Abstract
This review article provides an in-depth examination of various aspects related to tirzepatide, a synthetic peptide given the positive signal by the “United States Food and Drug Administration” for managing type 2 diabetes. Beginning with an overview of diabetes introduction, epidemiology, and issues related to β-cell dysfunction, is explored in the narrative. It further delves into the significance of incretins in the context of diabetes and introduces tirzepatide as a novel "twincretin" owing to its dual agonistic activity on the glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP) receptors. The document delves into the chemistry and structure of tirzepatide, providing insights into its unique composition of 39 amino acids derived from native GLP-1, GIP, and semaglutide sequences. Tirzepatide's pharmacology, with a focus on its pharmacokinetic characteristics, and its mechanism of action (MOA) are extensively discussed. Notably, tirzepatide exhibits a preference for GIP receptors, leading to effective reduction of hyperglycemia and positive effects on pancreatic β-cells. Clinical trials examining tirzepatide's impact on glycemic and obesity control are detailed, demonstrating its efficacy in reducing body weight and appetite. Furthermore, the review article explores tirzepatide's effects on cardiovascular and kidney function, highlighting potential renal benefits for diabetic and elevated cardiovascular risk individuals. The narrative also addresses the association between tirzepatide and NAFLD, emphasizing potential benefits in mitigating NAFLD outcomes. Additionally, a comprehensive overview of tirzepatide's side effects, supported by scientific trials, is presented in this article. In conclusion, this review article provides a thorough examination of tirzepatide’s multifaceted impact on diabetes management, shedding light on its pharmacological properties, clinical implications, and potential side effects. The information presented contributes to a comprehensive understanding of tirzepatide's role in the evolving landscape of T2D therapeutics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diabetes is a heterogeneous, complicated metabolic illness defined by high blood glucose concentrations across the world, and its occurrence is triggered by either insufficient secretion of insulin, insulin resistance, or two of them together [1]. Mineral and electrolyte imbalances, as well as alterations in protein and lipid metabolism, are linked to insulin insufficiency and/or resistance. Type 2 Diabetes (T2D) formerly regarded as a disorder of middle and late adulthood-is now diagnosed with increasing frequency among both adolescents and young adults, resulting in prolonged exposure to hyperglycemia, insulin resistance, and attendant morbidities [2]. The TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study showed that 60.1% of young T2D patients had at least one micro-vascular complication after an average follow-up of 13.3 years, with 28.4% having at least two complications [3]. The global diabetes epidemic has seen significant disparities, with higher susceptibility noted among individuals from India, China, the Pacific Islands, and Native Americans. The International Diabetes Federation (IDF) reported that in 2021, diabetes affected 8.8% of adults globally, with men at a higher risk (9.6%) than women (9.0%). The prevalence is expected to rise dramatically, with projections indicating 783 million people will have diabetes by 2045 [4]. In India alone, the diabetic population was around 77 million in 2019 and is projected to exceed 134 million by 2045, with a significant portion remaining undiagnosed [5].
Particularly, T2D is associated with numerous multi-organ problems linked to micro and macro-vascular complications, encompassing liver, kidney, heart, pancreas ailments, among others [6, 7]. Diabetes has emerged as one of the greatest worldwide health problems of the twenty-first century, ranking among the top 10 causes of death, together with cancer, respiratory disorders, and cardiovascular diseases. WHO classified diabetes as a non-communicable disease (NCD) that caused 1.6 million deaths globally in 2019, ranking it as the world's ninth most common cause of death [8]. The increasing burden of diabetes underscores the need for comprehensive strategies to improve diagnosis, treatment, and prevention globally. Efforts to manage and treat T2D include lifestyle modifications, nutritional interventions, and pharmacological therapies. Recent advancements in diabetes treatment involve novel medications like GLP-1 receptor agonists and the newly approved tirzepatide.Tirzepatide is an innovative anti-diabetic medication that acts as a dual agonist for both glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors [9]. It is different from other treatments because of its dual activity, especially those that solely target the GLP-1 receptor, like dulaglutide [10]. Tirzepatide’s notable effects on weight loss and glycemic control make it a superior treatment option over current ones. Research has indicated that tirzepatide is more effective than dulaglutide in lowering body weight and glycated hemoglobin A1c (HbA1c) levels. In a 26-week study, patients treated with tirzepatide experienced dose-dependent decreases in HbA1c of up to 2.4%, while those treated with dulaglutide reported reductions of up to 1.1% [11]. Likewise, tirzepatide caused body weight decreases ranging from 4.8 to 11.3 kg, which were much greater than the 2.7 kg decrease seen with dulaglutide. Additionally, tirzepatide has demonstrated significant enhancements in insulin sensitivity and beta-cell activity. Better insulin processing and less beta-cell stress were shown by the significant increases in the homeostatic model assessment for beta-cell function (HOMA2-B) and the reduction in the proinsulin/insulin and proinsulin/C-peptide ratios [10, 12]. The fact that weight loss was only partially responsible for these increases in insulin sensitivity suggests that tirzepatide’s dual receptor agonism provides additional mechanisms for glycemic control beyond what weight loss alone can give. In present review, we had made efforts in order to discuss the latest detail about the tirzepatide's with mechanism, experimental studies and adverse effects.
2 Methodology
We adopted a comprehensive approach to gathering data in order to formulate the methodology for our review article, drawing from a diverse array of credible sources spanning various sectors. Our primary sources encompassed materials from esteemed organizations such as the National Institutes of Health (NIH), clinicaltrial.gov, and MDPI, along with renowned academic databases like Scopus, Elsevier, Science Direct, and PubMed. In addition to mainstream scientific resources like Sci-Hub, Science.gov, and Springer, we also consulted specialized repositories such as those focusing on diabetes research, such as Diabetes journals and Pharm compass. Moreover, we utilized platforms like Research Gate, academic.oup.com, and Encyclopedia.pub, which foster intellectual discourse. Expanding our scope, we incorporated data from other sources such as the National Center for Toxicological Research-Center for Regulatory Science (NCTR-CRS) under the FDA, World Wide Science.org, Med gadget, Cornell Medicine, journals from the European Respiratory Society, New Drug Approvals.org, assets, and Vedanta. Our objective was to leverage the wealth of data provided by these various sources to ensure a comprehensive and robust analysis in our review article.
2.1 Cellular mechanism of β- cell dysfunction
Beta cell malfunction in type 2 diabetes involves complex interactions between environmental factors and molecular pathways (Fig. 1). Over nutrition, obesity, elevated free fatty acids (FFAs), hyperglycemia, and hyperlipidemia promote insulin resistance (IR) and chronic inflammation, exerting harmful pressures on beta cells such as metabolic/oxidative stress, ER stress, inflammatory stress, impeding Ca2 + mobilization, triggering pro-apoptotic signals and amyloid stress. These stresses, influenced by genetic susceptibility, can lead to islet integrity loss; triggering apoptotic unfolded protein response (UPR) pathways and beta cell malfunction [13]. High saturated FFAs disrupt ER homeostasis, activate IP3 receptors, or inhibit SERCA, affecting ER Ca2 + mobilization. Persistent high glucose levels increase islet amyloid polypeptides (IAAP) and proinsulin production, resulting in misfolded insulin and IAAP accumulation, and elevated reactive oxygen species (ROS) [14]. This mobilization affects ER Ca2 + , stimulates pro-apoptotic signals, degrades insulin mRNA, and releases interleukin (IL)-1β, attracting macrophages and intensifying local islet inflammation [13]. Precise insulin secretion regulation is crucial for metabolic demands, and islet integrity preservation is essential. Pathological mechanisms disrupt islet organization, hindering cell-to-cell communication, impairing insulin and glucagon release, and exacerbating hyperglycemia [15] Beta cell failure and insulin secretory dysfunction are fundamental in type 2 diabetes, arising from synthesis or secretion process errors. Reduced glucose transporter-2 (GLUT-2) expression and improper proinsulin folding are linked to diminished insulin production and diabetes [16].
3 Incretin hormone
Incretins are hormones derived from the intestine released in response to nutrients. They enhance or regulate islet hormone secretion, glucose levels, appetite, body weight, gut motility, immune functions, and lipid metabolism [17]. The two most studied incretin hormones are GLP-1 and GIP, produced in response to nutrients entering the gastrointestinal tract, mediated by G-protein-coupled receptors on β-cells [18].
GIP is produced as a 42-amino acid peptide precursor known as pro-GIP. This synthesis takes place in entero-endocrine K cells, primarily located in the duodenum and proximal jejunum, the propeptide is cleaved into GIP by post-translation processing, and that GIP is secreted whenever they receive stimulus [18, 19]. The production and expression of GIP is also noted in the central nervous system [18]. Gut GIP encourages glucose-dependent insulin production, but neutralizing GIP immune responses reduce glucose-stimulated insulin secretion [20]. In isolated mouse islets, neutralizing GIP immune responses led to a reduction in glucose-stimulated insulin secretion, aligning with the idea of local release of an insulinotropic GIP peptide from α-cells [20]. Additionally, in mice with a targeted deletion of the GCG gene, the ectopic production of physiologically active GIP has also been identified in β-cells [21]. Regulating factor X6 (Rfx6) was found to be a significant determinant of GIP production and release from K cells through investigation of gene expression in isolated K cells, However, the molecular regulation of GIP production in gut K cells remains poorly understood [22]. GLP-1 is synthesized by the pre-proglucagon peptide encoded by the GCG gene in entero-endocrine L cells in the small and large intestine [22, 23]. Emerging data indicates that L cells co-express multiple peptide hormones. The biologically active forms of GLP-1 are GLP-1 [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36 amide] and GLP-1 [7, 24, 37]. GLP-1 expression is also seen in rodent circumvallate papillae, adjacent salivary glands, and the CNS [25]. The production of GLP-1 is triggered by Interleukin-6 (IL-6) and luminal nutrients like proteins, lipids, and carbs [26, 27].
GIP and GLP-1 both are degraded by Dipeptidyl-peptidase 4 (DPP-4) [28] and have a short half-life. GLP-1has a half-life of two to five minutes, while GIP has a half-life of seven to eight minutes [29]. The release of GLP-1 after a meal is complex, with the earliest peak about 10–15 min post-eating, likely due to vagal stimulation or stomach distention, and the second peak 30–60 min post-eating due to direct nutrient interaction [30]. GLP-1 triggers insulin secretion in response to glucose, enhances insulin gene expression, and hinders glucagon release from α-cells [31]. GLP-1 agonists also promote satiety and weight loss.GLP-1 reduces glucagon secretion during hyperglycemia, while GIP stimulates it at lower glucose concentrations. The absolute glycemic threshold for GLP-1 to stimulate insulin secretion is approximately 66 mg/dL. Intravenous glucose reduces glucagon levels more effectively than oral glucose, likely due to GLP-2 and GIP being released only in response to oral glucose [32].
The contributions of GIP and GLP-1 to the incretin effect were measured by Gasbjerg et al. [33] According to their research, glucose itself provides 26%, 45%, and 29% of the total C-peptide production, GIP, and GLP-1. Incretin action post-oral glucose was mostly mediated by GIP, with negligible contribution from GLP-1, consistent with GLP-1's role in slowing stomach emptying. GLP-1 and GIP revealed more equivalent contributions to meal-induced insulin production when stomach emptying effects were taken out of the picture. GIP continues to be largely responsible for the majority of the incretin impact [33].
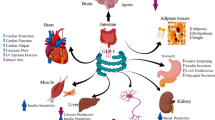
Incretins significantly influence the regulation of numerous physiological processes in different organs. In the pancreas, they enhance insulin secretion from beta cells and inhibit glucagon release from alpha cells, crucial for maintaining glucose homeostasis [34]. In liver, it helps in regulate blood sugar levels after meals by reducing the generation of glucose in the liver in an indirect manner. Incretins increase insulin sensitivity and lipid metabolism in adipose tissue, which improves glucose absorption and storage [18]. Through its hypothalamic effect, GLP-1 promotes satiety and reduces food intake in the brain, hence influencing appetite and body weight regulation. It can also influence cognitive functions and show neuroprotective effects. GLP-1 encourages natriuresis and diuresis in the kidneys, which helps control blood pressure and fluid balance. Additionally, incretins have anti-inflammatory effects in the immune system, reducing inflammation and potentially benefiting conditions associated with chronic inflammation, such as diabetes [35]. These diverse actions make incretins valuable targets for therapeutic interventions in metabolic diseases like type 2 diabetes (Table 1).
4 Tirzepatide
Tirzepatide, also recognized as LY3298176, is a 39 amino acids synthetic peptide. Developed from the natural sequence of the GIP receptor, it serves as a uni-molecular, dual agonist targeting both the glucagon-like peptide-1 (GLP-1) receptor and the glucose-dependent insulinotropic polypeptide (GIP) receptor [41, 42]. This unique dual agonist property makes it a ‘‘twincretin,’’ a term coined by Finan et al. to describe the combined effect of a substance on the secretion of insulin [43, 44]. On May 13, 2022, the (U.S.F.D.A.) granted approval for tirzepatide to be used in the treatment of type 2 diabetes (T2DM) under the trade name Mounjaro. This approval distinguished it as the first dual gut hormone-based agonist sanctioned for diabetes treatment. Tirzepatide, developed by Eli Lilly and Company, has the ability to lower body weight and regulate blood sugar levels [39]. While its affinity for the GLP-1R is approximately five times lower than that of indigenous GLP-1, it shares an affinity for the GIP receptor equivalent to that of natural GIP [45]. This dual action on both receptors contributes to its effectiveness in managing blood sugar.
In addition to its glycemic control benefits, tirzepatide has shown positive effects on other cardiovascular risk markers, including blood pressure, waist circumference, low-density lipoprotein (LDL), and circulating triglycerides [46, 47]. It is conjugated to an albumin-attached 20C fatty di-acid moiety, which prolongs its half-life to 5 days, allowing for once-weekly dosing [48, 49] Tirzepatide is administered through a subcutaneous injection once per week, with the dosage adjusted to meet blood sugar management goals. Both preclinical and clinical researches (Table 2) have indicated significant glucose-lowering effects. This innovative medication represents a first-in-class approach, stimulating both GIP and GLP-1 receptors to improve blood sugar management in T2D individuals.
5 Clinical trials
5.1 Chemistry of tirzepatide
5.1.1 Synthesis
The chemistry of tirzepatide involves a hybrid solid-phase peptide synthesis/liquid-phase peptide synthesis (SPPS/LPPS) strategy, as described by Michael O. Frederick and colleagues at Eli Lilly and Company [63]. This hybrid approach combines the benefits of solid-phase peptide synthesis (SPPS) and liquid-phase peptide synthesis (LPPS) to achieve a scalable and efficient manufacturing process. Solid-phase peptide synthesis (SPPS) is utilized to produce well-characterized intermediates and shorter peptide fragments with high purity, minimizing the risks and purity issues associated with longer SPPS builds. In the case of tirzepatide, four suitable fragments were selected from those synthesized using the SPPS technique. The selected fragments were then linked together in the liquid-phase peptide synthesis (LPPS) using a plug-flow reactor (PFR) in a four-step process [64]. The LPPS phase involved coupling the chosen fragments in a stepwise manner. Separate solutions of fragments in dimethylsulfoxide (DMSO)/acetonitrile (ACN), [Ethyl-cyano (hydroxyimino) acetato-O2] tri-1-pyrrolidinylphosphonium hexafluorophosphate (PyOxim) in ACN, and N,N0-diisopropylethylamine (DIPEA) were prepared and combined in continuous flow within the PFR. The reactions were monitored in real-time using high-performance liquid chromatography (HPLC). After coupling,deprotection was accomplished with diethyl amine (DEA), and the liberated product underwent purification through nano-filtration [63]. The process continued with the coupling of the deprotected product fromstep 1 with fragment 3 in step 2, and this pattern continued into step 3 with the coupling of fragment 4. In the ultimate phase, 19 acid-labile protecting groups were removed utilizing tri-fluoroacetic acid (TFA). This process yielded 8.71 kg of pure tirzepatide, achieving an 81% overall yield. The hybrid methodology not only reduced manufacturing risks but also established a stable and continuous manufacturing process with excellent yield and purity (Fig. 2). Real-time analytical monitoring and nano-filtration, a membrane-based technique, played crucial roles in achieving high purity (97.5–99.5%) and yield during the synthesis of tirzepatide [63].
6 Structure
Tirzepatide is a synthetic linear peptide, serves as a distinctive agonist for GIP/GLP-1 receptors, fashioned from the native sequences of GIP, GLP-1, and semaglutide, with the incorporation of distinct residues [44]. Specifically, tirzepatide's structure (Fig. 3) is derived from the native GIP sequence and incorporates a C20 fatty di-acid moiety (eicosanedioic acid) connected to a lysine residue at position C20 through hydrophilic linkers (γ-Glu-2xAdo, gamma glutamate, and bis-amino diethoxyacetyl) [65, 66]. Notably, the peptide sequence of tirzepatide includes two non-coded amino acid residues (α-amino isobutyric acid, or AIB) at positions 2 and 13, contributing to its prolonged half-life (116.7 h) and strong affinity for albumin [44]. The peptide’s C-terminus is amidated. Tirzepatide emerges as a groundbreaking medication with promising effects against both obesity and type-2 diabetes. With a molecular mass of 4810.52 Daltons and a chemical formula of C225H348N48O68, tirzepatide marks a significant advancement in the field [48, 49].
6.1 Pharmacology of tirzepatide
Tirzepatide administered via subcutaneous injection, exhibits unique pharmacokinetic properties.
Achieving Cmax takes 8 to 72 h. The calculated mean half-life was 116.7 h, and the Tmax was observed within 1–2 days of administration [67]. It has an elimination half-life of around 5 days, allowing for once-weekly dosing. Steady-state concentrations are typically achieved after 4 weeks of administration. The drug is primarily metabolized via β-oxidation of the C20 fatty diacid motif, proteolytic cleavage of the peptide backbone, and amide hydrolysis [65]. Tirzepatide metabolites are primarily excreted through urine and feces. In urine or feces, no intact tirzepatide is found. Tirzepatide has an apparent population mean clearance of 0.061 L/h and half-life of elimination about 5 days, allowing for once in a week dosage [44, 65].
6.2 Mechanism of action (MOA)
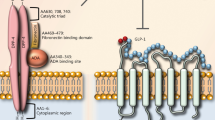
Tirzepatide (Fig. 4), a dual-agonist drug, exhibits a notable preference for the GIP receptor over GLP-1 receptors. In mechanistic studies, it has shown a strong affinity for human GIP receptors (4.23 + /0.23) compared to GLP-1 receptors (0.135 + /0.020) [48], leading to more effective reduction of hyperglycemia than other agonists of the GLP-1 receptor, and contributes to its therapeutic effectiveness. This higher affinity can be attributed to its molecular design, which integrates GLP-1 activity into the GIP sequence [68]. This imbalance is critical for maximizing efficacy because: GIPR activation promotes insulin release from pancreatic β-cells, leading to improved glycemic control. The lower affinity for GLP-1R reduces dose-limiting gastrointestinal adverse effects like nausea and vomiting, which are frequently linked to high GLP-1R activity. Tirzepatide’s pharmacokinetic characteristics, including albumin-binding properties due to a C20 unsaturated di-acid acyl-chain, enables sustained receptor activation and once-weekly dosage, maximizing therapeutic effects while minimizing administration frequency [68, 69].
Additionally, preclinical data has highlighted various positive effects of tirzepatide, including increased survival and proliferation of pancreatic β-cells, enhanced insulin synthesis and secretion, and elevated levels of adiponectin—a key adipokine involved in regulating lipid and glucose metabolism. Tirzepatide has demonstrated effects beyond glycemic control, showing reductions in appetite and food intake, resulting in weight loss [44, 70]. Additionally, studies indicate potential benefits in cognitive performance, decreased bone reabsorption, and increased cardio protection [71]. In population diagnosed with type 2 diabetes, a study with a 15 mg dose of tirzepatide demonstrated enhancements in β-cell function, glucagon secretion, and insulin sensitivity compared to a placebo. Further mechanistic research is warranted to explore tirzepatide's effects on energy intake, resting metabolic rate, and appetite in individuals without diabetes. Pharmacokinetic comparisons between diabetic individuals and healthy individuals have shown that tirzepatide delays gastric emptying, particularly after a single dose, and demonstrates a decrease in effectiveness or responsiveness with repeated once-weekly dosing, referred to as tachyphylaxis [44, 72]. Importantly, intrinsic factors indicated by tirzepatide’s properties suggest that patients with hepatic and renal impairment may not require dosage adjustments, adding to the drug’s potential versatility and applicability across a range of patient populations.
6.3 Effect on glycemic control
The mechanistic investigations carried out in preclinical trials shed light on tirzepatide’s distinctive role as a dual-agonist drug, exhibiting a robust affinity for GIP and GLP-1 receptors. This unique signaling preference directs the production of cyclic adenosine monophosphate (cAMP) while likely reducing the recruitment of β-arrestin [46]. Notably, tirzepatide's activation of the GLP-1 receptor proves to be a crucial factor in intensifying the insulinotropic impact on pancreatic beta cells. Mice studies further support tirzepatide’s potential in reducing insulin resistance, both in weight-dependent and independent subjects [73]. Preliminary human research suggests that tirzepatide may enhance insulin sensitivity more efficiently than selective GLP-1 receptor agonists, challenging the notion that weight loss is the sole contributor to improved insulin sensitivity. The subsequent disclosure of tirzepatide's mechanism through preclinical and clinical studies provides compelling data, strongly indicating its pharmacological properties and affirming its effects on glycemic control. This is underscored by concurrent improvements in β-cell function, insulin sensitivity, and α-cell function [74, 75]. Clinical trials involving tirzepatide reveal a dose-dependent effect on HbA1c across all five trials, with mean baseline decreases ranging from 1.87% to 2.59%. Dose-dependent reductions in HbA1c caused by tirzepatide (5, 10, and 15 mg) are significantly higher than those caused by various comparators, including placebo, semaglutide, insulin degludec, insulin glargine, and placebo with background insulin [75].
Meal tolerance tests illustrate tirzepatide's efficacy in reducing meal-stimulated glucagon secretion and fasting, showcasing its multifaceted impact on glucose metabolism.Research conducted by M. K. Thomas and colleagues at Eli Lilly and Company, [76] investigate the impact of tirzepatide on beta-cell function and insulin sensitivity in individuals with type 2 diabetes.
Significant improvements in beta-cell activity are indicated by HOMA2-B percent change from baseline increases ranging from 93 to 163% for tirzepatide (5, 10, and 15 mg doses), outperforming dulaglutide and placebo effects. Evaluation of intact proinsulin levels reveals significant decreases with tirzepatide at 5, 10, and 15 mg doses, indicating potential positive changes in insulin processing.Furthermore, tirzepatide therapy dramatically lowers glucose-adjusted glucagon levels, emphasizing improvements in glucose regulation, beta-cell activity, insulin processing, and reduced glucagon levels [74].
6.4 Effect on obesity control
Tirzepatide reduces obesity through a multifaceted approaches including suppressing appetite, reducing food intake, enhancing satiety, decreasing fat mass, and potentially increasing energy expenditure. These combined effects lead to significant and sustained weight loss, making tirzepatide an effective therapy for obesity management. Tim Heise and colleagues [76] conducted a secondary analysis study to assess the effect of tirzepatide 15 mg (N = 45), semaglutide 1 mg (N = 44), and placebo (N = 28) effects on body composition, appetite, and energy intake. Both tirzepatide and semaglutide significantly reduced body weight from baseline (P < 0.001). Differences emerged as early as the fifth week, the reduced body weigh with tirzepatide (2.6 kg) compared to placebo (1.0 kg) and semaglutide (1.9 kg). By week 28, tirzepatide resulted in an average weight loss of 11 kg, compared to 0 kg with placebo and 7 kg with semaglutide. Tirzepatide also induced greater reductions in fat mass compared to placebo and semaglutide. Additionally, both tirzepatide and semaglutide significantly decreased energy intake, increased satiety, reduced hunger, and lowered future meal consumption. Appetite reduction was more significant with tirzepatide compared to placebo [76]. In the SURPASS-2 study, over 280 days, tirzepatide (5 to 15 mg) led to weight loss ranging from 7 to 9.5 kg, whereas the placebo group experienced a weight loss of 0.7 kg. In comparison to semaglutide 1 mg, which resulted in a weight loss of – 6.2 kg [51]. After 52 weeks of treatment, starting insulin degludec resulted in 2.3 kg of weight gain. In SURPASS-3, tirzepatide resulted in a weight reduction ranging from 7.5 to 12.9 kg [52]. Following a 52-week period, individuals with severe type 2 diabetes who started insulin glargine while taking other glucose-lowering drugs.
In contrast to its effect on obesity, tirzepatide has shown remarkable results in neuroprotective qualities. A study conducted by Fontanella et al. [77] discovered that tirzepatide therapy drastically reduced the expression of the apoptotic marker BAX/Bcl2 ratio while up regulating markers such as MAP2, GAP-43, and AGBL4, all of which are important for neuronal plasticity and differentiation. Furthermore, tirzepatide increased the levels of miR-212 and miR-29c, microRNAs that regulate gene expression linked to neuronal survival and function. These molecular modifications emphasize tirzepatide's ability to rescue neurons from hyperglycemia-induced damage while also supporting its role in cognitive function improvement. The data indicate that tirzepatide could be an effective therapeutic treatment for treating diabetes-related neurodegenerative diseases.
6.5 Effects on cardiovascular outcomes
The effects of tirzepatide on cardio metabolic risk factors and vascular consequences have been investigated in the context of the SURPASS trials. Tirzepatide was found to lower diastolic blood pressure by 1–5.6 mmHg and systolic blood pressure by 2.8 to 12.6 mmHg.There was an observed average rise in pulse rate ranging from 0.7 to 8.3 beats per minute [54, 78]. Tirzepatide caused a decrease in waist circumference ranging from 6.9 to 9.9 cm in the SURPASS-2 trials. A similar drop in waist circumference were noted in SURPASS-4, and this reduction was sustained through 104 weeks [51, 53]. Additionally, after 40 weeks in SURPASS-2, individuals treated with tirzepatide exhibited lower triglyceride levels, ranging from 19.0% to 24.8%, in comparison to semaglutide 1 mg, which showed an 11.5% reduction. Higher levels of (HDL-C) (6.8–7.9% vs. 4.4%) were observed with tirzepatide.The impact on total cholesterol and low-density lipoprotein cholesterol (LDL-C) were similar between tirzepatide and semaglutide [51]. Furthermore, tirzepatide was linked to noteworthy reductions in volumes of abdominal visceral and subcutaneous adipose-tissue.Meanwhile, these volumes experienced an increase in the insulin degludec group.
The study conducted by Taktaz et al. [79] emphasizes tirzepatide's potential benefits through a meta-analysis of large randomized clinical trials, in vitro tests employing human cardiac AC16 cells in high glucose settings, and detailed bioinformatics analyses. The meta-analysis found that tirzepatide therapy significantly reduced the incidence of major adverse cardiovascular events (MACE) (hazard ratio = 0.59). In vitro tests demonstrated that tirzepatide inhibits high glucose-induced adverse effects on cardiac cells, including enhanced fibrosis, hypertrophy, and cell death markers. Furthermore, bioinformatics validated these findings by demonstrating the interaction of tirzepatide with important pathways that influence cardiac cell death, fibrosis, and contractility. Overall, these findings reveal that tirzepatide can reduce diabetes-related cardiac damage, implying therapeutic potential for heart failure therapy. These findings suggest that tirzepatide may have favorable effects on cardio metabolic risk variables such as blood pressure, waist circumference, and lipid profile [77]. More research is needed to properly understand the long-term effects of tirzepatide on cardiovascular health and other outcomes.
6.6 Effects on renal functions
The result of clinical trials of tirzepatide on kidney function indicates potential renal advantages, especially in individuals with type-2 diabetes (T2D) and a heightened cardiovascular risk.In the SURPASS-4 (NCT03730662) trial, tirzepatide was observed to reduce occurrence of the composite kidney endpoint (like eGFR decline of at least 40% from baseline, end-stage kidney disease, death due to kidney failure, or new-onset macro albuminuria) in comparison to those receiving insulin glargine. The average rate of decline in eGFR was markedly lower in the tirzepatide groups when compared to the insulin glargine group (-1.4 mL/min/1.73 m2/year vs. -3.6 mL/min/1.73 m2/year).The urine albumin-to-creatinine ratio (UACR) rose from baseline with insulin glargine but exhibited no increase with tirzepatide, suggesting a positive impact on albuminuria [80].
A clinical-trial (NCT03482024) conducted by Shweta Urva et al. assessed the impact of weekly tirzepatide on renal dysfunction in subjects with different renal statuses, primarily with T2DM. The study included subjects with mild, moderate, and life-threatening kidney impairment, and end-stage kidney disease requiring dialysis, andindividuals with regular kidney function.
Results indicated thatexposure to tirzepatide remained consistent across all groups, and there were no significant impacts on its pharmacokinetics.Reported adverse events were mostly mild and related to the gastrointestinal system in the renal impairment groups [81]. Overall, although there were accounts suggesting a potential risk of renal damage with tirzepatide, the SURPASS-4 trial and the additional study (Clinical Trials NCT03482024) indicate favorable effects on renal function,involving a decrease in the rate of eGFR decline and a decreased incidence of the composite kidney endpoint [80, 81]. It is essential to consider the overall context of these findings and consult healthcare professionals for personalized advice and monitoring.
6.7 Effect onNAFLD (non-alcoholic fatty liver disease) outcomes
The literature extensively outlines the connection between NAFLD and extra-hepatic manifestations that hold clinical significance.Younossi et al. emphasized a strong correlation between NAFLD and a range of cardio metabolic comorbidities, including obesity, Type 2 Diabetes (T2D), hyperlipidemia, hypertension, and metabolic syndrome [82]. This connection is ascribed to either the secondary consequences of obesity or the direct pathophysiological influence of insulin resistance in NAFLD [83]. A recent comprehensive review and meta-analysis, involving 101,028 individuals, disclosed that in the overweight population, the occurrence rate of NASH (Non-Alcoholic Steatohepatitis) and NAFLD was 33.5% and 70% respectively. The population with obesity showed similar prevalence rates, with 33.7% for NASH and 75.3% for NAFLD. Overweight patients with NAFLD exhibited clinically significant fibrosis (stages F2–4) at a rate of 20.3%, while obese patients had a slightly higher prevalence at 21.6%. Additionally, advanced fibrosis (stages F3–4) was identified in 6.7% of overweight individuals and 6.9% of obese individuals with NAFLD [84]. Individuals with diabetes, particularly those with T2D, not only showed an increased occurrence of steatosis but also notable liver fibrosis. A meta-analysis focusing on the worldwide epidemiology of NAFLD/NASH among individuals with T2D revealed a prevalence of 55.5% and 37.3%, respectively. The estimated incidence of advanced fibrosis was reported to be 17.0% [85].
The economic and clinical burden of NASH in individuals with Type 2 Diabetes (T2D) underscores the need for effective treatments. Hartman et al. performed a phase 2 trial, to investigate the impact of tirzepatide, on biomarkers related to NASH and liver fibrosis in individuals with Type 2 Diabetes (T2D). The study demonstrated significant reductions in AST, ALT, Pro-C3, and CK-18 levels at 182 days, particularly with tirzepatide doses of 10 and 15 mg, demonstrated the potential benefits for NASH and elevated adiponectin levels. Despite the promising outcomes, there is no published data on tirzepatide's impact on liver biopsy-evaluated histological characteristics in NASH patients. The ongoing SYNERGY-NASH trial aims to provide comprehensive evidence on tirzepatide's efficacy in improving hepatic outcomes for NAFLD and reversing NASH [62]. The SURPASS-3MRI trial, which is a subsidiary study of the phase 3 SURPASS-3 trial, explored the effects of tirzepatide exhibited a notable decrease in liver fat content in comparison to insulin degludec (8.09% vs. 3.38%) [86]. However, the extent of this benefit and its independence from weight loss remain unknown, necessitating further research to elucidate the underlying pathophysiological mechanism.
7 Adverse events associated with tirzepatide
Tirzepatide has significant therapeutic benefits, but its use comes along with various adverse effects that must be carefully considered. According to the results of the SURPASS and SURMOUNT clinical trials, tirzepatide is associated with a number of side effects (Table 3). During the initial stages of treatment, gastrointestinal issues such as nausea, vomiting, and diarrhea are the most often reported short-term adverse effects [46, 65]. Usually, these symptoms go away as the body adjusts to the medication. Additionally, individuals may have localized reactions at the injection site, which are often mild and transient and include pain, redness, or edema. Acute pancreatitis can also happen rapidly after beginning tirzepatide therapy; however, this is not frequent.
Concerns about long-term adverse consequences vary depending on the organ. The effects of tirzepatide over an extended period of time on cardiovascular events are being studied [60]. Although the data is still in its early stages, prolonged tirzepatide use may raise the risk of chronic pancreatitis, making the pancreas another essential organ system that needs close monitoring [65]. The thyroid gland requires special consideration due to the potential danger of medullary thyroid cancer from prolonged use, which has been noted with other GLP-1 receptor agonists [39]. Vigilant monitoring and additional investigation are required due to this potential risk. Additionally, there is a long-term risk of gallstone formation (cholelithiasis), which may be brought on by modifications in the drug's effects on bile composition and gallbladder motility.
8 Conclusion and future prospective
The use of incretin-based therapy, particularly GLP-1 receptor agonists, has exhibited encouraging outcomes in addressing obesity and associated conditions such as diabetes mellitus type-2, cardiovascular disease, and NAFLD (non-alcoholic fatty liver disease). Long-term studies demonstrate significant benefits in the context of losing weight and HbA1c reduction involving GLP-1RAs. The text introduces a new class of medication, tirzepatide, an agent that activates both GLP-1 and GIP receptors, which shows weight reduction and dominant blood glucose regulation compared to single-agonist therapies. Along with the SURMOUNT and SURPASS research trial programs, they support the effectiveness and assurance of LY3298176 (tirzepatide) in individuals with diabetes type 2 and/or obesity. However, during the writing of this article, we discovered the need for more research to understand the interplay between the nervous system and gastrointestinal hormones, unexplored GLP-1 or GIP receptors, genetic background, endocrine cells, and the composition of dietary intake. Future studies, like the SURPASS-CVOT study (NCT04255433), will assess the cardiovascular (CV) safety and efficacy of tirzepatide against dulaglutide (1.5 mg) in people with established coronary heart disease (ASCVD) and type 2 diabetes. Additional trials of SYNERGY-NASH (NCT04166773) will look into tirzepatide's effectiveness in nonalcoholic steatohepatitis (NASH) and its effect on those who are obese and have heart failure with maintained ejection fractions. According to the literature, incretins (GIPR and GLP-1) are found in a variety of organs; however, tirzepatide's effects on these organs have not been well investigated. Future research may look into these potential connections further.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- GLP-1:
-
Glucagon-like peptide-1
- GIP:
-
Gastric inhibitory polypeptide
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein- cholesterol
- SPPS:
-
Solid-phase peptide synthesis
- PFR:
-
Plug-flow reactor
- DMSO:
-
Dimethyl sulfoxide
- HPLC:
-
High-performance liquid chromatography
- EGP:
-
Endogenous Glucose Production
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- 6- OHDA:
-
6-Hydroxydopamine
- RCTs:
-
Randomized clinical Trials
- ROS:
-
Reactive oxygen species
- CSVD:
-
Cerebral small vessel disease
- FFAs:
-
Free fatty acids
References
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013. https://doi.org/10.2337/dc14-s081.
D. J. Magliano, E. J. Boyko, and IDF Diabetes Atlas 10th edition scientific committee, IDF DIABETES ATLAS, 10th ed. in IDF Diabetes Atlas. Brussels: International Diabetes Federation, 2021. Accessed: 09, Dec. 2023. http://www.ncbi.nlm.nih.gov/books/NBK581934/.
Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance-cell failure, or both? Diabetes Care. 2005;28(3):638–44. https://doi.org/10.2337/diacare.28.3.638.
Alsifri, S., Almaghamsi A.M., Mahfouz A.S., Shehab-Eldin W. &Rakha S. 2021. International Diabetes Federation (2021) IDF Diabetes Atlas. 10th Edition. —References —Scientific Research Publishing. https://www.scirp.org/reference/referencespapers?referenceid=3434373
Zhao M, Song L, Sun L, Wang M, Wang C, Yao S, Li Y, Yun C, Zhang S, Sun Y, Hou Z, Wu S, Xue H. Associations of type 2 diabetes onset age with cardiovascular disease and mortality: the Kailuan study. Diabetes Care. 2021;44(6):1426–32. https://doi.org/10.2337/dc20-2375.
Xie J, Wang M, Long Z, Ning H, Li J, Cao Y, Liao Y, Liu G, Wang F, Pan A. Global burden of type 2 diabetes in adolescents and young adults, 1990–2019: systematic analysis of the global burden of disease study 2019. BMJ. 2022;379: e072385. https://doi.org/10.1136/bmj-2022-072385.
Sun B, Luo Z, Zhou J. Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications. Cardiovasc Diabetol. 2021. https://doi.org/10.1186/s12933-020-01200-7.
World Health Organization 2020. The Top 10 Causes of Death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
Liarakos AL, Koliaki C. Novel dual incretin receptor agonists in the spectrum of metabolic diseases with a focus on tirzepatide: real game-changers or great expectations? Narrative Rev Biomed. 2023;11(7):1875. https://doi.org/10.3390/biomedicines11071875.
Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, Milicevic Z, Haupt A, Robins DA. Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. J Clin Endocrinol Metab. 2020. https://doi.org/10.1210/clinem/dgaa863.
Forzano I, Varzideh F, Avvisato R, Jankauskas SS, Mone P, Santulli G. Tirzepatide: a systematic update. Int J Mol Sci. 2022;23(23):14631. https://doi.org/10.3390/ijms232314631.
Lin F, Yu B, Ling B, Lv G, Shang H, Zhao X, Jie X, Chen J, Li Y. Weight loss efficiency and safety of tirzepatide: a systematic review. PLoS ONE. 2023;18(5): e0285197. https://doi.org/10.1371/journal.pone.0285197.
Lauffenburger JC, Lewey J, Jan S, Lee J, Ghazinouri R, Choudhry NK. association of potentially modifiable diabetes care factors with glycemic control in patients with insulin-treated type 2 diabetes. JAMA Netw Open. 2020;3(1): e1919645. https://doi.org/10.1001/jamanetworkopen.2019.19645.
Christensen AA, Gannon M. The beta cell in type 2 diabetes. Current Diabetes Rep. 2019. https://doi.org/10.1007/s11892-019-1196-4.
Yamamoto WR, Bone RN, Sohn P, Syed F, Reissaus CA, Mosley AL, Wijeratne AB, True JD, Tong X, Kono T, Evans-Molina C. Endoplasmic reticulum stress alters ryanodine receptor function in the murine pancreatic β cell. J Biol Chem. 2019;294(1):168–81. https://doi.org/10.1074/jbc.ra118.005683.
Hoang DO, Thorn P. Insulin secretion from beta cells within intact islets: Location matters. Clin Exp Pharmacol Physiol. 2015;42(4):406–14. https://doi.org/10.1111/1440-1681.12368.
Liu M, Weiss MA, Arunagiri A, Yong J, Rege N, Sun J, Haataja L, Kaufman RJ, Arvan P. Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes Obes Metab. 2018. https://doi.org/10.1111/dom.13378.
Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–37. https://doi.org/10.1016/j.cmet.2013.04.008.
Yip RG-C, Boylan MO, Kieffer TJ, Wolfe MM. Functional GIP receptors are present on adipocytes. Endocrinology. 1998;139(9):4004–7. https://doi.org/10.1210/endo.139.9.6288.
Adriaenssens AE, Biggs EK, Darwish T, Tadross J, Sukthankar T, Girish M, Polex-Wolf J, Lam BY, Zvetkova I, Pan W, Chiarugi D, Yeo GSH, Blouet C, Gribble FM, Reimann F. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metab. 2019;30(5):987-996.e6. https://doi.org/10.1016/j.cmet.2019.07.013.
Fukami A, Seino Y, Ozaki N, Yamamoto M, Sugiyama C, Sakamoto-Miura E, TatsuhitoHimeno TY, Tsunekawa S, Ali S, Drucker DJ, Murata Y, Seino Y, Oiso Y, Hayashi Y. Ectopic expression of GIP in pancreatic β-cells maintains enhanced insulin secretion in mice with complete absence of proglucagon-derived peptides. Diabetes. 2013;62(2):510–8. https://doi.org/10.2337/db12-0294.
Suzuki K, Harada N, Yamane S, Nakamura Y, Sasaki K, Nasteska D, Joo E, Shibue K, Harada T, Hamasaki A, Toyoda K, Nagashima K, Inagaki N. Transcriptional regulatory factor X6 (Rfx6) increases gastric inhibitory polypeptide (GIP) expression in enteroendocrine K-cells and Is involved in GIP hypersecretion in high fat diet-induced obesity. J Biol Chem. 2012;288(3):1929–38. https://doi.org/10.1074/jbc.m112.423137.
Weiss M., Steiner D.F. &Philipson L.H. 2000. Insulin Biosynthesis, Secretion, Structure, and Structure-Activity Relationships, in Feingold K.R., Anawalt B., Boyce A., Chrousos G., De Herder W.W., Dhatariya K., Dungan K., Hershman J.M., Hofland J., Kalra S., Kaltsas G., Koch C., Kopp P., Korbonits M., Kovacs C.S., Kuohung W., Laferrère B., Levy M., McGee E.A. & McLachlan R. (eds.). https://pubmed.ncbi.nlm.nih.gov/25905258/
Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, Morley TCE, Yeo GSH, Reimann F, Gribble FM. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153(7):3054–65. https://doi.org/10.1210/en.2011-2170.
Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–65. https://doi.org/10.1016/j.cmet.2006.01.004.
Marchetti P, Lupi R, Bugliani M, Kirkpatrick CL, Sebastiani G, Grieco FA, Del Guerra S, D’Aleo V, Piro S, Marselli L, Boggi U, Filipponi F, Tinti L, Salvini L, Wollheim CB, Purrello F, Dotta F. A local glucagon-like peptide 1 (GLP-1) system in human pancreatic islets. Diabetologia. 2012;55(12):3262–72. https://doi.org/10.1007/s00125-012-2716-9.
Diakogiannaki E, Gribble FM, Reimann F. Nutrient detection by incretin hormone secreting cells. Physiol Behav. 2012;106(3):387–93. https://doi.org/10.1016/j.physbeh.2011.12.001.
Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab. 2012;15(4):421–31. https://doi.org/10.1016/j.cmet.2011.12.019.
Singh A. Dipeptidyl peptidase-4 inhibitors: novel mechanism of actions. Indian J Endocrinol Metab. 2014;18(6):753. https://doi.org/10.4103/2230-8210.141319.
Sharma A, Paliwal G, Upadhyay N, Tiwari A. RETRACTED ARTICLE: Therapeutic stimulation of GLP-1 and GIP protein with DPP-4 inhibitors for type-2 diabetes treatment. J Diabetes Metab Disorders. 2015. https://doi.org/10.1186/s40200-015-0143-4.
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–57. https://doi.org/10.1053/j.gastro.2007.03.054.
Seghieri M, Rebelos E, Gastaldelli A, Astiarraga BD, Casolaro A, Barsotti E, Pocai A, Nauck M, Muscelli E, Ferrannini E. Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia. 2012;56(1):156–61. https://doi.org/10.1007/s00125-012-2738-3.
Gasbjerg LS, Helsted MM, Hartmann B, Jensen MH, Gabe MBN, Sparre-Ulrich AH, Veedfald S, Stensen S, Lanng AR, Bergmann NC, Christensen MB, Vilsbøll T, Holst JJ, Rosenkilde MM, Knop FK. Separate and combined glucometabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 in healthy individuals. Diabetes. 2019;68(5):906–17. https://doi.org/10.2337/db18-1123.
Tan Q, Akindehin SE, Orsso CE, Waldner RC, DiMarchi RD, Müller TD, Haqq AM. Recent advances in incretin-based pharmacotherapies for the treatment of obesity and diabetes. Front Endocrinol. 2022. https://doi.org/10.3389/fendo.2022.838410.
Huang X-M, Zhong X, Du Y-J, Guo Y-Y, Pan T-R. Effects of glucagon-like peptide-1 receptor agonists on glucose excursion and inflammation in overweight or obese type 2 diabetic patients. World J Diabetes. 2023;14(8):1280–8. https://doi.org/10.4239/wjd.v14.i8.1280.
Ferhatbegović L, Mršić D. The benefits of GLP1 receptors in cardiovascular diseases. Front Clin Diabetes Healthcare. 2023. https://doi.org/10.3389/fcdhc.2023.1293926.
Michałowska J, Miller-Kasprzak E, Bogdański P. Incretin hormones in obesity and related cardiometabolic disorders: the clinical perspective. Nutrients. 2021;13(2):351. https://doi.org/10.3390/nu13020351.
Ussher JR, Greenwell AA, Nguyen M-A, Mulvihill EE. cardiovascular effects of incretin-based therapies: integrating mechanisms with cardiovascular outcome trials. Diabetes. 2022;71(2):173–83. https://doi.org/10.2337/dbi20-0049.
Eli Lilly and Company 2022. FDA approves Lilly’s MounjaroTM (tirzepatide) injection, the first and only GIP and GLP-1 receptor agonist for the treatment of adults with type 2 diabetes | Eli Lilly and Company. https://investor.lilly.com/news-releases/news-release-details/fda-approves-lillys-mounjarotm-tirzepatide-injection-first-and [Accessed Nov 2023]
Bethel MA, Diaz R, Castellana N, Bhattacharya I, Gerstein HC, Lakshmanan MC. HbA1c change and diabetic retinopathy during GLP-1 receptor agonist cardiovascular outcome trials: a meta-analysis and meta-regression. Diabetes Care. 2020;44(1):290–6. https://doi.org/10.2337/dc20-1815.
Sun B, Willard FS, Feng D, Alsina-Fernandez J, Chen Q, Vieth M, Ho JD, Showalter AD, Stutsman C, Ding L, Suter TM, Dunbar JD, Carpenter JW, Mohammed FA, Aihara E, Brown RA, Bueno AB, Emmerson PJ, Moyers JS, Kobilka TS. Structural determinants of dual incretin receptor agonism by tirzepatide. Proc Natl Acad Sci. 2022. https://doi.org/10.1073/pnas.2116506119.
Zhao F, Zhou Q, Cong Z, Hang K, Zou X, Zhang C, Chen Y, Dai A, Liang A, Ming Q, Wang M, Chen L-N, Xu P, Chang R, Feng W, Xia T, Zhang Y, Wu B, Yang D, Zhao L. Structural insights into multiplexed pharmacological actions of tirzepatide and peptide 20 at the GIP, GLP-1 or glucagon receptors. Nature Commun. 2022. https://doi.org/10.1038/s41467-022-28683-0.
Finan B, Ma T, Ottaway N, Müller TD, Habegger KM, Heppner KM, Kirchner H, Holland J, Hembree J, Raver C, Lockie SH, Smiley DL, Gelfanov V, Yang B, Hofmann S, Bruemmer D, Drucker DJ, Pfluger PT, Perez-Tilve D, Gidda J. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci Transl Med. 2013. https://doi.org/10.1126/scitranslmed.3007218.
Chavda VP, Ajabiya J, Teli D, Bojarska J, Apostolopoulos V. Tirzepatide, a new era of dual-targeted treatment for diabetes and obesity: a mini-review. Molecules. 2022;27(13):4315. https://doi.org/10.3390/molecules27134315.
Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, Van der Velden WJ, Stutsman C, Cardona GR, Urva S, Emmerson PJ, Holst JJ, D’Alessio DA, Coghlan MP, Rosenkilde MM, Campbell JE, Sloop KW. — Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI insight. 2020;5(17): 140532. https://doi.org/10.1172/jci.insight.140532.
De Block C, Bailey C, Wysham C, Hemmingway A, Allen SE, Peleshok J. Tirzepatide for the treatment of adults with type 2 diabetes: an endocrine perspective. Diabetes Obes Metab. 2022;1:3–17. https://doi.org/10.1111/dom.14831.
Min T, Bain SC. The role of tirzepatide, dual GIP and GLP-1 receptor agonist, in the management of type 2 diabetes: the surpass clinical trials. Diabetes Therapy. 2020. https://doi.org/10.1007/s13300-020-00981-0.
Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, Cui X, Briere DA, Cabrera O, Roell WC, Kuchibhotla U, Moyers JS, Benson CT, Gimeno RE, D’Alessio DA, Haupt A. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: From discovery to clinical proof of concept. Mol Metab. 2018;18:3–14. https://doi.org/10.1016/j.molmet.2018.09.009.
Frías JP. Tirzepatide: a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) dual agonist in development for the treatment of type 2 diabetes. Expert Rev Endocrinol Metab. 2020;15(6):379–94. https://doi.org/10.1080/17446651.2020.1830759.
Eli Lilly and Company 2021. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT03954834?tab=table [Accessed 1 Mar 2024]
Eli Lilly and Company 2021. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT03987919?tab=table [Accessed 1 Mar 2024]
ELi Lilly and Company 2022. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT03882970 [Accessed 21 Jan 2024]
Eli Lilly and Company 2021. — ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT03730662 [Accessed 1 Mar 2024]
Eli Lilly and Company 2021. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04039503 [Accessed 1 Mar 2024]
Eli Lilly and Company 2022. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04537923 [accessed 1 March 2024]
Eli Lilly and Company 2021. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT03861052 [accessed 21 November 2023]
Eli Lilly and Company 2021. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT03861039 [Accessed 14 Dec 2023]
Eli Lilly and Company 2022. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04184622 [Accessed 1 Mar 2024]
Eli Lilly and Company 2021. — CTG Labs – NCBI. https://clinicaltrials.gov/study/NCT04093752 [Accessed 29 Aug 2023]
Eli Lilly and Company 2023. ClinicalTrials.gov. https://clinicaltrials.gov/study/NCT04255433 [Accessed 23 Feb 2024]
Eli Lilly and Company 2023. CTG Labs – NCBI. Available from https://clinicaltrials.gov/study/NCT04847557 [Accessed 17 Dec 2023]
Eli Lilly and Company 2023. ClinicalTrials.gov. Available from https://clinicaltrials.gov/study/NCT04166773 [Accessed 1 Mar 2024]
Frederick MO, Boyse RA, Braden TM, Calvin JR, Campbell BM, Changi SM, Coffin SR, Condon C, Gowran O, McClary GJ, Groskreutz SR, Harms ZD, Humenik AA, Kallman NJ, Klitzing ND, Kopach ME, Kretsinger JK, Lambertus GR, Lampert JT, Maguire LM. Kilogram-scale GMP manufacture of tirzepatide using a hybrid Spps/Lpps approach with continuous manufacturing. Org Process Res Dev. 2021;25(7):1628–36. https://doi.org/10.1021/acs.oprd.1c00108.
May SA, Johnson MD, Buser JY, Campbell AN, Frank SA, Haeberle BD, Hoffman PC, Lambertus GR, McFarland AD, Moher ED, White TD, Declan Hurley D, Corrigan AP, Gowran O, Kerrigan NG, Kissane MG, Lynch RR, Sheehan P, Spencer RD, Pulley SR. Development and manufacturing GMP scale-up of a continuous ir-catalyzed homogeneous reductive amination reaction. Org Process Res Dev. 2016;20(11):1870–98. https://doi.org/10.1021/acs.oprd.6b00148.
Ali R, Virendra SA, Chawla PA. Bumps and humps in the success of Tirzepatide as the first GLP1 and GIP receptor agonist. Health Sci Rev. 2022;4: 100032. https://doi.org/10.1016/j.hsr.2022.100032.
Alam S, Hasan MK, Neaz S, Hussain N, Hossain MF, Rahman T. Diabetes mellitus: insights from epidemiology, biochemistry, risk factors, diagnosis. Compl Compre Manag Diabetol. 2021;2(2):36–50. https://doi.org/10.3390/diabetology2020004.
Furihata K, Mimura H, Urva S, Oura T, Ohwaki K, Imaoka T. A phase 1 multiple-ascending dose study of tirzepatide in Japanese participants with type 2 diabetes. Diabetes Obes Metab. 2021;24(2):239–46. https://doi.org/10.1111/dom.14572.
Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, Van derVelden WJ, Stutsman C, Cardona GR, Urva S, Emmerson PJ, Holst JJ, D’Alessio DA, Coghlan MP, Rosenkilde MM, Campbell JE, Sloop KW. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI insight. 2020;5(17): 140532. https://doi.org/10.1172/jci.insight.140532.
Wang L. Designing a dual GLP-1R/GIPR agonist from tirzepatide: comparing residues between tirzepatide, GLP-1, and GIP. Drug Des Dev Ther. 2022;16:1547–59. https://doi.org/10.2147/dddt.s358989.
Sattar N, McGuire DK, Pavo I, Weerakkody GJ, Nishiyama H, Wiese RJ, Zoungas S. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022;28(3):591–8. https://doi.org/10.1038/s41591-022-01707-4.
Szayna M, DoyleM E, Betkey JA, Holloway HW, Spencer RGS, Greig NH, Egan JM. Exendin-4 decelerates food intake, weight gain, and fat deposition in zucker rats. Endocrinology. 2000;141(6):1936–41. https://doi.org/10.1210/endo.141.6.7490.
Urva S, Coskun T, Loghin C, Cui X, Beebe E, O’Farrell L, Briere DA, Benson C, Nauck MA, Haupt A. The novel dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 ( GLP -1) receptor agonist tirzepatide transiently delays gastric emptying similarly to selective long-acting GLP -1 receptor agonists. Diabetes Obes Metab. 2020;22(10):1886–91. https://doi.org/10.1111/dom.14110.
Samms RJ, Christe ME, Collins KAL, Pirro V, Droz BA, Holland AK, Friedrich JL, Wojnicki S, Konkol DL, Cosgrove R, Furber EPSC, Ruan X, O’Farrell LS, Long AM, Dogra M, Willency JA, Lin Y, Ding L, Cheng CC, Cabrera O. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. J Clin Investig. 2021. https://doi.org/10.1172/JCI146353.
Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, Milicevic Z, Haupt A, Robins DA. Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. J Clin Endocrinol Metab. 2020. https://doi.org/10.1210/clinem/dgaa863.
Heise T, Mari A, DeVries JH, Urva S, Li J, Pratt EJ, Coskun T, Thomas MK, Mather KJ, Haupt A, Milicevic Z. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 2022. https://doi.org/10.1016/S2213-8587(22)00085-7.
Heise T, DeVries JH, Urva S, Li J, Pratt EJ, Thomas MK, Mather KJ, Karanikas CA, Dunn J, Haupt A, Milicevic Z, Coskun T. Tirzepatide reduces appetite, energy intake, and fat mass in people with type 2 diabetes. Diabetes Care. 2023. https://doi.org/10.2337/dc22-1710.
Fontanella RA, Ghosh P, Pesapane A, Taktaz F, Puocci A, Franzese M, Feliciano MF, Tortorella G, Scisciola L, Sommella E, Ambrosino C, Paolisso G, Barbieri M. Tirzepatide prevents neurodegeneration through multiple molecular pathways. J Trans Med. 2024. https://doi.org/10.1186/s12967-024-04927-z.
Sinha R, Papamargaritis D, Sargeant JA, Davies MJ. Efficacy and safety of tirzepatide in type 2 diabetes and obesity management. J Obesity Metab Syndrome. 2023. https://doi.org/10.7570/jomes22067.
Taktaz F, Scisciola L, Fontanella RA, Pesapane A, Ghosh P, Franzese M, Tortorella G, Puocci A, Sommella E, Signoriello G, Olivieri F, Barbieri M, Paolisso G. Evidence that tirzepatide protects against diabetes-related cardiac damages. Cardiovasc Diabetol. 2024. https://doi.org/10.1186/s12933-024-02203-4.
Heerspink JLH, Sattar N, ImrePavo HA, Duffin KL, Yang Z, Wiese RJ, Wilson JM, Hemmingway A, David ZI. Effects of tirzepatide versus insulin glargine on cystatin c-based kidney function: a surpass-4 post hoc analysis. Diabetes Care. 2023. https://doi.org/10.2337/dc23-0261.
Urva S, Quinlan T, Landry J, Martin J, Loghin C. Effects of renal impairment on the pharmacokinetics of the dual gip and glp-1 receptor agonist tirzepatide. Clin Pharmacokinet. 2021;60(8):1049–59. https://doi.org/10.1007/s40262-021-01012-2.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. https://doi.org/10.1002/hep.28431.
Tariq R, Axley P, Singal AK. Extra-hepatic manifestations of nonalcoholic fatty liver disease: a review. J Clin Exp Hepatol. 2020;10(1):81–7. https://doi.org/10.1016/j.jceh.2019.07.008.
Quek J, Chan KE, Wong ZY, Tan C, Tan B, Lim WH, Tan DJH, Tang ASP, Tay P, Xiao J, Yong JN, Zeng RW, Chew NWS, Nah B, Kulkarni A, Siddiqui MS, Dan YY, Wong VW-S, Sanyal AJ, Noureddin M. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(1):20–30. https://doi.org/10.1016/s2468-1253(22)00317-x.
Younossi ZM, Golabi P, De Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. https://doi.org/10.1016/j.jhep.2019.06.021.
Gastaldelli A, Cusi K, FernándezLandó L, Bray R, Brouwers B, Rodríguez Á. — Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022. https://doi.org/10.1016/s2213-8587(22)00070-5.
Author information
Authors and Affiliations
Contributions
S and SG Conceptualization and literature review, S, SD, L, D and SB: formal analysis, S, SD, L: writing–original draft preparation, S, SD: writing–review and editing, SB, SG: All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sweta, Gupta, S., Bansal, S. et al. Tirzepatide a novel anti diabetic molecule unfold dual action. Discov Public Health 21, 75 (2024). https://doi.org/10.1186/s12982-024-00200-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12982-024-00200-2