Abstract
Background
Adolescents are lagging behind in the “third 95” objective of the Joint United Nations Program on HIV/AIDS requiring 95% of individuals on antiretroviral therapy (ART) to have viral load (VL) suppression. This study aimed to describe factors associated with viral non-suppression among adolescents in Mbale district, Uganda.
Methods
We conducted a retrospective review of routinely collected HIV programme records. Data such as age, education, ART Regimen, ART duration, WHO Clinical stage, comorbidities, etc., were extracted from medical records for the period January 2018 to December 2018. Descriptive analysis was done for continuous variables using means and frequencies to describe study sample characteristics, and to determine the prevalence of outcome variables. We used logistic regression to assess factors associated with VL non-suppression among adolescents.
Results
The analysis included 567 HIV-infected adolescents, with 300 (52.9%) aged between 13 to 15 years, 335 (59.1%) female, and mean age of 15.6 years (interquartile range [IQR] 13.5–17.8. VL non-suppression was 31.4% (178/567). Male sex (AOR = 1.78, 95% CI 1.06, 2.99; p < 0.01), age 16–19 years (AOR = 1.78, 95% CI 1.06, 2.99; p < 0.05), No formal education (AOR = 3.67, 95% CI 1.48–9.09; p < 0.01), primary education (AOR = 2.23, 95% CI 1.05–2.32; p < 0.01), ART duration of > 12 months to 5 years (AOR = 3.20, 95% CI 1.31–7.82; p < 0.05), ART duration > 5 years (AOR = 3.47, 95% CI 1.39– 8.66; p < 0.01), WHO Clinical Stage II (AOR = 0.48, 95% CI: 0.28, 0.82; p < 0.01), second-line ART regimen (AOR = 2.38, 95% CI 1.53–3.72; p < 0.001) and comorbidities (AOR = 3.28, 95% CI 1.20–9.00; p < 0.05) were significantly associated with viral non-suppression.
Conclusions
VL non-suppression among adolescents was almost comparable to the national average. VL non-suppression was associated with being male, age 16–19 years, education level, duration on ART therapy, WHO Clinical Staging II, second-line ART regimen, and presence of comorbidities. Adolescent-friendly strategies to improve VL suppression e.g. peer involvement, VL focal persons to identify and actively follow-up non-suppressed adolescents, patient education on VL suppression and demand creation for ART are needed, especially for newly-initiated adolescents and adolescents on ART for protracted periods, to foster attainment of the UNAIDS 95–95–95 targets.
Similar content being viewed by others

Introduction
Globally, as of 2020, of all people living with HIV, 84% knew their status, 73% were accessing treatment and 66% were virally suppressed in 2020 [1]. While this shows improvement, this progress is highly unequal, because Sub-Saharan Africa is lagging far behind with adolescents carrying the burden of viral-load non-suppression [1]. Adolescents, especially young women and girls in sub-Saharan Africa, continue to be disproportionately affected, contributing the largest part of the overall HIV non-suppression prevalence of 47% [2]. In Uganda, six years since the operationalization of viral load monitoring, viral suppression among adolescents is still sub-optimal, at 73.7%. However, information on viral load suppression among adolescents remains scarce in Sub-Saharan Africa [3].
Viral suppression is critical in HIV treatment success, ensuring a decrease AIDS-associated morbidity and mortality, and reduction in risk of viral transmission. Low- and middle-income countries, define viral suppression as a viral load (VL) < 1000 copies/ml. It is an indicator of ART effectiveness and treatment adherence. WHO defines 'Adolescents' as individuals in the 10–19 years age group [4]. Follow-up of virological suppression status among adolescents in crucial for quick identification of treatment failure, of patients in need of intensive adherence counseling, and prevents occurrence of drug resistance, leading to unwanted switch to costly and limited ART regimens [3].
The “third 95” of the UNAIDS 95/95/95 strategy to alleviate the epidemic seeks to have 95% of the HIV-infected individuals on ART virally suppressed by 2025 [2]. The “third 95” is critical in the battle to defeat HIV/AIDS because virally suppressed individuals can barely transmit the virus [5]. Unfortunately, as of 2020, merely 66% of PLHIV on ART were virally suppressed worldwide, with adolescents contributing a bulk of the unsuppressed [6].
As of 2017, only 70% of the HIV-infected in Uganda are virally suppressed, with children, adolescents, and young adults making up about 65% of the unsuppressed group [7]. Furthermore, due to the common mode of age disaggregation employed in Uganda where adolescents between 13 and 15 are grouped with children aged 0–15 while those aged 16–19 are considered adults, specific data on adolescent VL suppression is limited [7]. Consequently, while adolescents contribute a substantial number of virally non-suppressed patients, there is limited data on the viral outcomes of this demographic [8].
Moreso, adolescents face distinctive challenges in taking ART such as normal juvenile forgetfulness and failure to take such issues seriously, disclosure concerns, and stigma, all contributing to high non-suppression rates [9]. Likewise, until recently, there has been a paucity of adolescent-friendly services and research, leading to under-diagnosis of, and disregard for, their unique challenges causing high non-suppression rates [10]. The lack of adolescent-centric HIV research has contributed to a deficiency of data on the determinants of VL non-suppression among adolescents. The very few papers available used data from the largely urbanized central region, ignoring rural societies in the East such as Mbale district [8].
Additionally, non-suppressed adolescents are more prone to HIV-related morbidity and mortality that could be circumvented through IAC and support [11]. Also, IAC mitigates subpar adherence that is common among adolescents and leads to drug-resistant HIV strains, which require expensive second-line or third-line treatments [12].
The influence of various determinants of VL non-suppression varies among populations, age groups, and settings, requiring contextual data to inform remedial measures among adolescents [13]. Therefore, understanding the distinct determinants of VL non-suppression among adolescents will give all stakeholders new insight into HIV infection among adolescents. It will further motivate adolescents to adhere to medication, thus sustaining the relevance of affordable first-line treatments by preventing drug resistance and also and help attainment of the third UNAIDS 95–95–95 target [2, 14]. This study aimed to describe factors associated with non-viral suppression among adolescents in Mbale district, Uganda.
Methods
Study design
We conducted a retrospective review of routinely collected data that employed methods of secondary data analysis of routinely available program data on HIV-infected adolescents receiving ART in Mbale district for the period of January and December 2018.
Study setting
Mbale district (Fig. 1) [15] in Eastern Uganda boasts a population of 488,960 as of 2016. 51% are female, and over 90% of the population is rural. Mbale District is a largely impoverished district with a high HIV prevalence. While the general prevalence of HIV in Mbale is 5.3%, that of adolescents is 7% [16]. Primary care facilities in Uganda are organized by administrative division and include Health Center II (parish), Health Center III (sub-county), and Health Center IV (county). Mbale has numerous health facilities, including special clinics, HCIII, HCIV, and the regional referral hospital providing HIV services, backed by the government and private funders (Fig. 2) [15, 17]. In Uganda, since 2016, the test and treat strategy is employed for all newly HIV diagnosed persons as per the WHO recommendations. VL testing is performed after 6 months following ART initiation and every 6 months for those that have HIV VL suppression. At the enrollment into the HIV care clinics, socio-demographic data on adolescents is recorded on client treatment cards (blue card), and clinical history is also documented on these cards at each visit thereafter. VL test results received from the national laboratory center for each client who had a blood sample drawn for VL testing are recorded in viral load registers and blue cards by health facility staff and provided to adolescents at subsequent clinic visits [18]. HIV-positive adolescents at five purposively selected high-volume health centers of different levels (regional referral hospital, HCIV, HCIII, special clinic), and located in different geographical regions of the district were included in the study [16]. Because of the health center selection criteria, it transpired that the higher-level centres had higher volumes and thus would provide a large number of potential study participants. Furthermore, these higher level centres serve large catchment areas, coupled with patients or adolescents from far-flung areas of the district. On that basis, we could not ignore them for smaller health centres (which also usually have challenged of doing viral load tests), thus, we ended up with 5 health centres at three health centre levels.
Study population
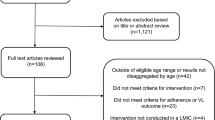
We included all adolescents aged 13 to 19, on ART for at least 6 months, and had VL tests at the selected facilities. We excluded those with an unknown duration on ART, those on ART for less than six months, and those who had no VL test results. After calculation of the sample size of 383 adolescents required for the study using the Leslie Kish equation for cross-sectional studies [19] sample size equation for cross-section studies, the proportionate allocation method was used to determine the number of participants to be randomly selected from each health facility [20] Socio-demographic and clinical data including age, sex, level of education, ART Regimen, ART duration, WHO Clinical stage, comorbidities were analyzed.
Ethical considerations
A district support letter was secured prior to attainment of local ethical approval from a local Research Ethics Committee (Mildmay Uganda REC; #REC REF 08,010-2019) and then from the University of Liverpool (Reference Number H00065262). Anonymized data was sent through a secure e-mail address. The raw data will be securely retained for five years, then discarded. The investigator was aware of the sensitive nature of the study, and that the necessary data for the study pertained to a vulnerable group. However, the anonymized data and the lack of access to participant files fully protected the group from any risk of breach of confidentiality.
Data collection
Data collection occurred over 2 weeks in January 2020 after obtaining all approvals. Data were abstracted from the sources below;
-
Paper registers: The VL registers
-
Electronic ART registers where applicable
-
Client treatment record (blue card) at all the health facilities.
Initially, a list of all adolescents on ART for at least 6 months at each facility was generated from the electronic database or entered into a Microsoft® Excel database 2007 version for health facilities that lacked electronic records. Each patient was given a unique study code by the facility staff, and the list of codes from each health facility was sent to the study investigator who then randomly selected the number of specified participants for each facility. The list of codes of selected participants was then posted back to health facility staff who linked the codes to participants whose records were eventually abstracted.
The health facility staff were trained on the specific indicators to be entered into the excel sheet, and the investigator visited the health facilities during the data collection process to oversee and ensure quality of the process. The study team reviewed all the records of the participants that were abstracted to ensure data quality, and corrections were performed on records with observed data errors.
Data on key demographic variables such as age and sex were abstracted from the registers and blue cards into the excel sheet. Data on the start date of ART was used to calculate the duration on ART at the time of the VL test. The date of VL testing, the date VL test results were received at the facility, and the date VL test results provided to the adolescents were all updated in a Microsoft® Excel database.
Data analysis
Data analysis was conducted based on the primary outcome of interest in the study using the Statistical Package for the Social Sciences (SPSS) version 20.0 (IBM Corp. Armonk, NY, USA). The primary outcome “VL non-suppression” was defined as an adolescent having a VL load above the WHO-recommended detectable threshold of 1000 copies/ml after six months on ART [21]. Socio-demographic variables like age, sex, level of education, and clinical variables like ART regimen, comorbidities, or duration on ART, served as independent variables. Descriptive analysis was done for continuous variables using means depending on the normality of the distribution of the variables, and frequencies to describe the characteristics of the study sample and to determine the prevalence (proportions) of the outcome variables. We used a multivariable logistic regression model to assess factors related to VL non-suppression. Pearson’s chi-square test was used to test relationships between categorical variables. Factors with p ≤ 0.2 in bivariate analyses were included in the multivariable model. p-values of ≤ 0.05 were considered statistically significant.
Results
We enrolled a total of 567 adolescents, more than half of the adolescents (52.9% /300) were aged between 13 and 15 years, and the mean age of the study participants was 15.6 ± 2.10. 335 (59.1%) of the participants were female. The majority of the study participants (336 or 59.3%) had attained primary education. Furthermore, 482 (85%) of the adolescents received ART services from the regional referral hospital. The mean duration on ART therapy for the study participants was 74.3 ± 41.7 months, with the largest proportion of the participants (323 or 57%) on ART therapy for over 5 years. The majority of participants (414 or 73%) were receiving 1st line of the ART regimen, and the presence of comorbidities was observed among 19 participants. About half of the adolescents (279 or 49.3%) were in WHO Clinical disease stage II (refer to Table 1).
Out of the total 567 adolecents enrolled in the study, 178 (31.4%) had VL load non-suppression. Being male (odds ratio [OR] = 1.21; 95% CI 1.12, 2.48; p ≤ 0.01), lacking formal education (OR = 3.29; 95% CI 1.51, 7.13; p ≤ 0.01), having co-morbidities (OR = 3.12; 95% CI 1.23, 7.89; p ≤ 0.05), being on second line ART regimen (OR = 2.38; 95% CI 1.61, 3.51; p ≤ 0.001) were significantly associated with VL non-suppression in univariate analysis (Table 2). Similary, adolescents on ART > 5 years and those on ART for > 12 months- < 5 years were three times (OR = 3.07; 95% CI 1.46, 6.47 p ≤ 0.05 < 0.01) and over two times (OR = 2.29; 95% CI 1.05, 4.98; p ≤ 0.01), respectively, more likely to be non-suppressed than those on ART for less than a year.
In the adjusted model, being male (adjusted OR [AOR] = (AOR = 1.78, 95% CI 1.06, 2.99; p < 0.01) lacking formal education (AOR = 3.67, 95% CI 1.48–9.09; p < 0.01), and attending primary education (AOR = 2.23, 95% CI 1.05–2.32; p < 0.01) were significantly associated with VL non-suppression. Also, relative to age; older adolescents aged 16–19 (AOR = 1.78, 95% CI 1.06, 2.99; p < 0.05), and those with WHO clinical stage II (AOR = 0.48, 95% CI 0.28, 0.82; p < 0.01), had higher odds of viral non-suppression. Longer duration on ART for > 5 years (AOR = 3.47, 95% CI 1.39–8.66; p < 0.01), and > 12 months to < 5 years (AOR = 3.20, 95% CI 1.31–7.82; p < 0.05), being on second line ART regimen (AOR = 2.38, 95% CI 1.53–3.72; p < 0.001) and having comorbidities (AOR = 3.28, 95% CI 1.20–9.00; p < 0.05) all remained significantly associated with VL non-suppression in the adjusted model (Table 2).
Discussion
In this cross-sectional study of 567 HIV-infected adolescents on ART in rural Uganda, in which the mean age was 15.6 years, we found that 31.7% of the adolescents had viral non-suppression during the study period. Being male, age 16–19 years, lack of formal education, primary education level, duration on ART therapy for > 12 months to ≤ 5 years and > 5 years, WHO Clinical Staging II, second-line ART regimen, and presence of comorbidities were significantly associated with viral non-suppression.
Our finding of a VL non-suppression rate of 31.4% found in this study is almost comparable to the national age-specific rate of 27.5% among adolescents in 2017/2018 [18]. A cross-sectional study by Natukunda et al. in Uganda [8] found the VL non-suppression prevalence among adolescents to be 34.5%, which is a little higher than this study’s rate. This study’s rate is also higher than that reported for low- and middle-income countries (29%) and a lot higher than that reported in South Africa (19%) [22]. Adolescents in Uganda, therefore, show a higher rate of viral non-suppression and are lagging behind other age groups in achieving the 95–95–95 targets, making them a weak link in the collective effort to reach the goal above [18]. The possibility of having good adherence yet with poor viral outcomes cannot be ruled out in a setting like Uganda where treatment options are not regularly updated and there is limited routine ART resistance testing [4]. Besides only reporting the proportion of adolescents with non-suppressed viral loads, the present study also identified independent predictors of viral non-suppression among this critical population. The findings in the study suggest the need for HIV clinical services to design and implement targeted interventions with a particular focus on male gender, co-morbidities, adolescents on second line ART and those on longer duration of ART.
The generally poorer adherence to ART among males is possibly related to a “background reluctance to access healthcare” and might lead to a higher likelihood of viral non-suppression [23]. The results are consistent with a cross-sectional study conducted in Malawi [24], a Mozambican case–control study [25], and an observational cohort study [26] in Cameroon where more males were non-suppressed than females. However, a study in Tanzania found that the female gender was independently associated with VL non-suppression [27].
Having a comorbidity, such as tuberculosis in most of the cases, increased the odds of being virally non-suppressed. The pill burden among such adolescents might probably explain the poor adherence and the subsequent non-suppression. Also, drug-to-drug interaction may affect the efficacy of ARVs, leading lead to non-suppression [28, 29]. These findings are in line with a study in Zimbabwe and in South Africa, which showed that adolescents on prolonged medication for another disease were likelier to be virally non-suppressed [28, 29].
Additionally, being on the second-line of ART increased the odds of being virally non-suppressed. Many adolescents on second-line regimens likely have a history of treatment disturbances like poor adherence, leading to virologic failure, and a switch to second-line. A study found that deficient adherence to first-line ART portended suboptimal adherence to second-line ART [30]. A retrospective cross-sectional study in Ethiopia [31] and a case–control study in Eswatini [32] found that EFV-based ART was more likely cause to VL suppression compared to NVP-based regimens.
Furthermore, the longer the adolescents were on ART, the higher their odds of VL non-suppression. It is plausible that since longer ART duration is typical of perinatally-infected adolescents, non-suppression may be a result of drug resistance over time, leading to non-suppression or treatment fatigue leading to reduced adherence to ART [8]. A systematic review [33] showed that longer ART duration was associated with higher levels of acquired ART resistance. This is consistent with studies in Uganda [8] and in Swaziland [35], where VL load non-suppression was higher among adolescents on ART for 5 years and above. However, a study in South Africa found that adolescents on ART between 6 months and one year were more likely to be non-suppressed compared to those on treatment for more extended periods [36].
Regarding older age and VL non-suppression, it is conceivable that because older adolescents usually have been on ART for longer periods, they suffer from medication fatigue and miss appointments, leading to poorer adherence [31, 37]. Additionally, older adolescents are in a complex, transitory life period, associated with an inability to make important life decisions, and more sensitivity to stigma, leading to poorer outcomes [38]. This finding was consistent with cross-sectional studies in Uganda [13] and Zimbabwe [38] which showed that older adolescents were likelier to be non-suppressed compared to younger adolescents. However, a retrospective study in Kenya [39] and a prospective cohort study in Tanzania [27] found no association between VL non-suppression and age; and that older adolescence was associated with VL non-suppression, respectively. In this study, because of the age disaggregation employed in Uganda where ages 0–14 are considered children while those 16–19 are considered adults [34], we found it appropriate to use similar disaggregation, thus the 16 years old cut off.
Also, adolescents in higher clinical stages likely have a history of treatment disturbances, which may continue and lead to increased occurrence of VL non-suppression [40]. A study in Ethiopia [31] also showed an increased likelihood of viral non-suppression in clinical stage II compared stage I, while another in Uganda [34] found no association between clinical stage and VL non-suppression. While a study in Swaziland [37] and another in Nigeria [41] found that stage 3 or stage 4 was significantly associated with VL non-suppression, the very few adolescents in stage 3 or 4 might explain why the analysis did not reveal any association between these stages and VL non-suppression in this study. Nevertheless, the findings are more-or-less congruent with the above-stated studies and point to a direct relationship between increasing clinical stage and VL non-suppression.
Lastly, less the education the adolescents had, the higher their odds of VL non-suppression. It is potentially because the more educated adolescents appreciated more the importance of taking medication, and these also seemed to have more parental support compared to the uneducated [42]. The findings are in line with a cross-sectional study in Ethiopia, where uneducated participants were more likely to be non-suppressed compared to educated ones [42]. However, a study in Uganda found that adolescents who attended school, especially higher school, had inferior ART adherence and this was attributed to the stigma and lack of privacy in a school setting for poor adherence and outcomes [43].
The strengths of this cross-sectional study include the use of five high-volume HIV programmes scattered across the district and use of probability proportional to size sampling to identify adolescents thus, making the sample more representative of adolescents in Eastern Uganda, and, subsequently, increasing the external validity of the study [44]. The major limitation of this study was the usage of routine data that was no collected for research purposes, which handicapped the researcher as regards the exposure variables that could be studied. Our limitation was that we did not analyze data on variables like household income or distance to the health facilities that would have allowed us to control for more concealed demographic confounders, because they are not routinely collected in public health facilities. Lastly, the cross-sectional design did not also allow for analysis of time-varying covariates.
Conclusion
In conclusion, we found that adolescents in Mbale District, continue to face worse HIV viral load outcomes with almost one-third of adolescents in Mbale District virally non-suppressed, a prevalence higher than the national average. The determinants of viral load non-suppression among adolescents are unique and thus require unique interventions. Urgent adolescent-friendly strategies to decrease non-suppression rates such a peer involvement, VL focal persions to identify VL non-suppression and actively follow up with these adolescents, patient education on VL non-suppression, and demand creation for ART especially for newly initiated adolescents and adolescents on ART for protracted periods, should be laid with these determinants in mind so that they (strategies) are targeted, if efforts to achieve HIV epidemic control in resource-limited settings are to be successful.
Availability of data and materials
The dataset used and analyzed during this study is available from the corresponding author on reasonable request.
Abbreviations
- AIDS:
-
Acquired Immune Deficiency Syndrome
- ART:
-
Anti-retroviral treatment
- DHO:
-
District Health Officer
- HIV:
-
Human Immunodeficiency Virus
- IAC:
-
Intensified Adherence Counselling
- MoH:
-
Ministry of Health
- PLHIV:
-
People Living with HIV
- UAC:
-
Uganda AIDS Commission
- UBOS:
-
Uganda Bureau of Statistics
- UNAIDS:
-
Joint United Nations Programme on HIV/AIDS
- USAID:
-
United States Agency for International Development
- VL:
-
Viral Load
- WHO:
-
World Health Organization
References
Joint United Nations Programme on HIV/AIDS (UNAIDS). Global Aids Update 2021: Confronting Inequalities. Lessons for pandemic responses from 40 years of AIDS. 2021 p. 386.
UNAIDS. Understanding Fast-Track: Accelerating Action to End the AIDS Epidemic by 2030. Author Geneva; 2015.
Katumba A. Sub-optimal hIV viral suppression and its determinants among adolescents in the post-viral load monitoring scale-up era in Mulago Iss Clinic and Bwizibwera Hiv Clinic in Uganda [thesis]. Makerere University; 2019 [2021 Sept 12]. http://makir.mak.ac.ug/handle/10570/7762
World Health Organization. Global Fund, US Centers for Disease Control and Prevention. HIV drug resistance report 2017. Geneva: World Health Organization; 2017. p. 82. https://apps.who.int/iris/handle/10665/255896. Accessed 12 Sept 2021.
Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–8.
UNAIDS. Ending AIDS: progress towards the 90–90–90 targets. Geneva: Joint United Nations Programme on HIV. AIDS. 2017;
Ministry of Health. Uganda HIV/AIDS country progress report July 2016-June 20187: Reaching men, girls and young women to reduce new HIV infections. 2017. https://www.unaids.org/sites/default/files/country/documents/UGA_2018_countryreport.pdf
Natukunda J, Kirabira P, Ong KIC, Shibanuma A, Jimba M. Virologic failure in HIV-positive adolescents with perfect adherence in Uganda: a cross-sectional study. Trop Med Health. 2019;47(1):8.
Davies M-A, Moultrie H, Eley B, Rabie H, Van Cutsem G, Giddy J, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa-The IeDEA Southern Africa Collaboration. J Acquired Immune Defic Syndr. 2011;56(3):270.
Nasuuna E, Kigozi J, Babirye L, Muganzi A, Sewankambo NK, Nakanjako D. Low HIV viral suppression rates following the intensive adherence counseling (IAC) program for children and adolescents with viral failure in public health facilities in Uganda. BMC Public Health. 2018;18(1):1048.
Chhim K, Mburu G, Tuot S, Sopha R, Khol V, Chhoun P, et al. Factors associated with viral non-suppression among adolescents living with HIV in Cambodia: a cross-sectional study. AIDS Res Ther. 2018;15(1):20.
Mbonye M, Seeley J, Ssembajja F, Birungi J, Jaffar S. Adherence to antiretroviral therapy in Jinja, Uganda: a six-year follow-up study. PLoS ONE. 2013;8(10):e78243.
Bulage L, Ssewanyana I, Nankabirwa V, Nsubuga F, Kihembo C, Pande G, et al. Factors associated with Virological non-suppression among HIV-positive patients on antiretroviral therapy in Uganda, august 2014–July 2015. BMC Infect Dis. 2017;17(1):326.
Rowley CF. Developments in CD4 and viral load monitoring in resource-limited settings. Clin Infect Dis. 2013;58(3):407–12.
Monje F, Erume J, Mwiine FN, et al. Knowledge, attitude and practices about rabies management among human and animal health professionals in Mbale District. Uganda One Health Outlook. 2020;2:24. https://doi.org/10.1186/s42522-020-00031-6.
Ministry of Health. Ministry of Health: Health Management Information System 106 Database. 2019.
Ministry of Health. A national health facility master list 2018: a complete list of all health facilities in Uganda. 2018. http://library.health.go.ug/publications/health-facility-inventory/national-health-facility-master-facility-list-2018.
Uganda AIDS Commission. Uganda AIDS Country Progress Report July 2017-June 2018: Enhancing HIV Mainstreaming towards ending AIDS as a Public Health threat in Uganda by 2030. 2018.
Kish L. Survey sampling. 1965.
Rao TJ. Optimum allocation of sample size and prior distributions: a review. International Statistical Review/Revue Internationale de Statistique. 1977;173–9.
WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization; 2016.
Boerma RS, Boender TS, Bussink AP, Calis JC, Bertagnolio S, Rinke de Wit TF, et al. Suboptimal viral suppression rates among HIV-infected children in low-and middle-income countries: a meta-analysis. Clin Infect Dis. 2016;ciw645.
Boullé C, Kouanfack C, Laborde-Balen G, Boyer S, Aghokeng AF, Carrieri MP, et al. Gender Differences in Adherence and Response to Antiretroviral Treatment in the Stratall Trial in Rural District Hospitals in Cameroon. JAIDS J Acquir Immune Defic Syndr. 2015;69(3):355–64.
Umar E. ART Adherence among Adolescents & Young People Living with HIV in Southern Malawi: A Conditional Process Analysis. 2017.
Penot P, Héma A, Bado G, Kaboré F, Soré I, Sombié D, et al. The vulnerability of men to virologic failure during antiretroviral therapy in a public routine clinic in Burkina Faso. J Int AIDS Soc. 2014;17(1):18646.
Njom Nlend AE, Motaze AN, Ndiang ST, Fokam J. Predictors of Virologic Failure on First-line Antiretroviral Therapy Among Children in a Referral Pediatric Center in Cameroon [Internet]. 2017 [cited 2020 Feb 5]. https://www.ingentaconnect.com/content/wk/inf/2017/00000036/00000011/art00014
Muri L, Gamell A, Ntamatungiro AJ, Glass TR, Luwanda LB, Battegay M, et al. Development of HIV drug resistance and therapeutic failure in children and adolescents in rural Tanzania: an emerging public health concern. AIDS. 2017;31(1):61–70.
Sithole Z, Mbizvo E, Chonzi P, Mungati M, Juru TP, Shambira G, et al. Virological failure among adolescents on ART, Harare City, 2017- a case-control study. BMC Infect Dis. 2018;18(1):469.
Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in Southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65–71.
Ramadhani HO, Bartlett JA, Thielman NM, Pence BW, Kimani SM, Maro VP, et al. Association of First-Line and Second-Line Antiretroviral Therapy Adherence. Open Forum Infectious Diseases. 2014;
Desta AA, Woldearegay TW, Futwi N, Gebrehiwot GT, Gebru GG, Berhe AA, et al. HIV virological non-suppression and factors associated with non-suppression among adolescents and adults on antiretroviral therapy in northern Ethiopia: a retrospective study. BMC Infect Dis. 2020;20(1):4.
Chouraya C, Ashburn K, Khumalo P, Mpango L, Mthethwa N, Machekano R, et al. Association of Antiretroviral Drug Regimen With Viral Suppression in HIV-positive Children on Antiretroviral Therapy in Eswatini. Paediatric Infect Dis J. 2019;38:835.
Stadeli KM, Richman DD. Rates of emergence of HIV drug resistance in resource-limited settings: a systematic review. Antivir Ther. 2013;18(1):115.
Natukunda J, Kirabira P, Ong KIC, Shibanuma A, Jimba M. Virologic failure in HIV-positive adolescents with perfect adherence in Uganda: a cross-sectional study. Tropical medicine and health. 2019;47(1):8.
Jobanputra K, Parker LA, Azih C, Okello V, Maphalala G, Jouquet G, et al. Impact and programmatic implications of routine viral load monitoring in Swaziland. J Acq Immune Deficiency Syndromes. 2014;67(1):45.
Joseph Davey D, Abrahams Z, Feinberg M, Prins M, Serrao C, Medeossi B, et al. Factors associated with recent unsuppressed viral load in HIV-1-infected patients in care on first-line antiretroviral therapy in South Africa. Int J STD AIDS. 2018;29(6):603–10.
Jobanputra K, Parker LA, Azih C, Okello V, Maphalala G, Kershberger B, et al. Factors Associated with Virological Failure and Suppression after Enhanced Adherence Counselling, in Children, Adolescents and Adults on Antiretroviral Therapy for HIV in Swaziland. PLoS ONE. 2015;10(2):e0116144.
Makadzange AT, Higgins-Biddle M, Chimukangara B, Birri R, Gordon M, Mahlanza T, et al. Clinical, Virologic, Immunologic Outcomes and Emerging HIV Drug Resistance Patterns in Children and Adolescents in Public ART Care in Zimbabwe. PLoS ONE. 2015;10(12):e0144057.
Mwangi AW. Factors associated with viral suppression among adolescents on antiretroviral therapy in Homabay County, Kenya. 2019. http://etd.uwc.ac.za/xmlui/handle/11394/6877. Accessed 12 Sept 2021.
Sovershaeva E. HIV-infection in children and adolescents in Zimbabwe: viral suppression, airway abnormalities and gut microbiota. 2019. https://munin.uit.no/handle/10037/16909. Accessed 12 Sept 2021.
Greig JE, du Cros PA, Mills C, Ugwoeruchukwu W, Etsetowaghan A, Grillo A, et al. Predictors of raised viral load during antiretroviral therapy in patients with and without prior antiretroviral use: a cross-sectional study. PLoS ONE. 2013;8(8):e71407.
Mekuria LA. Retention in care, viral suppression, treatment adherence and quality of life in a public antiretroviral therapy program in Addis Ababa, Ethiopia [PhD Thesis]. Universiteit van Amsterdam; 2016.
MacCarthy S, Saya U, Samba C, Birungi J, Okoboi S, Linnemayr S. “How am I going to live?”: exploring barriers to ART adherence among adolescents and young adults living with HIV in Uganda. BMC Public Health. 2018;18(1):1158.
Bruce N, Pope D, Stanistreet D. Quantitative methods for health research. Division of Public Health: University of Liverpool, UK; 2008. p. 1–538.
Acknowledgements
The authors thank the District Health Officer and other Mbale district personnel whose contributions made this work possible.
Funding
This study was solely funded by JM. The content is entirely the responsibility of the authors and does not necessarily represent the official views of Mbale district administration.
Author information
Authors and Affiliations
Contributions
JM designed the study and wrote the first draft along with RN. JM and MRK collected the data and performed the statistical analyses. NM, JAB, CNK, HK participated in the study conception and interpretation of results. AB supervised all stages of study implementation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval to conduct this retrospective chart review was obtained from the University of Liverpool Board of Ethics (H00065262), Mildmay Uganda Research Ethics Committee (08010-2019), and Uganda National Council for Science and Technology (HS501ES). Administrative clearance was obtained from the District Health Officer of Mbale district.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Maena, J., Banke-Thomas, A., Mukiza, N. et al. Determinants of viral load non-suppression among adolescents in Mbale District, Eastern Rural Uganda. AIDS Res Ther 18, 91 (2021). https://doi.org/10.1186/s12981-021-00408-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12981-021-00408-1