Abstract
Human Papillomavirus (HPV) infection poses a significant health burden in Latin America and the Caribbean (LAC), leading to various conditions from benign to malignant, including cervical, anal, and oropharyngeal cancers. This systematic review encompassed 24 studies with a total of 14,466 participants, exploring HPV vaccine acceptance in the region. It was conducted using the following databases: PubMed, Scopus, Ovid Medline, and Web of Science. The review reveals an 84% prevalence of HPV vaccine acceptance in the LAC. Factors influencing acceptance include education, income levels, and vaccine safety concerns. Peru and Honduras exhibited the highest acceptance rates, while the coronavirus disease 2019 (COVID-19) pandemic contributed to declining acceptance post-2019. The importance of educational campaigns and healthcare recommendations in promoting vaccine acceptance is highlighted, along with the impact of reduced vaccination access during the pandemic. This study underscores the critical role of ongoing educational initiatives and accessible healthcare in maintaining high HPV vaccine acceptance rates in LAC. Addressing the reduced acceptance during the pandemic is pivotal for reinstating effective vaccination programs. Findings emphasize the need of sustained efforts to ensure widespread vaccine acceptance, thereby mitigating the burden of HPV-related diseases in the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Human Papillomavirus (HPV) is a double-stranded circular DNA virus, belonging to the family Papillomaviridae. There are more than 100 types of HPV, which can be classified into two categories: low-risk HPVs (ie. 6, 11, 40, 42, 43, 44) and high-risk HPV (ie. 16, 18) [1,2,3], based on its oncogenic potential. HPV infection can lead to a variety of manifestations, from benign, such as anogenital or skin warts, to malignant, such as cervical, anal, and oropharyngeal cancer, in both men and women [4].
In the United States, around 14,000 women are diagnosed with cervical cancer each year, leading to about 6000 deaths. Approximately 3.9% of women test positive for HPV-16/18, which causes around 71.2% of invasive cervical cancer cases [5]. In contrast, in South America, Central America and the Caribbean there are about 59,439 new cases of cervical cancer are diagnosed annually [6]. These high infection rates are not only limited to the female population. A systematic review conducted in 2021 by Farahmand M, on the prevalence of penile HPV infection among men who have sex with men (MSM), revealed a prevalence rate of 36.2% across 13 countries worldwide, with Mexico having the highest prevalence at 94.0% [7].
HPV vaccines greatly reduce the risk of developing cervical cancer or other locations for which the virus has a predilection. Currently, there are 3 types of vaccines, the bivalent that covers HPV subtypes 16 and 18, the quadrivalent against subtypes 6, 11, 16 and 18, and finally the nine-valent which covers the aforementioned subtypes adding 31, 33, 45, 52 and 58 [8]. It is recommended that individuals receive the HPV vaccine before becoming sexually active [9].
According to the Centers for Disease Control and Prevention (CDC), vaccination is recommended for children aged 11–12, with a two-dose regimen administered 6–12 months apart. However, if vaccination starts after the age of 15, three doses are advised, with each dose spaced 6 months apart [9]. To note, the age ranges vary according to the vaccination scheme of each country.
Vaccine acceptability varies between group populations. In Yang, Z et al. meta-analysis performed in 2021, the level of approval among men who engage in sexual activity with other men (MSM) among 15 studies was 50%, and factors such possessing a college education or higher (OR = 1.62, 95% CI 1.35–1.95), openly discussing sexual orientation with healthcare providers (OR = 2.38, 95% CI 1.47–3.86), and being knowledgeable about HPV (SMD = 0.28, 95% CI 0.16–0.39), were described to promote HPV vaccine acceptance [10]. Moreover, a study carried out in 2021 by Seema, K et al., demonstrated a high acceptance rate (79.17%) for vaccination from a total of 384 girls, from Eastern UP [11]. In Latin America, a study carried out in 2017 by Munguia-Daza, F et al., on the attitude towards vaccination among parents from a school in a city of Peru found that about favorable in 24.7%, indifferent in 55.9% and unfavorable in 19.4% [12]. Similarly, a study conducted in 2016 by Chaparro, R et al. in Argentina revealed an overall acceptance rate of 46.6% (95% CI 34.8–58.6) [13].
Prior to the pandemic, in a 2019 comparative analysis, an estimated 73% (95 CI 50–83%) of adolescent girls in Latin America and the Caribbean, among 28 countries with available data, were vaccinated with at least one dose of HPV vaccine compared to an estimated 50% (95 CI 35–63%) among adolescent girls in high-income countries [14]. Despite these studies, there remains a gap in understanding the overall prevalence of HPV vaccine acceptance specifically in Latin America and the Caribbean. Therefore, the objective of this systematic review is to assess the prevalence of HPV vaccine acceptance in this region.
2 Methods
2.1 Study design, register and report guideline
We conducted this systematic review in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [15]. A concise outline of the protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration code CRD42023481925.
2.2 Data sources and searches
We conducted a comprehensive literature search to assess the acceptance of HPV vaccination in Latin America and the Caribbean. This search encompassed the following databases: PubMed, Scopus, Ovid Medline and Web of Science; searches were conducted on 3rd May 2023, and articles from inception until this date were included. However, we only found articles from 2001 to 2022 that met our selection criteria. We utilized both DeCS/MeSH keywords and free-text terms to enhance the search strategy. There were no language limitations. (Supplemental Material S1).
2.3 Study selection and data extraction
The articles retrieved from the aforementioned databases were transferred to the Rayyan data management software [16], eliminating duplicated studies. Three reviewers independently assessed each study (FES-V, EAA-B, JME-G) by examining the titles and abstracts based on the inclusion criteria. We included (1) Cross-sectional and Cohort studies that (2) assessed the acceptance proportion of vaccination against HPV in LAC in (3) Children and Adults. We excluded studies that were (1) Case reports (2) Narrative Reviews (3) Scoping Reviews (4) Conference Abstracts (5) Systematic Reviews, and Letters to Editors (6).
After completing this stage, we thoroughly reviewed each article in full text to determine if they met the predefined selection criteria. Articles were excluded if they did not meet these criteria. In cases where necessary information was missing, we attempted to reach out to the corresponding author for clarification. Additionally, we conducted a secondary bibliographic search based on the articles that passed the full-text review stage. If there were disagreements regarding whether to include or exclude an article, these were resolved by consensus. Subsequently, the relevant information from the selected articles was extracted and compiled into a standardized Excel spreadsheet. The extracted data encompassed various aspects such as title, author, country, publication year, study design, target population, demographic characteristics (age, sex), details of questionnaire administration, survey date, responses recorded for vaccine acceptance, and acceptance rates.
2.4 Assessment of study quality and publication bias
To assess the quality of the studies incorporated into the systematic review, we employed the Newcastle Ottawa Scale adapted for Cross-sectional studies (NOS-CS) and Newcastle Ottawa Scale adapted for Cohort studies [17]. A study with a rating of 7 or more stars was classified as having low risk of bias, while a study with fewer than 7 stars was deemed to have high risk of bias. The included studies were independently analyzed by seven reviewers (FES-V, EAA-B, JME-G, VAV-C, VJ-A, AMC-L, KLH-R), and any disagreement regarding study quality was resolved by a team consensus after examination of the article.
In this systematic review, we opted against assessing publication bias because conventional funnel plots and Egger’s tests were deemed inaccurate for proportional meta-analysis in previous studies. Two main reasons supported this decision. First, there was a lack of evidence indicating that proportions could be appropriately adjusted for these tests. Second, the tests employed to evaluate publication bias were constructed based on the premise that studies yielding positive results were published more frequently than those with negative outcomes. However, within the framework of this particular study design, there was no consensus on what constitutes a positive outcome.
2.5 Data synthesis and analysis
STATA 16.0 was used to combine the data obtained from the articles. A pooled analysis was conducted to determine the rates of acceptance. To account for the expected high heterogeneity between studies, a random effects model (DerSimonian and Laird) was employed for the quantitative analysis. The Clopper-Pearson method was utilized to calculate the 95% CI. Heterogeneity between studies was evaluated using the Cochran’s Q test and I2 statistic, with values over 60% indicating high heterogeneity for the I2 statistic and a P-value < 0.05 considered indicative of heterogeneity for the Cochran’s Q test. The studies were stratified into subgroups for meta-analysis based on participants’ age (adults vs. children), sexual orientation, gender, and parental status. We performed a sensitivity analysis excluding studies with low methodological quality.
3 Results
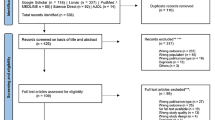
The systematic search retrieved 1610 studies, which were then imported into the data management tool “Rayyan QCRI.” Following this, duplicates (n = 851) were identified and removed. A total of 759 articles underwent assessment based on their titles and abstracts, resulting in the exclusion of 702 studies that did not fulfill the selection criteria outlined for this systematic review. In total, 57 articles were reviewed in their full-text, and 24 studies were included in the qualitative and quantitative synthesis. The selection process is summarized using the PRISMA flowchart (Fig. 1).
3.1 Study characteristics
Out of the 24 articles included in this study, 23 were cross-sectional studies [18,19,20,21,22, 24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41], and 1 was a cohort study [23]. A total of 14,466 participants were included. 77.29% were female, 21.42% were male, 1.23% were transwomen and 0.04% did not indicate their gender. Most of the questionnaires were administered face-to-face (21 out of 24), one was administered through an online survey, one was administered through phone call and finally one was administered through a combination of telephone/face-to-face questionnaire. Geographically, the studies were distributed as follows: 7 from Mexico [20, 30, 32, 38, 39, 41], 5 from Puerto Rico [23,24,25, 35, 40], 5 from Argentina [18, 19, 21, 22, 29], 3 from Brazil [26,27,28], 2 from Honduras [36, 37], 1 from Peru [33], and 1 from Haiti [31]. The different studies evaluated the acceptance rate of HPV vaccine in multiple populations. The publication years of these studies ranged from 2001 to 2022 (Table 1).
3.2 Assessment of study quality and publication bias
The quality assessment of the studies included in this meta-analysis was conducted utilizing the Newcastle–Ottawa Assessment Scale for both cross-sectional and cohort studies [17]. The quality scores ranged from 6 to 9 stars. Nineteen studies were classified as high-quality (≥ 7 stars), and five studies as low-quality (≤ 6 stars). Since most administered surveys were conducted in person, the selection risk is diminished compared to virtual surveys, which are only available to those with internet access and electronic devices, considering that some countries have high poverty levels (Tables 2 and 3).
3.3 Pooled estimates of the included studies
A meta-analysis was conducted to analyze the acceptability of the HPV vaccine in Latin America and the Caribbean. The pooled prevalence of HPV vaccine acceptability was 84% (95% CI 0.80–0.88, I2: 98.40%). It is important to note that 70.8% of the studies included in this meta-analysis are concentrated in three countries (Mexico, Argentina, and Puerto Rico), while the remaining 29.2% come from different parts of Latin America and the Caribbean (Fig. 2).
3.4 Subgroup analysis
Regarding subgroup analysis by country and study years, it was found that Honduras (92%, CI 95%: 0.90–0.94) and Peru (90%, CI 95%: 0.87–0.92) had the highest aggregated prevalence of HPV vaccine acceptance compared to Mexico (87%, CI 95%: 0.84–0.91, I2: 96.17%), Brazil (82%, CI 95%: 0.54–0.93), and Haiti (82%, CI 95%: 0.78–0.86), Argentina (81%, CI 95%: 0.68–0.95, I2: 99.30%) and Puerto Rico (73%, CI 95%: 0.01–0.06, I2: 98.73%) (Fig. 3).
In this meta-analysis, the analyzed articles were categorized into two time periods: 2001–2014 and 2015–2022. The highest pooled prevalence of HPV vaccine acceptability was observed during the years 2001–2014 (n = 11, 87%, 95% CI 0.83–0.91, I2: 93.76%). Comparatively, the pooled prevalence of HPV vaccine acceptability was lower during the years 2015–2022 (n = 13, 81%, 95% CI 0.76–0.86, I2: 98.88%) (Fig. 4).
3.5 Sensitivity analysis
After eliminating studies with a high risk of bias, there were no significant changes in heterogeneity or the POOLED prevalence of HPV vaccine acceptability (85%, 95% CI 0.81–0.89, I2: 98.15) (Fig. 5).
4 Discussion
According to the United Nations Development Programme, vaccine distribution globally is influenced by a complex array of factors, encompassing politics, economics, social dynamics, diplomacy, and public health considerations [42]. In line with this, the Pan American Health Organization endorsed the resolution titled “Reinvigorating Immunization as a Public Good for Universal Health” in 2021, aiming to narrow gaps in vaccine accessibility through six strategic approaches [43]. Currently, 47 countries and territories in the Americas have incorporated the HPV vaccine into their national immunization schedules [44]. The primary aim of our systematic review was to evaluate HPV vaccine prevalence in Latin America and the Caribbean, yielding an HPV vaccine acceptability rate of 84%. However, limitations in accessibility impede further increases in this percentage, particularly given evidence suggesting the viability of both two-dose and single-dose regimens, with the latter being available in only 10 countries and territories across the region [44]. It is imperative to underscore the importance of accurate and up-to-date data in guiding global endeavors toward achieving equitable vaccine distribution and addressing existing disparities [42].
These results are similar or even higher than acceptance prevalence figures worldwide. In Prue et al.’s systematic review performed in 2016 [45], individual studies from the regions of the US, Europe, Africa, Oceania, and Asia were evaluated, showing acceptance prevalence in adolescent males with average values ranging from 30 to 70%. Our results inform countries in Latin America and the Caribbean, as one of the regions with the highest prevalence of HPV, second only to Africa [46].
Our study can be compared to others worldwide. In Europe, an average acceptance of 64.3% (range 45.6–79.5%) in adolescents and 59.2% (range 32.2–65.6%) for parents has been observed, with the highest acceptance prevalence in countries like Iceland (90.9%), Sweden (90.5%), Denmark (80%), and the United Kingdom (76.7%) [47]. Cunningham et al.’s systematic review in Africa reported acceptability ranging from 59 to 100%, although a wider gap between African countries is evident in Gamagoun’s study, ranging from 20.4 to 99% [47]. Furthermore, studies included in Young’s meta-analysis, covering countries such as Australia, China, Malaysia, New Zealand, South Korea, Taiwan, and Thailand, revealed prevalences ranging from 31 to 88% [49].
Today, most countries in Latin America and the Caribbean have the same vaccination schedule for children aged 9–13. The schedule may be based on three doses (0, 1, 6) or two doses, as some countries do (0 and 6) [50]. The first country in the region to introduce the vaccine was Puerto Rico in 2006, followed by Panama in the same year. Other countries introduced it in the following 3 years, while some, like Venezuela, have yet to implement it [51]. Initially, the vaccine was intended for implementation in females only, but gradually, in some countries, it has also been implemented for males, as is the case in Peru. By 2021, according to the latest WHO reports, the country with the highest population coverage was the Dominican Republic, with 79% of the population protected, while Mexico had the lowest coverage at just 0.5%. Despite this, most countries in Latin America and the Caribbean have seen an increase in coverage [52].
The high prevalence of vaccine acceptance may be attributed to the increased education provided today, particularly to parents who allow vaccination of minors, as well as various implementation measures in countries. Vaccination during medical visits, the mandatory requirement of vaccination for secondary education enrollment (e.g., Puerto Rico), and the use of media with clear and concise messages are some of the implementations that have led to high coverage rates in some countries, such as Argentina [50]. Additionally, a greater fear among vaccine recipients and their guardians of the possibility of cervical cancer may be another reason. In 2020, according to the GLOBOCAN report, there were 604,000 new cases and 342,000 deaths worldwide, with 80% originating from Latin America, the Caribbean, sub-Saharan Africa, and Southeast Asia [50]. In Peru, there was an 11.5% increase in cervical cancer cases (4270 new cases) for the same year, making it the second most prevalent cancer in women, second only to breast cancer. It is estimated that by 2030, the incidence of cancer will increase by 32% more people diagnosed in the region [54].
Other factors associated with high HPV vaccine acceptance include sociodemographic factors such as being female, young parental age, high household income, and having completed other childhood vaccinations [47]. Race also plays a role; Chao et al. demonstrated that Hispanic female adolescents are more likely to complete the vaccination schedule than Black females but less likely than Caucasian females [55]. In males, receiving a recommendation for the vaccine from a doctor, having it provided for free, and having evidence of its safety in males from research studies increase acceptance [56].
However, certain factors hinder HPV vaccine acceptance in some countries. Lack of information about vaccine safety or misinterpretation of their daughters' freedom to engage in sexual activity is one of the main factors among Hispanics [57]. Completing another vaccination schedule late or not completing it is another associated factor; a negative reaction to the vaccine as an adverse effect did not significantly affect acceptance [58]. Additionally, in young women, lack of information about HPV infection susceptibility and severity, lower educational levels, and fewer visits to gynecology clinics are associated [59].
Finally, in our study, there was a decrease in HPV vaccination acceptance between the periods from 2001 to 2014 (87%) and from 2015 to 2022 (81%). One of the main reasons we believe this decrease was found is because 5 number of studies were conducted during the years of the COVID-19 public health emergency, from early 2020 to early 2023 according to the WHO, are included. During the pandemic, not only was there a decrease in access to regular vaccination services, but there were also several anti-vaccine campaigns that instilled fear of vaccination in both parents and independent adults [60]. Ackerson et al., performed in 2021, documented a 45% decrease in routine vaccine doses for children aged 0–23 months in early March 2022, compared to the same time range in 2019. For children aged 2 years or older, there was a 94% decrease in 2022 compared to 2019 [61]. On the other hand, a study conducted in 2021 by Díaz et al. found that vaccination coverage in children had significantly decreased even before the pandemic, due to factors such as reduced state funding for healthcare, poor access to healthcare services, inadequate vaccine supply, and a shortage of healthcare workers [62].
4.1 Limitations of the study
The study has several limitations. First, there is heterogeneity among the included studies in the meta-analysis, as they may have differences in study design, participant characteristics, and other factors that could affect the results. Second, meta-analyses tend to narrow their focus on specific research questions or variables, which may not fully capture the complexity and diversity of real-world phenomena. Additionally, confounding variables, which are not directly measured but can influence the relationship between the variables of interest, may not be fully accounted for in the meta-analysis. Lastly, the results of a meta-analysis may not be universally applicable, as they often pertain to specific populations or contexts and may not generalize to other populations or settings.
5 Conclusions
In conclusion, our study highlights the importance of ongoing educational campaigns and healthcare recommendations to promote HPV vaccine acceptance [63, 64]. These initiatives play a crucial role in maintaining high vaccine acceptance rates, especially considering LAC´s high prevalence of HPV [46]. Addressing the decline in acceptance during the pandemic is essential for reinstating effective vaccination programs. The decrease in acceptance observed during the COVID-19 pandemic underscores the urgent need to address challenges in vaccine access and combat anti-vaccine sentiments [65]. This underscores the critical role of accessible healthcare services and educational initiatives in sustaining high HPV vaccine acceptance rates in LAC [66].
Data availability
The datasets used and/or analyzed during the current study are not publicly accessible but can be requested from the corresponding author upon reasonable request.
References
Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27. https://doi.org/10.1056/nejmoa021641.
Kombe Kombe AJ, Li B, Zahid A, Mengist HM, Bounda G-A, Zhou Y, et al. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Front Public Health. 2021. https://doi.org/10.3389/fpubh.2020.552028.
Wang R, Pan W, Jin L, Huang W, Li Y, Wu D, et al. Human papillomavirus vaccine against cervical cancer: opportunity and challenge. Cancer Lett. 2020;471:88–102. https://doi.org/10.1016/j.canlet.2019.11.039.
Della Fera AN, Warburton A, Coursey TL, Khurana S, McBride AA. Persistent human papillomavirus infection. Viruses. 2021;13(2):321. https://doi.org/10.3390/v13020321.
Related Cancers, Fact Sheet. United States of America. Hpvcentre.net. https://hpvcentre.net/statistics/reports/USA_FS.pdf
Human Papillomavirus and Related Diseases Report. Hpvcentre.net. 2023. https://hpvcentre.net/statistics/reports/XWX.pdf
Farahmand M, Monavari SH, Tavakoli A. Prevalence and genotype distribution of human papillomavirus infection in different anatomical sites among men who have sex with men: a systematic review and meta-analysis. Rev Med Virol. 2021. https://doi.org/10.1002/rmv.2219.
Nogueira-Rodrigues A. HPV vaccination in Latin America: global challenges and feasible solutions. Am Soc Clin Oncol Educ Book. 2019;39:e45-52. https://doi.org/10.1200/edbk_249695.
CDC. La vacuna contra el VPH para preadolescentes y adolescentes. Centers for Disease Control and Prevention. 2023 https://www.cdc.gov/vaccines/parents/diseases/hpv-sp.html
Zhao Y, Xin X, Deng H, Xu J, Weng W, Zhang M, et al. Improving the acceptability of human papillomavirus vaccines among men who have sex with men according to the associated factors: a systematic review and meta-analysis. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2021.600273.
Kumari S, Singh A, Sangal R, Sharma NR. KAP study on cervical cancer and human papillomavirus vaccine acceptability among adolescent girls of Eastern UP: a cross sectional study. Int J Reprod Contracept Obstet Gynecol. 2021;10(5):2031. https://doi.org/10.1820/2320-1770.ijrcog20211533.
Munguia-Daza F, Huaranga-Santiago E. Aceptación de la vacuna contra el virus del papiloma humano en padres de familia de niñas de primaria. Huánuco, 2017. Rev Peru Investig Salud. 2019;3(2):62–7. https://doi.org/10.3583/repis.3.2.261.
Aceptación de la vacuna contra el virus del papiloma humano y los factores asociados en la ciudad de Resistencia, Chaco. Arch Argent Pediatr. 2016 https://doi.org/10.5546/aap.2016.36
De Oliveira LH, Janusz CB, Da Costa MT, El Omeiri N, Bloem P, Lewis M, et al. HPV vaccine introduction in the Americas: a decade of progress and lessons learned. Expert Rev Vaccines. 2022;21(11):1569–80. https://doi.org/10.1080/14760584.2022.2125383.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1):b2700–b2700. https://doi.org/10.1136/bmj.b2700.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016. https://doi.org/10.1186/s13643-016-0384-4.
Ottawa hospital research institute. Ohri.ca. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Alder S, Gustafsson S, Perinetti C, Mints M, Sundström K, Andersson S. Mothers’ acceptance of human papillomavirus (HPV) vaccination for daughters in a country with a high prevalence of HPV. Oncol Rep. 2015;33(5):2521–8.
Alder S, Perinetti C, Mints M, Belkić K, Sundström K, Sandin S, et al. Acceptance of human papillomavirus (HPV) vaccination among young women in a country with a high prevalence of HPV infection. Int J Oncol. 2013;43(4):1310–8.
Allen-Leigh B, Rivera-Rivera L, Yunes-Díaz E, Portillo-Romero AJ, Brown B, León-Maldonado L, et al. Uptake of the HPV vaccine among people with and without HIV, cisgender and transgender women and men who have sex with men and with women at two sexual health clinics in Mexico City. Hum Vaccin Immunother. 2020;16(4):981–90.
Arrossi S, Maceira V, Paolino M, Sankaranarayanan R. Acceptability and uptake of HPV vaccine in Argentina before its inclusion in the immunization program: a population-based survey. Vaccine. 2012;30(14):2467–74.
Chaparro RM, Em Vargas V, Zorzo LR, Genero S, Cayre A. Acceptance of human papillomavirus vaccination and associated factors in the city of Resistencia, Argentina. Arch Argent Pediatr. 2016. https://doi.org/10.5546/aap.2016.eng.36.
Colón-López V, Medina-Laabes DT, Abreu RS, Díaz Miranda OL, Ortiz AP, Fernández ME, et al. Understanding parents’ views toward the newly enacted HPV vaccine school entry policy in Puerto Rico: a qualitative study. BMC Public Health. 2021. https://doi.org/10.1186/s12889-021-11952-w.
Colón-López V, Quiñones V, Del Toro-Mejías LM, Conde-Toro A, Serra-Rivera MJ, Martínez TM, et al. HPV awareness and vaccine willingness among Dominican immigrant parents attending a federal qualified health clinic in Puerto Rico. J Immigr Minor Health. 2015;17(4):1086–90.
Colón-López V, Del Toro-Mejías LM, Ortiz AP, Tortolero-Luna G, Palefsky JM. HPV awareness and willingness to HPV vaccination among high-risk men attending an STI clinic in Puerto Rico. P R Health Sci J. 2012;31(4):227–31.
de Oliveira MSF, Sorpreso ICE, Zuchelo LTS, da Silva ATM, de Gomes J, Silva BKRM, et al. Knowledge and acceptability of HPV vaccine among HPV-vaccinated and unvaccinated adolescents at Western Amazon. Rev Assoc Med Bras. 2020;66(8):1062–9. https://doi.org/10.1590/1806-9282.66.8.1062.
Ferreira GRON, da Formigosa JAS, de Lira ALBC, Reis RK, Gir E, Freitas WLS, et al. The family health strategy influence on the human papillomavirus vaccine acceptance in a peripheral community of the Brazilian Amazon region. Health Equity. 2022;6(1):852–61.
Gattegno MV, Vertamatti MAF, Bednarczyk RA, Evans DP. A cross-sectional survey of parental attitudes towards human papillomavirus vaccination exclusion categories in Brazil. BMC Int Health Hum Rights. 2019. https://doi.org/10.1186/s12914-019-0195-5.
Gentile A, Pacchiotti AC, Giglio N, Nolte MF, Talamona N, Rogers V, et al. Vaccine hesitancy in Argentina: validation of WHO scale for parents. Vaccine. 2021;39(33):4611–9.
Godoy Verdugo MK, Zonana Nacach A, Anzaldo Campos MC. Acceptance of the vaccine against human papilloma virus from mothers to daughters aged 9 to 13 years old. Ginecol Obstet Mex. 2013;81(11):645–51.
Halliday D, Butler R, Francis D, Thompson J, Joseph M, Ragin CC. Knowledge and attitudes toward HPV and the HPV vaccines in the Bahamas. West Indian Med J. 2014. https://doi.org/10.7727/wimj.2012.318.
Lazcano-Ponce E, Rivera L, Arillo-Santillán E, Salmerón J, Hernández-Avila M, Muñoz N. Acceptability of a human papillomavirus (HPV) trial vaccine among mothers of adolescents in Cuernavaca, Mexico. Arch Med Res. 2001;32(3):243–7.
Lee FH, Paz-Soldan VA, Carcamo C, Garcia PJ. Knowledge and attitudes of adult Peruvian women vis-à-vis human papillomavirus (HPV), cervical cancer, and the HPV vaccine. J Low Genit Tract Dis. 2010;14(2):113–7. https://doi.org/10.1097/lgt.0b013e3181c08f5e.
León-Maldonado L, Cabral A, Brown B, Ryan GW, Maldonado A, Salmerón J, et al. Feasibility of a combined strategy of HPV vaccination and screening in Mexico: the FASTER-Tlalpan study experience. Hum Vaccin Immunother. 2019;15(7–8):1986–94.
Morales-Campos DY, Vanderpool RC. Examining differences in HPV awareness and knowledge and HPV vaccine awareness and acceptability between U.S. Hispanic and island Puerto Rican women. J Health Dispar Res Pract. 2017;10(3):1–18.
Perkins RB, Langrish SM, Cotton DJ, Simon CJ. Maternal support for human papillomavirus vaccination in Honduras. J Womens Health. 2011;20(1):85–90.
Perkins RB, Mehta PK, Langrish SM. Fathers’ intentions to accept human papillomavirus vaccination for sons and daughters: exploratory findings from rural Honduras. Int J Public Health. 2012;57(1):143–8.
Portillo-Romero AJ, León-Maldonado L, Allen-Leigh B, Brown B, Magis C, García-Fuentes NB, et al. HPV vaccine acceptance is high among adults in Mexico, particularly in people living with HIV. Salud Publica Mex. 2018;60(6):658.
Ramírez-Rios AD, Bonnez W. Attitudes affecting the potential use of human papillomavirus vaccination: a survey of health promotion students in Mexico city. J Community Health. 2014;39(2):266–73.
Roura-Monllor J, Nieves-Muñoz J, Ortiz AP, Romaguera J. HPV knowledge, vaccine knowledge, and vaccine acceptance in women with cervical cytology anomalies attending colposcopy clinics in Puerto Rico. Int J Gynaecol Obstet. 2018;143(1):52–8.
Sánchez Anguiano LF, Lechuga Quiñones AM, Milla Villeda RH, Lares Bayona EF. Knowledge and acceptance of vaccine against human papillomavirus among mothers of students from Durango city, Mexico. Ginecol Obstet Mex. 2013;81(2):77–85.
Global dashboard for vaccine equity. UNDP. https://data.undp.org/insights/vaccine-equity
May 28. CE168/14 - reinvigorating immunization as a public good for universal health. Paho.org. https://www.paho.org/en/documents/ce16814-reinvigorating-immunization-public-good-universal-health
Barnabas RV, Brown ER, Onono MA, Bukusi EA, Njoroge B, Winer RL, et al. Efficacy of single-dose human papillomavirus vaccination among young African women. NEJM Evid. 2022. https://doi.org/10.1056/EVIDoa2100056.
Prue G, Shapiro G, Maybin R, Santin O, Lawler M. Knowledge and acceptance of human papillomavirus (HPV) and HPV vaccination in adolescent boys worldwide: a systematic review. J Cancer Policy. 2016;10:1–15. https://doi.org/10.1016/j.jcpo.2016.09.009.
Papilomavirus humano y cáncer. Who.int. https://www.who.int/es/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer
López N, Garcés-Sánchez M, Panizo MB, de la Cueva IS, Artés MT, Ramos B, et al. HPV knowledge and vaccine acceptance among European adolescents and their parents: a systematic literature review. Public Health Rev. 2020. https://doi.org/10.1186/s40985-020-00126-5.
Al Mandhari A. WHO regional office for the Eastern Mediterranean. Working together to improve lives in the Eastern Mediterranean region. East Mediterr Health J. 2018;24(6):503–503. https://doi.org/10.2671/2018.24.6.503.
Young A. HPV vaccine acceptance among women in the Asian Pacific: a systematic review of the literature. Asian Pac J Cancer Prev. 2010;11(3):641–9.
Salazar Fajardo LJ, Benavides Delgado MR, Boogaard S, Marín Y. Estrategias latinoamericanas para la vacunación contra el virus del papiloma humano—una revisión temática. Hacia Promoc Salud. 2017;22(2):129–43. https://doi.org/10.1715/hpsal.2017.22.2.10.
Luciani S, Bruni L, Agurto I, Ruiz-Matus C. HPV vaccine implementation and monitoring in Latin America. Salud Publica Mex. 2018;60(6):683. https://doi.org/10.2114/9090.
IM Coverage. Paho.org. https://ais.paho.org/imm/IM_JRF_COVERAGE.asp
Martínez V. Cáncer de cuello uterino: tendencias para el 2030 en América Latina. Oceano Medicina. Océano Medicina; 2022. https://pe.oceanomedicina.com/nota/actualidad-pe/cancer-cuello-uterino-tendencias-2030/
Cervical cancer is the third most common cancer among women in Latin America and the Caribbean, but it can be prevented. Paho.org. https://www.paho.org/en/news/1-2-2019-cervical-cancer-third-most-common-cancer-among-women-latin-america-and-caribbean-it
Chao C, Velicer C, Slezak JM, Jacobsen SJ. Correlates for human papillomavirus vaccination of adolescent girls and young women in a managed care organization. Am J Epidemiol. 2010;171(3):357–67. https://doi.org/10.1093/aje/kwp365.
Ferris DG, Waller JL, Miller J, Patel P, Price GA, Jackson L, et al. Variables associated with human papillomavirus (HPV) vaccine acceptance by men. J Am Board Fam Med. 2009;22(1):34–42. https://doi.org/10.3122/jabfm.2009.01.080008.
Jeudin P, Liveright E, del Carmen MG, Perkins RB. Race, ethnicity and income as factors for HPV vaccine acceptance and use. Hum Vaccin Immunother. 2013;9(7):1413–20. https://doi.org/10.4161/hv.24422.
Marlow LAV, Waller J, Wardle J. Trust and experience as predictors of HPV vaccine acceptance. Hum Vaccin. 2007;3(5):171–5. https://doi.org/10.4161/hv.3.5.4310.
Restivo V, Costantino C, Fazio T, Casuccio N, D’Angelo C, Vitale F, et al. Factors associated with HPV vaccine refusal among young adult women after ten years of vaccine implementation. Int J Environ Res Public Health. 2018;15(4):770. https://doi.org/10.3390/ijerph15040770.
Saxena S, Skirrow H, Bedford H. Routine vaccination during covid-19 pandemic response. BMJ. 2020. https://doi.org/10.1136/bmj.m2392.
Ackerson BK, Sy LS, Glenn SC, Qian L, Park CH, Riewerts RJ, et al. Pediatric vaccination during the COVID-19 pandemic. Pediatrics. 2021. https://doi.org/10.1542/peds.2020-047092.
Díaz-Badillo Á, Garibay-Nieto GN, Navas-Figueroa AL, Perales-Torres AL, Morales-Gómez MC, López-Alvarenga JC. La vacunación en el contexto de la pandemia de COVID-19. Cir Cir. 2021. https://doi.org/10.2487/ciru.21000487.
Levy MS, Finch L, Lindsay KA, Jeudin P, Huang M. Leveraging teachable moments in cancer prevention by improving HPV vaccination in health professional students (HPS): a systematic review. Front Oncol. 2022. https://doi.org/10.3389/fonc.2022.978843.
Chyderiotis S, Sicsic J, Raude J, Bonmarin I, Jeanleboeuf F, Le Duc Banaszuk A-S, et al. Optimising HPV vaccination communication to adolescents: a discrete choice experiment. Vaccine. 2021;39(29):3916–25. https://doi.org/10.1016/j.vaccine.2021.05.061.
Daniels V, Saxena K, Roberts C, Kothari S, Corman S, Yao L, et al. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: a model based analysis. Vaccine. 2021;39(20):2731–5. https://doi.org/10.1016/j.vaccine.2021.04.003.
Sichero L, Picconi MA, Villa LL. The contribution of Latin American research to HPV epidemiology and natural history knowledge. Braz J Med Biol Res. 2020;53(2):e9560.
Acknowledgements
None.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
F.E.S.V: Conceptualization, Methodology, Project administration, Formal analysis, Methodology, Validation, Visualization, Writing—original draft. E.A.A.B: Conceptualization, Resources, Software, Supervision, Validation, Writing—review & editing. J.M.E.G: Conceptualization, Formal analysis, Supervision, Validation, Witing—original draft. V.A.V.C: Investigation, Data curation, Writing—original draft. K.H.R: Investigation, Data curation, Writing—original draft. A.C.L: Investigation, Data curation, Writing—original draft. V.J.A: Investigation, Data curation, Writing – original draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Salazar-Valdivia, F.E., Alarcon-Braga, E.A., Estrada-Grossmann, J.M. et al. HPV vaccine acceptance in Latin America and the Caribbean: a systematic review and meta-analysis. Discov Public Health 21, 22 (2024). https://doi.org/10.1186/s12982-024-00146-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12982-024-00146-5