leg cycling and hybrid exercise in spinal cord injured subjects. Arch Phys Med Rehabil. 1997;78(7):712–8.
Nash MS, Bilsker S, Marcillo AE, Isaac SM, Botelho LA, Klose KJ, et al. Reversal of adaptive left ventricular atrophy following electrically-stimulated exercise training in human tetraplegics. Paraplegia. 1991;29:590–9.
Nash MS, Montalvo BM, Applegate B. Lower extremity blood flow and responses to occlusion and sedentary. Arch Phys Med Rehabil. 1996;77(December):1260–5.
Pacy PJ, Evans RH, Halliday D. Effect of anaerobic and aerobic exercise promoted by computer regulated functional electrical stimulation (FES) on muscle size, strength and histology in paraplegic males. Prosthet Orthot Int. 1987;11(2):75–9.
Pacy PJ, Hesp R, Halliday DA, Katz D, Cameron G, Reeve J. Muscle and bone in paraplegic patients, and the effect of functional electrical stimulation. Clin Sci (Lond). 1988;75(5):481–7.
Panisset MG, El-Ansary D, Dunlop SA, Marshall R, Clark J, Churilov L, et al. Factors influencing thigh muscle volume change with cycling exercises in acute spinal cord injury—a secondary analysis of a randomized controlled trial. J Spinal Cord Med. 2020. https://doi.org/10.1080/10790268.2020.1815480.
Petrofsky JS, Laymon M. The effect of previous weight training and concurrent weight training on endurance for functional electrical stimulation cycle ergometry. Eur J Appl Physiol. 2004;91(4):392–8.
Petrofsky JS, Stacy R. The effect of training on endurance and the cardiovascular responses of individuals with paraplegia during dynamic exercise induced by functional electrical stimulation. Eur J Appl Physiol Occup Physiol. 1992;64(6):487–92.
Phillips CA, Danopulos D, Kezdi P, Hendershot D. Muscular, respiratory and cardiovascular responses of quadriplegic persons to an FES bicycle ergometer conditioning. Int J Rehab Res. 1989;12(2):147–57.
Phillips CA, Petrofsky JS, Hendershot DM, Stafford D. Functional electrical exercise: a comprehensive approach for physical conditioning of the spinal cord injured patient. Orthopedics. 1984;7(7):1112–23.
Pollack SF, Spielholz N, Haas F, Ragnarsson KT. Induced lower extremity exercises in spinal cord injured people. Arch Phys Med Rehabil. 1989;70(March):214–9.
Ragnarsson KT, Pollack SP, O’Daniel W Jr, Edgar R, Petrofsky J, Nash MS. Clinical evaluation of computerized functional electrical stimulation after spinal cord injury: a multicenter pilot study. Arch Phys Med Rehabil. 1988;69(October):672–7.
Ralston KE, Harvey LA, Batty J, Lee BB, Ben M, Cusmiani R, et al. Functional electrical stimulation cycling has no clear effect on urine output, lower limb swelling, and spasticity in people with spinal cord injury: a randomised cross-over trial. J Physiother. 2013;59(4):237–43.
Rayegani SM, Shojaee H, Sedighipour L, Soroush MR, Baghbani M, Amirani OoB. The effect of electrical passive cycling on spasticity in war veterans with spinal cord injury. Front Neurol. 2011;20(2:39):1–7.
Reichenfelser W, Hackl H, Hufgard J, Kastner J, Gstaltner K, Gfohler M. Monitoring of spasticity and functional ability in individuals with incomplete spinal cord injury with a functional electrical stimulation cycling system. J Rehabil Med. 2012;44(5):444–9.
Robergs RA, Appenzeller O, Qualls C, Ausenbrey J, Krauss J, Kopriva L, et al. Increased endothelin and creatine kinase after electrical stimulation of paraplegic muscle. J Appl Physiol. 1985;75(6):2400–5.
Sadowsky CL, Hammond ER, Strohl AB, Commean PK, Eby SA, Damiano DL, et al. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J Spinal Cord Med. 2013;36(6):623–31.
Scremin AM, Kurta L, Gentili A, Wiseman B, Perell K, Kunkel C, et al. Increasing muscle mass in spinal cord injured persons with a functional electrical stimulation exercise program. Arch Phys Med Rehabil. 1999;80(12):1531–6.
Sijobert BB, Fattal C, Daubigney A, Azevedo-Coste C. Participation to the first Cybathlon: an overview of the FREEWHEELS team FES-cycling solution. Eur J Transl Myol. 2017;27(4):265–71.
Skold C, Lonn L, Harms-Ringdahl K, Hultling C, Levi R, Nash M, et al. Effects of functional electrical stimulation training for six months on body composition and spasticity in motor complete tetraplegic spinal cord-injured individuals. J Rehabil Med. 2002;34(1):25–32.
Sloan KE, Bremner LA, Byrne J, Day RE, Scull ER. Musculoskeletal effects of an electrical stimulation induced cycling programme in the spinal injured. Paraplegia. 1994;32(6):407–15.
Stampacchia G, Olivieri M, Rustici A, D’Avino C, Gerini A, Mazzoleni S. Gait rehabilitation in persons with spinal cord injury using innovative technologies: an observational study. Spinal Cord. 2020;58(9):988–97.
Thrasher TA, Ward JS, Fisher S. Strength and endurance adaptations to functional electrical stimulation leg cycle ergometry in spinal cord injury. NeuroRehabilitation. 2013;33(1):133–8.
Tong RKY, Wang X, Leung KWC, Lee GTY, Lau CCY, Wai HW, et al. How to prepare a person with complete spinal cord injury to use surface electrodes for FES trike cycling. IEEE Int Conf Rehabil Robot. 2017;2017:801–5.
Twist DJ, Culpepper-Morgan JA, Ragnarsson KT, Petrillo CR, Kreek MJ. Neuroendocrine changes during functional electrical stimulation. Am J Phys Med Rehabil. 1992;71(3):156–63.
Van Duijnhoven N, Hesse E, Janssen T, Wodzig W, Scheffer P, Hopman M. Impact of exercise training on oxidative stress in individuals with a spinal cord injury. Eur J Appl Physiol. 2010;109(6):1059–66.
Yasąr E, Ylmaz B, Gktepe S, Kesikburun S. The effect of functional electrical stimulation cycling on late functional improvement in patients with chronic incomplete spinal cord injury. Spinal Cord. 2015;53(12):866–9.
Zbogar D, Eng JJ, Krassioukov AV, Scott JM, Esch BTA, Warburton DER. The effects of functional electrical stimulation leg ergometry training on arterial compliance in individuals with spinal cord injury. Spinal Cord. 2008;46(11):722–6.
Belavy DL, Miokovic T, Rittweger J, Felsenberg D. Estimation of changes in volume of individual lower-limb muscles using magnetic resonance imaging (during bed-rest). Physiol Meas. 2011;32(1):35–50.
Biering-Sorensen B, Kristensen IB, Kjaer M, Biering-Sorensen F. Muscle after spinal cord injury. Muscle Nerve. 2009;40(4):499–519.
Castro MJ, Apple DF Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80(4):373–8.
Smit CA, de Groot S, Stolwijk-Swuste JM, Janssen TW. Effects of electrical stimulation on risk factors for developing pressure ulcers in people with a spinal cord injury: a focused review of literature. Am J Phys Med Rehabil. 2016;95(7):535–52.
Buchholz AC, Pencharz PB. Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care. 2004;7(6):635–9.
Bailey KA, Gammage KL, van Ingen C, Ditor DS. Managing the stigma: exploring body image experiences and self-presentation among people with spinal cord injury. Health Psychol Open. 2016;3(1):2055102916650094.
Burnham R, Martin T, Stein R, Bell G, MacLean I, Steadward R. Skeletal muscle fibre type transformation following spinal cord injury. Spinal Cord. 1997;35(2):86–91.
Thijssen DH, Heesterbeek P, van Kuppevelt DJ, Duysens J, Hopman MT. Local vascular adaptations after hybrid training in spinal cord-injured subjects. Med Sci Sports Exerc. 2005;37(7):1112–8.
Gregory CM, Dixon W, Bickel CS. Impact of varying pulse frequency and duration on muscle torque production and fatigue. Muscle Nerve. 2007;35(4):504–9.
Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–8.
World Health Organization. WHO global recommendations on physical activity for health. Available at: http://www.who.int/dietphysicalactivity/factsheet_recommendations/en/. Accessed 30 Apr 2021. 2010.
Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, et al. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43(7):408–16.
Cragg JJ, Noonan VK, Krassioukov A, Borisoff J. Cardiovascular disease and spinal cord injury: results from a national population health survey. Neurology. 2013;81(8):723–8.
Sachdeva R, Gao F, Chan CCH, Krassioukov AV. Cognitive function after spinal cord injury: a systematic review. Neurology. 2018;91(13):611–21.
Phillips AA, Ainslie PN, Krassioukov AV, Warburton DE. Regulation of cerebral blood flow after spinal cord injury. J Neurotrauma. 2013;30(18):1551–63.
Petitti DB, Teutsch SM, Barton MB, Sawaya GF, Ockene JK, DeWitt T. Update on the methods of the U.S. Preventive Services Task Force: insufficient evidence. Ann Intern Med. 2009;150(3):199–205.
Martin Ginis KA, Hicks AL. Exercise research issues in the spinal cord injured population. Exerc Sport Sci Rev. 2005;33(1):49–53.
Andrews JC, Schunemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013;66(7):726–35.
Cochrane. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). Available at: http://handbook.cochrane.org/chapter_9/9_4_11_use_of_vote_counting_for_meta_analysis.htm. Accessed 30 Apr 2021. 2011.
Battistuzzo CR, Callister RJ, Callister R, Galea MP. A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J Neurotrauma. 2012;29(8):1600–13.
Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45(3):190–205.
Cragg JJ, Noonan VK, Dvorak M, Krassioukov A, Mancini GB, Borisoff JF. Spinal cord injury and type 2 diabetes: results from a population health survey. Neurology. 2013;81(21):1864–8.
Adriaansen JJ, Ruijs LE, van Koppenhagen CF, van Asbeck FW, Snoek GJ, van Kuppevelt D, et al. Secondary health conditions and quality of life in persons living with spinal cord injury for at least ten years. J Rehabil Med. 2016;48(10):853–60.
Piatt JA, Nagata S, Zahl M, Li J, Rosenbluth JP. Problematic secondary health conditions among adults with spinal cord injury and its impact on social participation and daily life. J Spinal Cord Med. 2016;39(6):693–8.
Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103(3):249–57.
Post MW, van Leeuwen CM. Psychosocial issues in spinal cord injury: a review. Spinal Cord. 2012;50(5):382–9.
Martin Ginis KA, Jetha A, Mack DE, Hetz S. Physical activity and subjective well-being among people with spinal cord injury: a meta-analysis. Spinal Cord. 2010;48(1):65–72.
Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol. 2008;586(20):5003–12.
Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, Donaldson NN, et al. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone. 2008;43(1):169–76.
Williamson P, Altman D, Blazeby J, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13(1):132.
Kayagil TA, Grimes JP, Grill WM. Mechanisms underlying reversal of motor unit activation order in electrically evoked contractions after spinal cord injury. Muscle Nerve. 2008;37(2):210–8.
Thomas CK, Nelson G, Than L, Zijdewind I. Motor unit activation order during electrically evoked contractions of paralyzed or partially paralyzed muscles. Muscle Nerve. 2002;25(6):797–804.
Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther. 2005;85(4):358–64.
Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869.
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687.
CONSORT. Extensions of the CONSORT Statement. Available at: http://www.consort-statement.org/extensions. Accessed 30 Apr 2021.
Acknowledgements
We thank Dr Christof Leicht (School of Sport, Exercise and Health Sciences, Loughborough University), Dr Emily Dunford (Department of Kinesiology, McMaster University), Dr Jasmin Ma (Health and Exercise Sciences, University of British Columbia), David Ankomah (Faculty of Human Movement Sciences, VU University) and Richard Xiang (Faculty of Medicine, University of British Columbia) for their contributions to study eligibility screening, data extraction, and study quality appraisal.
Funding
The study was financed by the University of Alberta Spinal Cord Injury Research Chair Endowment Funds and delivered in collaboration with Loughborough University. The study was not industry-sponsored. JWvdS is based in The Healthcare Improvement Studies Institute (THIS Institute), University of Cambridge. THIS Institute is supported by the Health Foundation, an independent charity committed to bringing about better health and healthcare for people in the UK.
Author information
Authors and Affiliations
Contributions
JWvdS: Study concept and design, data collection, analysis and interpretation of data, drafted the manuscript for intellectual content. VLG-T: Analysis and interpretation of data, revised the manuscript for intellectual content. SEV: Data collection, revised the manuscript for intellectual content. GMD: Analysis and interpretation of data, revised the manuscript for intellectual content. CHH: Study concept and design, analysis and interpretation of data, revised the manuscript for intellectual content. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
van der Scheer, J.W., Goosey-Tolfrey, V.L., Valentino, S.E. et al. Functional electrical stimulation cycling exercise after spinal cord injury: a systematic review of health and fitness-related outcomes. J NeuroEngineering Rehabil 18, 99 (2021). https://doi.org/10.1186/s12984-021-00882-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12984-021-00882-8