Abstract
Background
The maintenance of species and the promotion of speciation are closely related to chromosomal rearrangements throughout evolution. Decapoda represents the most species-rich order among crustaceans and, despite its ecological and economic importance, little is known about decapod karyology. We aim at cytogenetically characterizing two sympatric prawn species.
Results
Analysis of mitotic metaphases and meiotic diakinesis of the common prawn Palaemon serratus and the rockpool prawn P. elegans, revealed considerable differences between their karyotypes including chromosome numbers and sex determination systems. The cytogenetic data for P. serratus showed a diploid number of 56 and the putative absence of heteromorphic sex chromosomes. However, the diploid chromosome number in P. elegans was 90 for females and 89 for males. The karyotype of the females consisted of the three largest acrocentric pairs and 42 submetacentric and metacentric pairs, while the karyotype of the males comprised a clearly identifiable large metacentric chromosome and two acrocentric pairs as well as the smaller 42 pairs. These results highlight the presence of the X1X1X2X2/X1X2Y multiple sex chromosome system in P. elegans, which constitute the only sexual system for Decapoda reported cytogenetically using modern techniques. The origin of this sex chromosome system is discussed. We hypothesize that the chromosome evolution within the genus could involve several fusion events giving rise to a reduction on the chromosome number in P. serratus. In both species, the major ribosomal genes were located in two chromosome pairs and hybridization signals of the telomeric sequences (TTAGGG)n were visualized at the telomeres of all chromosomes. C-banding revealed that, when present, constitutive heterochromatin had a predominantly telomeric distribution and no centromeric constitutive heterochromatin was observed.
Conclusions
Although more comparative cytogenetic analyses are needed to clarify our hypotheses, the findings of this work indicate that the prawns of the genus Palaemon represent a promising model among Decapoda representatives to investigate the karyotype evolution and the patterns of sex chromosome differentiation.
Similar content being viewed by others
Background
Decapoda is the most species-rich order within Crustacea. This extremely diverse group plays a key role in the aquatic trophic relationships [1, 2] and many of these species have a significant commercial importance since they are exploited for human consumption in different countries around the world [3, 4]. However, despite the importance of this group, the limited knowledge of decapod crustacean karyology constitutes an obstacle to elucidate different modes of sex determination, the occurrence of chromosomal rearrangements along their evolution or clarify phylogenetic relationships between related species. To our knowledge, during the last 25 years karyological data have only been reported in 46 species of decapods belonging to 10 families (for a review, see [5]). This scarcity of studies is mostly caused by decapod chromosomes peculiarities, usually small-size, numerous and highly condensed [6].
The family Palaemonidae comprises 981 species [7] of which only 13 belonging to three genera (Palaemon, Exopalaemon and Macrobrachium) have been studied at the cytogenetic level. These species show a wide karyotypic diversity and remarkable differences in their diploid chromosome number (Table 1). The existence of sex chromosomes was never determined cytogenetically in any species of the genera of Palaemonidae family and only rarely in Decapoda.
The genus Palaemon Weber, 1795 (Crustacea: Decapoda) is a group of caridean prawns of the family Palaemonidae. Recently, phylogenetic and taxonomic revisions changed the status of the genus Palaemon [8,9,10,11] as well as the number of its species. The genus Palaemon currently comprises 86 species, two of which have been recently described (Palaemon minos sp. nov. and Palaemon colossus sp. nov.) [10].
The selected species, the common prawn P. serratus and the rockpool prawn P. elegans, have a wide geographical distribution from the North Sea to Mauritania and Namibia, respectively, including the Mediterranean and Black Seas [12, 13]. These species differ in physiology, life history strategies and larval development [14,15,16]. They are both marine prawns, but whereas P. serratus inhabits estuaries in the reproductive season, P. elegans is common in tidal rockpools, Zostera, Posidonia and Cymodocea meadows and it also can be found in slightly brackish water close to river mouths [17].
Whilst the species are morphologically similar, it is unknown whether they share chromosome number and morphology. The karyotype of P. serratus was recently described. In our previous study, the karyotype of P. serratus was described [5].
Here, we aim at: (i) extending the previous knowledge on the cytogenetics of P. serratus; (ii) providing the first karyological data for P. elegans and compare them with what is known about P. serratus and (iii) identifying their sex chromosome systems. For this purpose we have studied the mitotic and meiotic chromosomes of both species and applied conventional staining and banding techniques, fluorescence in situ hybridization (FISH) with 18S–5.8S-28S rDNA and telomeric (TTAGGG)n, (TTAGG)n and (TAACC)n probes.
Methods
Biological material and chromosome preparation
Specimens of P. serratus and P. elegans used in this study were collected from the Artabro Gulf (43° 25′N, 8°20′W) in the northwest of Spain. Animals were captured with a fish trap and carried alive to the laboratory. Animals were kept at 18 °C in an aerated aquarium and fed with frozen brine shrimp for 24 h. Individuals were sorted into species [13] and the sex was determined by the presence (in males) or absence (in females) of the masculine appendix on the endopodite of the second pleopod [18]. Metaphase chromosome spreads were obtained according to previously described protocol [5]. Briefly, adult shrimps were injected at the epimeral line with 0.005% colchicine solution (5 μl/g body weight) 3–5 h before anesthetization by exposure to ethyl ether. Cefalothorax content (including gonad, circulatory tissue, digestive tissue and muscular tissue) was removed from each individual and then immersed into a hypotonic solution of 0.56% KCl for 10 min at room temperature. The tissue was then fixed four times in freshly prepared ethanol/glacial acetic acid (3:1) for 20 min each time at 4 °C, followed by overnight incubation in a fresh fixative at 4 °C. The following day a piece of about 3 mm of the heterogeneous fixed material was dissolved in 45% acetic acid and a cell suspension was obtained. Then, 4–5 drops of this suspension were pipetted onto pre-heated slides at 43 °C and air-dried.
Chromosome staining and fluorescence in situ hybridization
The slides were stained with anti-fade medium Vectashield (Vector Laboratories) containing 1.5 μL/mL 4′, 6-diamidino-2-phenylindole (DAPI). C-banding was performed on metaphase plates following Sumner [19].
To locate the position and number of the 18S–5.8S-28S rDNA sites we used the DNA probe pDm 238 from Drosophila melanogaster [20] labeled with FITC by using Prime-It Fluor fluorescence labeling kit (Stratagene) following the manufacturer’s instructions.
Chromosome mapping of the telomeric sequences was carried out using a (TTAGGG)n Cy3-labeled pan-telomeric probe (Cambio) according to the instructions of the manufacturer; a PCR generated pentanucleotide (TTAGG)n repeat according to Ijdo et al. [21] labeled with rhodamine-dUTP and the (TAACC)2 probe was synthesized and directly 5′ labeled with Cy3 (Isogen Life Science).
In situ hybridization was performed as described in González-Tizón et al. [22] with minor pre-hybridization and post-hybridization modifications. The slides were pretreated with DNAse-free RNAse (100 μg/mL in 2 x SSC) for 30 min at 37 °C, washed in 2 x SSC for 5 min and dehydrated in a graded ethanol series. Post-hybridization washes consisted of two 5-min incubations in 2 × SSC at 37 °C and at room temperature, respectively, followed by a 5-min incubation wash in 0.1 M Tris, 0.15 M NaCl and 0.05% Tween-20 at room temperature. Chromosomes were counterstained with 40 μL of anti-fade medium Vectashield containing 1.5 μL/mL DAPI.
Images were captured using a Nikon Microphot-FXA epifluorescence microscope equipped with a Nikon DS-Qi1Mc digital camera and processed with the NIS-Elements D 3.10 software.
The cytogenetic analyses described above were performed on P. serratus and P. elegans with the exception of the 45S rDNA chromosomal location in P. serratus, characterized in a previous work [5].
Results
Karyotypes, heterochromatin distribution and Fluorochrome staining
Mitotic and meiotic metaphases were obtained from 18 P. elegans specimens (8 females and 10 males) and 10 P. serratus specimens (6 females and 4 males). At least 15 metaphases per individual were observed, specifically 126 in P. elegans females, 153 in P. elegans males, 92 in P. serratus females and 31 in P. serratus males.
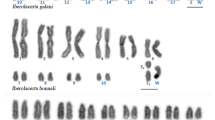
The diploid chromosome number in P. elegans was 90 for females and 89 for males (Fig. 1a, b; Table 1). The karyotype consisted of 43 autosomal chromosome pairs: 5 metacentric/submetacentric, 4 subtelocentric/telocentric, and 34 hardly distinguishable due to size similarities (Fig. 2). The karyotype of the females also included two large telocentric sex chromosome pairs (Fig. 2a), while that of the males included one clearly identifiable large metacentric chromosome and two telocentric chromosomes (Fig. 2b). Thus, male heterogamety is evidenced by a metacentric chromosome present only in the male karyotype (Y chromosome) which is the largest element of the complement. During meiotic diakinesis, each arm of the large metacentric Y is terminally associated with one acrocentric chromosome (X1 and X2) forming a trivalent (X1X2Y, Fig. 1d). Therefore, in diakinetic plates males exhibited 43 autosomal bivalents and one sex trivalent while females showed 45 undistinguished bivalents (Fig.1d, c).
In P. serratus, the karyotype was identical to that previously described (2n = 56) [5]. At meiotic diakinesis 28 bivalents in both sexes were observed (Fig. 1e).
Fluorochrome staining with DAPI revealed bright centromeric/pericentromeric AT-rich blocks on all chromosomes in P. elegans and P. serratus (Fig. 1) whereas interstitial bands were observed on the four largest chromosomes of P. serratus. In P. elegans chromosomes DAPI-bands were noticed in some terminal regions, always weaker than those found at the centromeres. We also detected large telomeric DAPI faint segments in a few chromosomes.
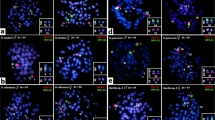
C-banding revealed that, when present, constitutive heterochromatin had a predominantly telomeric distribution in both species of Palaemon (Fig. 3a, b). Furthermore, no centromeric constitutive heterochromatin was observed. A large heterochromatic block was also found in the telomeres of four small size chromosomes in both species. In P. elegans the two X chromosomes and the Y chromosome were C-negative (Fig. 3a). In P. serratus, small weak bands of heterochromatin were also localized in interstitial positions of the large metacentric chromosomes. Slides containing C-banded chromosomes were previously stained with DAPI (Fig. 3c, d).
C-banded plates of (a) meiotic diakinesis of P. elegans and (b) mitotic metaphase of P. serratus males. Single arrows show C-band blocks, double arrow shows the sex trivalent (c, d) The same meiotic diakinesis of P. elegans and mitotic metaphase of P. serratus males, stained with DAPI. Chromosomal localization of the 18S–5.8S-28S rDNA genes of (e) P. elegans male and (f) female. Chromosomal localization of the (TTAGG)n telomeric sequences in (g) P. elegans male and (h) P. serratus male. Asterisks in a and c indicate the sex trivalent. The bar equals 10 μm
Chromosomal mapping of the 18S–5.8S-28S rDNA genes
In situ hybridization of the 18S–5.8S-28S rDNA genes on meiotic chromosomes of both sexes of P. elegans revealed four sites of probe hybridization (Fig. 3e, f). The rDNA probe mapped the free telomeres of two bivalents paired at one end (dumbbell-shape bivalents). Both 18S–5.8S-28S rDNA-bearing chromosome pairs were heteromorphic showing different hybridization intensity of the homologous chromosomes. FISH signals coincided with the heterochromatic blocks observed.
Chromosomal location of the telomeric probes
In situ hybridization of the (TTAGGG)n, (TTAGG)n and the (TAACC)n telomeric sequences were made in P. serratus and P. elegans. No hybridization signals were detected with the (TTAGGG)n or the (TAACC)n probes while FISH with the (TTAGG)n pentanucleotide repeat produced discrete fluorescence signals at the telomeres of all chromosomes in P. serratus and in all the diakinetic bivalents in P. elegans (Fig. 3g, h).
Discussion
Chromosome number and karyotypes
The diploid chromosome number obtained in this study for P. elegans falls within the range of the published chromosome numbers in other members of the family Palaemonidae, with P. serratus displaying the lowest number in the family (2n = 56).
The lack of cytogenetic studies in other members of the genus Palaemon hinders the definition of clear trends in karyotype evolution in these species. However, some evidence supports the hypothesis that the chromosome evolution within the genus could involve several fusion events giving rise to a reduction on the chromosome number in P. serratus: i) We observed interstitial DAPI-bright bands on the large metacentric chromosomes of P. serratus, being DAPI-positive bands that are characteristic of centromeric regions in both Palaemon species, as observed in other families of decapods such as Astacidae [23, 24], Cambaridae [25], Nephropidae [26], Scyllaridae [27] and Palinuridae [28]. ii) The presence of interstitial C-bands on these chromosomes may represent a chromosome fusion event. In general, decapod species on which this technique has been performed to date showed positive C-bands at the centromeres of almost all chromosomes (e.g. [29,30,31,32]), with the only exception of P. serratus and P. elegans wherein heterochromatin is located, mainly, in the telomeres. Macgregor and Sessions [29] postulated that the heterochromatin expansion is originated in the centromeres and then is dispersed towards the telomeres. Hence, according to this theory, dispersed distributions of heterochromatin (interstitial or telomeric) have an older phylogenetic status. Iii) Recent molecular phylogenetic studies have suggested that genus Exopalaemon should be included within Palaemon [8, 33]. Among Exopalaemon, karyological analysis of E. modestus and E. carinicauda, have shown a diploid chromosome number of 90 [34, 35]. More recently, the determination of the Palaemon khori karyotype was performed showing 2n = 96 [36].
In the light of our results, the phylogeny and the chromosome numbers found in the family Palaemonidae (Table 1), it seems likely that the high chromosome number detected represents the ancestral condition in this lineage whereas the reduced chromosome number of 2n = 56 observed in P. serratus constitutes a derived character. According to that, it seems plausible that the fusions constitute the main mechanism responsible for the origin of the P. serratus karyotype, which was also suggested for Astacidae and Parastacidae among Decapoda [24]. Further cytogenetic studies are still necessary in order to determine the mechanisms underlying the karyotype evolution in this group of species.
Ribosomal loci
As previously reported in P. serratus [5], P. elegans revealed four sites of 18S–5.8S-28S rDNA probe hybridization corresponding to two loci. Given the divergence observed between both karyotypes, this may constitute a plesiomorphic condition for genus Palaemon. In all cases, the ribosomal clusters were located in terminal positions on two small chromosome pairs. In addition, conspicuous heterochromatin blocks were located in the major ribosomal genes sites, closely related to large telomeric DAPI faint segments, highlighting the rDNA GC-richness as reported for a wide variety of organisms (e.g. [37] and references therein).
Moreover, in P. elegans both rDNA-bearing chromosome pairs showed heteromorphism in size of the 18S–5.8S-28S rDNA locus between homologous as observed in males of some species of the Astacidae [23, 24]. Mlinarec et al. [24] have speculated from these findings that the heteromorphic chromosome pair could represent male sex chromosomes suggesting the presence of an XX-XY sex determination system, even though the karyological characterization of females is a pending issue. Conversely, our results show that in P. elegans the heteromorphic rDNA-bearing chromosome pairs correspond to autosomes, which have been reported for many animal groups (e.g. [38,39,40,41]).
Telomeric repeats
This study shows for the first time the presence of the TTAGG repeat, known as the ancestral motif of arthropod telomeres, in the family Palaemonidae [42]. Since the presence of this repeat has not been demonstrated in most decapod families, it is interesting to confirm the constant presence of this motif within Decapoda, particularly when some animal groups have lost the TTAGG repeat during their evolution such as the crustacean species Asellus aquaticus (Isopoda) [43].
FISH with the (TTAGGG)n probe found in all vertebrates [44] and the (TAACC)n probe identified in the shrimp Penaeus vannamei [45] gave no hybridization signals. On the contrary, in both P. serratus and P. elegans, the hybridization signals of the (TTAGG)n probe were located at the telomeres of all chromosomes. Nonetheless, no interstitial telomeric signals were found as evidence of structural reorganizations occurring throughout chromosomal evolution. However, the fusion sites of ancestral chromosomes do not always preserve the telomeric sequences, and when retained these non-functional repeats could undergo a progressive degeneration or reduction [46], that could impede their detection by FISH.
Sex chromosomes
The comparative analysis between the karyotypes of both sexes of P. elegans in addition to their meiotic behaviour showed a heteromorphism between males and females, which is compatible with the presence of an X1X1X2X2/X1X2Y sex chromosome system, in which the Y chromosome would correspond to the large metacentric chromosome exclusive to males, and the X1 and X2 chromosomes would correspond to two of the largest acrocentric chromosomes of the complement. According to this system females of P. elegans have 2n = 90 (86 + X1X1X2X2) whereas males have 2n = 89 (86 + X1X2Y).
Interestingly, the C-banding technique revealed a lack of constitutive heterochromatin in the sex chromosomes, not even in the Y chromosome which also turned out to be remarkably large.
Typically, during the evolution of sex chromosomes from autosomes, the reduction of recombination between the sex-determining regions is the first step to produce simple sex chromosome systems (XY or ZW). Then, the differential accumulation of repetitive sequences and deleterious mutations favour the heteromorphism between the X and Y (or Z and W), either in size, morphology or through banding techniques [47], and the recombination is kept in the pseudoautosomal regions of the sex chromosomes. In regard to the multiple sex chromosome systems, the initial stage of differentiation seems to be associated with chromosomal rearrangements between the chromosomes bearing sex-determining genes and an autosome (e.g. [48,49,50]). Consequently, due to rearrangements, even newly evolved sex chromosomes can be heteromorphic [51] and not necessarily involve heterochromatin increase [49]. These considerations may explain the existence of meiotic recombination between the P. elegans X and Y chromosomes, the lack of heterochromatin in them and the size of the euchromatic Y chromosome; indicating the possibility that the multiple sex chromosome system in this prawn species is a result of recent evolution. In light of this possibility, and bearing in mind male and female karyotypes and their meiotic behaviour, the initial step of sex chromosome differentiation in this species could be a centric fusion between two nonhomologous acrocentric chromosomes, forming the large metacentric neo-Y and leading to two acrocentric chromosomes without homologous in males (neo-X1 and X2 chromosomes). Accordingly, during meiosis, the recently formed neo-Y would pair with the neo- X1 at one end and with the neo-X2 at the other end, which would lead to the formation of a trivalent such as we observed.
In neither this nor our previous report [5], did we identify sex chromosomes in P. serratus. Also, we did not find differences in the constitutive heterochromatin pattern between sexes. Even so, the results demonstrated that the sex chromosome systems of both congeneric species are different since mitotic and meiotic metaphases displayed the same chromosome number in both P. serratus males and females, making a multiple sex determination system impossible in that species. In this regard, future studies involving comparative genomic hybridization would be helpful in investigating the putative absence of heteromorphic sex chromosomes in detail in the aforementioned species.
A review of the literature suggests that the multiple sex chromosome system X1X1X2X2/X1X2Y found in P. elegans may be unprecedented among decapods with the exception of Cervimunida princeps [52]. However, without additional studies using current techniques, the C. princeps sex determination system formulated in 1959 is questionable considering that it was based on male chromosome number (2n = 109) and the presence of three univalents at meiotic metaphase I, observations that could correspond for instance to an XX/XY1Y2 system.
The present data show the first karyotype with distinguishable heteromorphic sex chromosomes within the family Palaemonidae, where a ZZ/ZW sex chromosome system had been suggested for Macrobrachium rosenbergii, in which it is believed that the female is the heterogametic sex on the basis of molecular studies [53]. In fact, the ZZ/ZW sex-determining mechanism was never determined cytogenetically in any member of Decapoda although its existence has also been inferred in the crayfish species Cherax quadricarinatus (infraorder Astacidea) [54] and some penaeid shrimps (for a review, see [55, 56]). In contrast, male crabs (infraorder Brachiura) are reported to be the heterogametic sex based on their karyotype, with an XX/XY sex chromosome system and even an XX/XO system being observed (see the reviews [6, 57]). Notwithstanding, due to the inherent limitations of the techniques used at the time, we should be cautious as to the reliability of these studies. Recently, the ZZ/ZW sex determination system was proposed for the Chinese mitten crab Eriocheir sinensis (infraorder Brachiura) based on QTL mapping and confirmed by triploid induction experiments [58].
Our results on Palaemon sex determination systems and our bibliographic review reveal a large variability within Decapoda. They also show the difficulty of identifying sex chromosomes in this order using cytogenetic methods. The absence of heterochromatic blocks in the sex chromosomes in P. elegans could be a widespread characteristic in decapods. Besides, the high chromosome number and their small and homogenous size complicate the identification of sex chromosome pairs, especially if the meiotic stage, where the homologous are connected and the chromatin more condensed, is not analyzed.
Conclusions
This and our previous study [5] show that the congeners P. serratus and P. elegans present a high degree of diversity in their chromosome number, karyotype and sex determination system, ranging from the putative absence of heteromorphic sex chromosomes to the multiple chromosome system (X1X1X2X2/X1X2Y). Such variability, even between species so closely related, makes this genus a promising model among Decapoda to investigate not only the karyotype evolution but also the patterns of sex chromosome differentiation.
In this perspective, future comparative cytogenetic analyses comprising other Palaemon species are needed to clarify the hypothesis developed in this work where fusions events would constitute the main mechanism of karyotype evolution in the genus. Likewise, the sex determination system in P. serratus and the existence of additional sex chromosome systems in the genus that shed light on the genus sex chromosome evolution are interesting aspects to be elucidated in further studies.
References
Hobbs HH, Jass PJ, Huner JV. A review of global crayfish introductions with particular emphasis on two north American species (Decapoda, Cambaridae). Crustaceana. 1989;56:299–316.
Stergiou KI, Karpouzi VS. Feeding habits and trophic levels of Mediterranean fish. Rev Fish Biol Fisher. 2002;11:217–54.
Holthuis LB. FAO Species Catalogue. Shrimps and prawns of the world. An annotated catalogue of species of interest to fisheries. FAO Fish Synop. 1980;1:1–271.
Anger K. The biology of the decapod crustacean larvae. In: Anger K, editor. Crustacean issues, vol. 14. Rotterdam: AA Balkema; 2001. p. 13–40.
González-Tizón AM, Rojo V, Menini E, Torrecilla Z, Martínez-Lage A. Karyological analysis of the shrimp Palaemon Serratus (Decapoda: Palaemonidae). J. Crust. Biol. 2013;33:843–8.
Lécher P, Defaye D, Noel P. Chromosomes and nuclear DNA of Crustacea. Invertebr Reprod Dev. 1995;27:85–114.
De Grave S, Fransen CHJM. Carideorum catalogus: the recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda). Zool Meded. 2011;85:195.
Ashelby CW, Page TJ, De Grave S, Hughes JM, Johnson ML. Regional scale speciation reveals multiple invasions of freshwater in Palaemoninae (Decapoda). Zool Scr. 2012;41:293–306.
De Grave S, Ashelby CW. A re-appraisal of the systematic status of selected genera in Palaemoninae (Crustacea: Decapoda: Palaemonidae). Zootaxa. 2013;3734:331–44.
Tzomos T, Koukouras A. Redescription of Palaemon Antennarius H. Milne Edwards, 1837 and Palaemon Migratorius (Heller, 1862) (Crustacea, Decapoda, Palaemonidae) and description of two new species of the genus from the circum-Mediterranean area. Zootaxa. 2015;3905:027–51.
Carvalho F, De Grave S, Mantelatto FL. An integrative approach to the evolution of shrimps of the genus Palaemon (Decapoda, Palaemonidae). Zool Scr. 2017;46:473–85.
d'Udekem d'Acoz C. Inventaire et distribution des crustacés décapodes de l'Atlantique nord-oriental, de la Méditerranée et des eaux continentales adjacentes au nord de 25°N. Patrimoines Naturels (MNHN/SPN). 1999;40:1–383.
Holthuis, LB. The recent genera of the caridean and stenopodidean shrimps (class Crustacea, order Decapoda, supersection Natantia) with keys for their determination. Brill, 1955.
Fincham AA. Larval development of British prawns and shrimps (Crustacea: Decapoda: Natantia). 4. Palaemon Serratus (pennant, 1777) and functional morphology of swimming. Bull Br Mus Nat Hist. (Zool.). 1983; 44: 125-161.
González-Ortegón E, Pascual E, Cuesta JA, Drake P. Field distribution and osmoregulatory capacity of shrimps in a temperate European estuary (SW Spain). Estuar Coast Shelf Sci. 2006;67:293–302.
González-Ortegón E, Giménez L. Environmentally mediated phenotypic links and performance in larvae of a marine invertebrate. Mar Ecol Prog Ser. 2014;502:185–95.
Cuesta JA, González-Ortegón E, Rodríguez A, Baldó F, Vilas C, Drake P. The decapod crustacean community of the Guadalquivir estuary (SW Spain): seasonal and inter-year changes in community structure. Hydrobiologia. 2006;557:85–95.
González-Ortegón E, Cuesta JA. An illustrated key to species of Palaemon and Palaemonetes (Crustacea: Decapoda: Caridea) from European waters, including the alien species Palaemon Macrodactylus. J Mar Biol Assoc U K. 2006;86:93.
Sumner AT. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 1972;75:304–6.
Roiha H, Miller JR, Woods LC, Glover DM. Arrangements and rearrangements of sequences flanking the two types of rDNA insertion in D. Melanogaster. Nature. 1981;290:749–53.
Ijdo JW, Wells RA, Baldini A, Reeders ST. Improved telomere detection using a telomere repeat probe (TTAGGG) n generated by PCR. Nucl Acids Res. 1991;19:4780.
González-Tizón AM, Martínez-Lage A, Rego I, Ausió J, Méndez J. DNA content, karyotypes, and chromosomal location of 18S-5.8 S-28S ribosomal loci in some species of bivalve molluscs from the Pacific Canadian coast. Genome. 2000;43:1065–72.
Mlinarec J, Mcžić M, Pavlica M, Šrut M, Klobučar G, Maguire I. Comparative karyotype investigations in the European crayfish Astacus Astacus and A. Leptodactylus (Decapoda, Astacidae). Crustaceana. 2011;84:1497–510.
Mlinarec J, Porupski I, Maguire I, Klobučar G. Comparative karyotype investigations in the white-clawed crayfish Austropotamobius pallipes (Lereboullet, 1858) species complex and stone crayfish a. Torrentium (Schrank, 1803) (Decapoda: Astacidae). J Crust Biol. 2016;36:87–93.
Salvadori S, Coluccia E, Deidda F, Cau A, Cannas R, Lobina C, et al. Karyotype, ribosomal genes, and telomeric sequences in the crayfish Procambarus Clarkii (Decapoda: Cambaridae). J Crust Biol. 2014;34:525–31.
Salvadori S, Coluccia E, Milia A, Cannas R, Deiana AM. Study of mitotic and meiotic chromosomes of Homarus Gammarus (Crustacea, Decapoda). Biol. Mar. Medit. 2002;9:889–91.
Deiana AM, Libertini A, Cau A, Cannas R, Coluccia E, Salvadori S. Genetics of slipper lobsters. In: Lavalli KL, Spanier E, editors. The biology and fisheries of the slipper lobster. Boca Raton: CRC Press; 2007. p. 53–67.
Cannas R, Deiana AM, Salvadori S, Coluccia E. Dati preliminari sulla cariologia di Panulirus regius De Brito Capello, 1864 (Crustacea, Decapoda). Biol Mar Medit. 2004;11:721–3.
Macgregor HC, Sessions SK. The biological significance of variation in satellite DNA and heterochromatin in newts of the genus Triturus: an evolutionary perspective. Philos T Roy Soc B. 1986;312:243–59.
Deiana AM, Coluccia E, Milia A, Salvadori S. Supernumerary chromosomes in Nephrops norvegicus L. (Crustacea, Decapoda). Heredity. 1996;76:92-9.
Coluccia E, Cau A, Cannas R, Milia A, Salvadori S, Deiana AM. Mitotic and meiotic chromosomes of the American lobster Homarus Americanus (Nephropidae, Decapoda). Hydrobiologia. 2001;449:149–52.
Salvadori S, Coluccia E, Deidda F, Cau A, Cannas R, Deiana AM. Comparative cytogenetics in four species of Palinuridae: B chromosomes, ribosomal genes and telomeric sequences. Genetica. 2012;140:429–37.
Cuesta JA, Drake P, Martínez-Rodríguez G, Rodríguez A, Schubart CD. Molecular phylogeny of the genera Palaemon and Palaemonetes (Decapoda, Caridea, Palaemonidae) from a European perspective. Crustaceana. 2012;85:877–88.
Jiang YQ, Xie SH, Zhou Q, Lan WZ. Chromosome Karyotype in freshwater Prown Exopalaemon Modestus [J]. Fish Sci. 2008;9:013.
Li Y, Liu P, Li J, Li JT, Gao BQ. The chromosome preparation and karyotype in ridgetail white prawn Exopalaemon Carinicauda. J Dalian Ocean Univ. 2012;5:015.
Hassan H, Leitao A, Al-Shaikh I, Al-Maslamani I. Karyotype of Palaemon khori (Decapoda: Palaemonidae). Vie Milieu. 2015;65:151–5.
Sumner AT. Chromosome banding. Australia: Allen & Unwin; 1990.
Cross I, Vega L, Rebordinos L. Nucleolar organizing regions in Crassostrea Angulata: chromosomal location and polymorphism. Genetica. 2003;119:65–74.
Vidotto AP, Swarça AC, Fenocchio AS, Dias AL. Cytogenetic studies in three Pimelodella Meeki populations (Pisces, Pimelodidae) from Tibagi river basin (Brazil). J Hered. 2004;95:517–20.
Bonifácio HL, Da Silva VM, Martin AR, Feldberg E. Molecular cytogenetic characterization of the Amazon River dolphin Inia Geoffrensis. Genetica. 2012;140:307–15.
Eler ES, Da Silva MNS, Silva CEF, Feldberg E. Comparative cytogenetics of spiny rats of the genus Proechimys (Rodentia, Echimyidae) from the Amazon region. Genet Mol Res. 2012;11:830–46.
Vítková M, Král J, Traut W, Zrzavý J, Marec F. The evolutionary origin of insect telomeric repeats, (TTAGG)n. Chromosom Res. 2005;13:145–56.
Pelliccia F, Volpi EV, Lanza V, Gaddini L, Baldini A, Rocchi A. Telomeric sequences of Asellus Aquaticus (crust. Isop.). Heredity. 1994;72:78–80.
Meyne J, Ratliff RL, Moyzis RK. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci. 1989;86:7049–53.
Alcivar-Warren A, Meehan-Meola D, Wang Y, Guo X, Zhou L, Xiang J, et al. Isolation and mapping of telomeric pentanucleotide (TAACC)n repeats of the Pacific whiteleg shrimp, Penaeus Vannamei, using fluorescence in situ hybridization. Mar Biotechnol. 2006;8:467–80.
Ruiz-Herrera A, Nergadze SG, Santagostino M, Giulotto E. Telomeric repeats far from the ends: mechanisms of origin and role in evolution. Cytogenet Gen Res. 2009;122:219–28.
Charlesworth D, Charlesworth B, Marais G. Steps in the evolution of heteromorphic sex chromosomes. Heredity. 2005;95:118–28.
Král J. Evolution of multiple sex chromosomes in the spider genus Malthonica (Araneae: Agelenidae) indicates unique structure of the spider sex chromosome systems. Chromosom Res. 2007;15:863–79.
Cioffi MB, Moreira-Filho O, Almeida-Toledo LF, Bertollo LAC. The contrasting role of heterochromatin in the differentiation of sex chromosomes: an overview from Neotropical fishes. J Fish Biol. 2012;80:2125–39.
López-López A, Hudson P, Galián J. Recent origin of a chiasmatic sex trivalent in Australian Pseudotetracha tiger beetles. J Zool. 2013;289:262–9.
Charlesworth D, Mank JE. The birds and the bees and the flowers and the trees: lessons from genetic mapping of sex determination in plants and animals. Genetics. 2010;186:9–31.
Niiyama H. A comparative study of the chromosomes in decapods, isopods and amphipods, with some remarks on cytotaxonomy and sex-determination in the Crustacea. Mem Fac Fish Hokkaido Univ. 1959;7:1–60.
Jiang XH, Qiu GF. Female-only sex-linked amplified fragment length polymorphism markers support ZW/ZZ sex determination in the giant freshwater prawn Macrobrachium Rosenbergii. Anim Genet. 2013;44:782–5.
Parnes S, Khalaila I, Hulata G, Sagi A. Sex determination in crayfish: are intersex Cherax quadricarinatus (Decapoda, Parastacidae) genetically females? Genet Res. 2003;82:107–16.
Sellars MJ, Li F, Preston NP, Xiang J. Penaeid shrimp polyploidy: global status and future direction. Aquaculture. 2010;310:1–7.
De Vos S, Bossier P, Van Stappen G, Vercauteren I, Sorgeloos P, Vuylsteke M. A first AFLP-based genetic linkage map for brine shrimp Artemia Franciscana and its application in mapping the sex locus. PLoS One. 2013;8:e57585.
Ginsburger-Vogel T, Charniaux-Cotton H. Sex determination. In: Abele LG, editor. The biology of Crustacea 2: 257–281. New York: Academic Press; 1982.
Cui Z, Hui M, Liu Y, Song C, Li X, Li Y, et al. High-density linkage mapping aided by transcriptomics documents ZW sex determination system in the Chinese mitten crab Eriocheir Sinensis. Heredity. 2015;115:206–15.
Indy JR, Arias-Rodriguez S, Paramo-Delgadillo J, Hernandez-Guzman AL, D Artola Barcelo, Contreras-Sanchez W. Cytogenetic studies of invertebrate species from Tabasco, Mexico. Proceedings of the World Aquaculture Meeting, Veracruz, Mexico, September 25–September 29, 2009, UJAT, Mexico.
Gaofeng Q. Studies on chromosomes of Macrobrachium superbum Heller (Crustacea, Decapoda). J Fish Sci Ch. 1997;1:1.
Mittal OP, Dhall U. Chromosome studies in three species of freshwater decapods (Crustacea). Cytologia. 1971;36:633–8.
Qiu G, Du N, Lai W. Chromosomal and karyological studies on the freshwater prawn Macrobrachium Nipponense (Crustacea, Decapoda). Oceanol Limnol Sin. 1994;25:493–8.
Lakra WS, Kumar P. Studies on the chromosomes of two freshwater prawns, Macrobrachium Idella and M. Scabriculum (Crustacea, Decopoda, Palaemonidae). Cytobios. 1995;84:147–56.
Vishnoi DN. Studies on the chromosomes of some Indian Crustacea. Cytologia. 1972;37:43–51.
Chavez JC, Murofushi M, Aida K, Hanyu I. Karyological studies on the freshwater prawn Macrobrachium Rosenbergii. Aquaculture. 1991;97:327–34.
Choudhary N, Sharma R, Asthana S, Vyas P, Rather MA, Reddy AK, et al. Development of Karyotype and localization of cytogenetic markers in Dimua River prawn, Macrobrachium Villosimanus (Tiwari, 1949). J Biol Sci. 2013;13:507.
Acknowledgements
This research was supported by grants CTM2014-53838-R (Ministerio de Economía, Industria y Competitividad, Spain) and GRC2014/050 (Xunta de Galicia, Spain). The funding to Z. T. was provided by a ʽFPU’ fellowship from the Ministerio de Educación, Cultura y Deporte (Spain), and A. P. by a ´FPI´ fellowship from the Ministerio de Educación, Cultura y Deporte (Spain). Special thanks are due to Dr. Verónica Rojo for ideas and discussions, and Dr. Manuel Pimentel and Dr. Mark Walton, for reviewing the linguistic quality of the manuscript. We also thank the two anonymous reviewers for their improvements in the manuscript.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
AGT and AML designed the study and helped to draft the manuscript; ZT, AP and AML collected samples; EGO identified specimens; ZT performed the experiments and wrote the manuscript. All authors revised the manuscript critically and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No approval by an ethical committee was required to achieve the goals of the present study because experimental work was accomplished with an unregulated marine invertebrate.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Torrecilla, Z., Martínez-Lage, A., Perina, A. et al. Comparative cytogenetic analysis of marine Palaemon species reveals a X1X1X2X2/X1X2Y sex chromosome system in Palaemon elegans . Front Zool 14, 47 (2017). https://doi.org/10.1186/s12983-017-0233-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12983-017-0233-x