Abstract
Background
Reduction of the reservoir of latent HIV-infected cells might increase the possibility of long-term remission in individuals living with HIV. We investigated factors associated with HIV-1 proviral DNA levels in children receiving different antiretroviral therapy (ART) strategies in the children with HIV early antiretroviral therapy (CHER) trial.
Methods
Infants with HIV < 12 weeks old with CD4% ≥ 25% were randomized in the CHER trial to early limited ART for 40 or 96 weeks (ART-40 W, ART-96 W), or deferred ART (ART-Def). For ART-Def infants or following ART interruption in ART-40 W/ART-96 W, ART was started/re-started for clinical progression or CD4% < 25%. In 229 participants, HIV-1 proviral DNA was quantified by PCR from stored peripheral blood mononuclear cells from children who had received ≥ 24 weeks ART and two consecutive undetectable HIV-1 RNA 12–24 weeks apart. HIV-1 proviral DNA was compared between ART-Def and ART-96 W at week 96, and in all arms at week 248. Factors associated with HIV-1 proviral DNA levels were evaluated using linear regression.
Findings
Longer duration of ART was significantly associated with lower HIV-1 proviral DNA at both 96 (p = 0.0003) and 248 weeks (p = 0.0011). Higher total CD8 count at ART initiation was associated with lower HIV-1 proviral DNA at both 96 (p = 0.0225) and 248 weeks (p = 0.0398). Week 248 HIV-1 proviral DNA was significantly higher in those with positive HIV-1 serology at week 84 than those with negative serology (p = 0.0042).
Intepretation
Longer ART duration is key to HIV-1 proviral DNA reduction. Further understanding is needed of the effects of “immune-attenuation” through early HIV-1 exposure.
Funding
Wellcome Trust, National Institutes of Health, Medical Research Council.
Similar content being viewed by others
Introduction
An estimated 3.3 million children under 15 years of age live with human immunodeficiency virus (HIV) worldwide, over 90% in sub-Saharan Africa [1, 2]. The introduction of antiretroviral therapy (ART) early in life has substantially reduced morbidity and mortality [3, 4] and optimised CD4 cell reconstitution [5]. Although HIV-1 virological suppression is achievable in most children, as in adults, HIV remains latent and integrated within the host genome in subpopulations of infected cells [6]. This reservoir, commonly estimated by quantitative measures of HIV-1 proviral DNA [7, 8], occurs in many cell types including CD34 stem cells, CNS macrophages, astrocytes and dendritic cells [9,10,11]: however resting CD4 memory cells are considered a critical reservoir due to their longevity, homeostatic cell division and potential for reactivation on antigen encounter [12].
There is increasing interest in viral reservoirs following reports of HIV remission (also known as functional cure) in adults and children treated soon after infection [13]. Remission is defined as lack of detectable virus in blood and a functional immune system without the need for ART, despite detectable HIV using sensitive assays. The case report of the “Mississippi Baby” who received ART from 31 hours of age focused attention on very early ART in children. The mother discontinued ART around 15–18 months of age and when retested at 23 months old, the infant had undetectable plasma HIV-RNA and only traces of proviral DNA just above detectable limits at 24 and 26 months of age. The infant maintained virological suppression for 27.6 months before viral resurgence [14, 15]. One of 227 early-treated children from the children with HIV early antiretroviral therapy (CHER) trial stopped ART after 40 weeks, as per trial randomization, and remained negative for HIV diagnostic tests at the age of 9.5 years [16]. Virus persists at very low levels in plasma and is detectable as low levels of cell-associated DNA, but immunologically he is not unlike healthy children of similar age. There is also a French teenager who received ART from 3 months of age, after a 6 week course of zidovudine was started at birth. After ART was discontinued at approximately 6 years of age, she has remained virologically suppressed for 11 years [17].
Novel approaches to attain HIV remission, including depletion of T-cell subsets known to have integrated HIV-1 DNA, elimination of latent reservoir through activation and clearance mechanisms, and interference with memory CD4 T-cell homeostasis are being pursued [12, 18, 19]. However, as these are not presently considered viable options, early ART initiation remains the therapeutic focus to reduce reservoir size.
Formation and stability of the HIV-1 reservoir in the presence of ART is not well understood [20,21,22], particularly whether its persistence is primarily due to longevity of latently infected cells or low-level replication [23,24,25]. Although earliest possible ART initiation is considered optimal in vertically infected children [26,27,28], it is unclear how timing of initiation, ART duration or ART-interruption within early childhood years, impacts on viral reservoirs and immune responses [29]. Further knowledge in these areas could inform practical approaches towards functional cure; yet even in the absence of a functional cure, the long-term impact of different ART-strategies on reservoir size is of considerable interest with potential to inform future ART management strategies in childhood.
In this sub-study of the CHER trial [3, 4], we compare peripheral HIV-1 proviral DNA in children who received early ART but interrupted ART temporarily versus deferred but continuous ART. We examine the effect of ART-interruption and factors associated with low HIV-1 proviral DNA up until 5 years of age.
Materials and methods
Participants
The CHER trial compared early limited ART (zidovudine, lamivudine and lopinavir-ritonavir) for 40 or 96 weeks (ART-40W or ART-96W) with deferred ART (ART-Def) in HIV-infected infants < 12weeks old with baseline CD4 ≥ 25% [3, 4] enrolled between 2005 and 2008. HIV was diagnosed by HIV DNA PCR and confirmed with RNA viral load (VL) > 1000 copies/ml. For infants on deferred ART or following ART interruption after 40 or 96 weeks, ART was started/re-started for clinical progression (protocol-defined CDC severe stage B/C disease) or CD4% < 25% in infants and < 20% in older children.
HIV-1 proviral DNA was measured by quantitative PCR using DNA extracted from 322 samples of cryopreserved peripheral blood mononuclear cells (PBMCs) collected at 12-weekly time-points from 40 to 248 weeks of the trial in 229 participants (Table 1). The use of stored samples was approved by the Human Research Ethics Committees of Stellenbosch University and the University of the Witwatersrand (M12/01/005 and 040703) for the two trial sites: Children’s Infectious Disease Clinical Research Unit (KIDCRU, now the Family Center for Research with Ubuntu) and The Perinatal HIV Research Unit (PHRU). To minimise inclusion of episomal DNA, samples were restricted to those available from all children on ART who were virally suppressed for at least 24 weeks with two consecutive viral loads below 400 copies/ml 12–24 weeks apart [30]. In addition, children must have adhered to the CHER ART-strategies. Using these criteria, all available specimens were used from trial weeks 40 (ART-96W only), weeks 96 (ART-Def and ART-96W) and week 248 (all arms). Children from ART-Def who fulfilled these criteria at 3 or 4 time-points on continuous ART (96, 156, 204, 248 and 252 weeks) were also analysed to illustrate the trajectory of HIV-1 proviral DNA decline on continuous ART. Together these samples were used to answer the following questions: (1) What factors are associated with low levels of HIV-1 proviral DNA at 96 and 248 weeks of ART? (2) What is the effect of early ART followed by interruption on HIV-1 proviral DNA levels at 248 weeks compared to deferred ART without interruption i.e., comparing all CHER ART-strategies: ART-Def, ART-40W and ART-96W?
We explored the relationship between HIV-1 proviral DNA and clinical and immunological characteristics available from the CHER trial, including baseline (randomisation) viral loads, CMV serostatus and quantification at randomisation, and HIV-1 serostatus and quantitative HIV-specific antibodies (anti-gp120 IgG at week 84 in ART-Def and ART-96W [31]) at trial week 84. Week 84 was the closest available timepoint to week 96 where serum samples were available for analysis from children on ART [31].
DNA extraction of PBMCs
PBMCs were isolated from whole blood using standard Ficoll separation and cryopreserved in liquid nitrogen in 10% dimethyl sulfoxide and 90% fetal calf serum. Cryopreserved PBMCs were thawed to room temperature and DNA extracted using the QIAGEN® QIAmp DNA extraction kit (Hilden, Germany). Extracted DNA was eluted from the mini spin column, quantified on the nanodrop (Thermo Scientific, Massachusetts, USA), and stored at – 20 °C until PCR.
Quantification of total HIV-1 DNA
As described by Smith et al. [32], primers and probe were used to detect total HIV-1 DNA by amplifying the region between LTR and gag. Additionally, primers and probe for human pyruvate dehydrogenase (PDH) were duplexed in the reaction as an internal control. A standard curve was generated using a 6-point logarithmic scale of DNA extracted from 8E5 cells [33] (ATCC), which contain one copy of HIV provirus per cell.
HIV-1 proviral DNA was quantified by real-time PCR using Applied Biosystems 7900HT Fast Real-Time PCR System (TaqMan, Life technologies). For each 25 μl PCR reaction the assay included 12.5 μl of QIAgen Multiplex PCR Master Mix, 0.25 μl of each primer PDH or LTR (concentration 10 μM or 20 μM), and 0.25 μl of each PDH and LTR probe at a concentration of 10μM. PCR conditions were 95°C for 15 minutes, then 45 cycles of 94 °C and 60 °C for 1 minute each. To maximize assay sensitivity, 600 ng of extracted DNA from patient samples was added to each reaction well, with both samples and standards run in triplicate. The lowest limit of detection was 10 proviral copies per 106 PBMCs. Undetectable measures of proviral DNA were repeated for verification.
Statistical analysis
HIV-1 proviral DNA levels were log to base 10 transformed to approximate normality. Factors associated with log10 HIV-1 proviral DNA levels at weeks 96 and 248 were investigated using linear regression. Regression diagnostics were examined to ensure that all model assumptions were met.
Factors investigated included age at ART start, birthweight, sex, duration of ART by weeks 96 and 248, CDC stage, initial ART regimen, baseline VL and immunological data (CD4% and count, CD8% and count), time to initial VL suppression, CMV serology, Prevention of mother to child transmission (PMTCT) received, HIV-specific antibody (anti-gp120 IgG) and HIV-1 serology measured at week 84. Variables significantly associated with log10 HIV-1 proviral DNA levels at p < 0.10 or plausible clinical factors such as CD8 count at week 248, were included in a multivariable model, using backwards stepwise elimination (exit probability p = 0.05) to reach the final model. Differences between two and three groups were tested using the Wilcoxon rank-sum and Kruskal-Wallis rank tests, respectively. Stata version 15.1 (Stata Corporation, College Station, Texas, USA) was used for all analyses.
Results
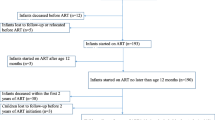
To compare HIV-1 proviral DNA levels between early and deferred ART we analysed 44 PBMC samples from 125 children randomised to ART-Def (35.2%), and 73 samples from 143 children in ART-96W (51%) at 96 weeks. To compare HIV-1 proviral DNA levels across all three arms we analysed 70 PBMC samples from 125 children randomised to ART-Def (56%), 56 samples from 143 children from ART-40W (39%), and 43 samples from 143 children from ART-96W (30%) at 248 weeks (Table 1). Figure 1 illustrates an overview of the CHER trial treatment strategies and median duration on ART within arms. Patient characteristics and follow-up immunology, virological suppression and reservoir size at all measured time-points are described in Tables 2, 3 respectively.
Overview of the CHER trial treatment strategies and median duration on ART within arms from participants analysed for HIV proviral DNA. Median time to ART initiation in the deferred arm, ART-Def, was 26.1 weeks (IQR 20–42.9, n = 79). Median duration of ART-interruption for ART-40 W was 31.3 (12.5–54.3, n = 56) and 52.3 (11.4–152, n = 94) weeks in ART-96 W
Factors associated with low levels of HIV-1 proviral DNA
Time spent on ART by week 96 was significantly shorter in ART-Def [median 84 (IQR 72–84) weeks] versus ART-96W [96 (IQR 96–96), p < 0.0001]. There was significantly more HIV-1 proviral DNA in ART-Def at 96 weeks [median 2415 (IQR 499–7450)] than ART-96W [325 (53–3670) copies of HIV-1 proviral DNA/106 PBMCs, p = 0.0019, Fig. 2A]. Figure 2B illustrates increasing reservoir size by age of initiating ART, and the range of HIV-1 proviral DNA at ART initiation. In multivariable analysis (Table 4a), at 96 weeks, longer duration of ART was significantly associated with lower levels of HIV-1 proviral DNA [ß = − 1.21 (95% CI − 1.85, − 0.57), p = 0.0003]. This suggests a reduction in HIV-1 proviral DNA percentage by 70% for every further year on ART. The same effect is evident at 248 trial weeks (Table 4b) whereby a reduction of 30% HIV-1 proviral DNA is seen for every further year on ART [ß = − 0.36 (95% CI − 0.15, − 0.15), p = 0.0011].
HIV-1 proviral DNA analysis from weeks 96 and 248 of the CHER trial. Y-axes represent HIV-1 proviral DNA (log10 copies per 106 PBMCs). Plot A: HIV-1 proviral DNA in ART-Def [n = 44, median 2415 (IQR 499–7450)] and ART-96 W (n = 73, median [325 (53–3670) copies of HIV-1 proviral DNA/106 PBMCs] at week 96 (p = 0.0019). Plot B: HIV-1 proviral DNA at week 96 by age of starting ART (n = 117). Plot C: HIV-1 proviral DNA in ART-Def (n = 70, ART-40 W (n = 56) and ART-96 W (n = 43) at 248 weeks (Kruskal–Wallis test p = 0.2553). Plot D: HIV-1 proviral DNA at week 248 by duration of ART received by 248 weeks. Plot E: HIV-1 proviral DNA at trial week 248 by weeks of continuous HIV-1 RNA suppression below 400 copies/ml
Higher total CD8 count at ART initiation was associated with lower HIV-1 proviral DNA at both 96 and 248 weeks [ß = − 0.08 (95% CI − 0.14, − 0.01), p = 0.0225 and ß = − 0.07 (95% CI − 0.14, − 0.00), p = 0.0398, respectively]. Therefore, for every 500 cell increase in total CD8 count, a reduction of approximately 7% in HIV-1 proviral DNA was demonstrated at weeks 96 and 248.
Compared to CDC stage N at enrolment, CDC stage B was associated with a 57% reduction in HIV-1 proviral DNA levels at 96 weeks [ß=− 0.84 (95% CI − 1.58, − 0.09), p = 0.0287]. This relationship was not seen at 248 weeks. There was 40% more reduction of HIV-1 proviral DNA levels at KIDCRU, the Cape Town trial study site compared to PHRU, the Johannesburg study site [ß = − 0.52 (95% CI − 0.85, − 0.18), p = 0.0031] at 248 weeks (but not 96 weeks). Multivariable analysis did not suggest that the different maternal or child PMTCT used at the two trial sites significantly affected total HIV-1 proviral DNA at either week 96 or 248, however cumulative time on ART at 248 weeks was significantly shorter in participants from PHRU [mean 204, median 220 (IQR 184–233)] versus KIDCRU [mean 220, median 220 (IQR 208–240) weeks, Wilcoxon rank-sum test p = 0.042].
At 248 weeks, HIV-1 proviral DNA levels were significantly higher in those with positive HIV-1 serology determined at 84 weeks compared to children with negative HIV-1 serology [ß = 0.73 (95% CI 0.23, 1.22), p = 0.0042]. The same relationship was evident between undetermined serology (not determined in 56 ART-40W, 9 ART-96W and 19 ART-Def children) and increased HIV-1 proviral DNA levels compared with negative HIV-1 serology. Age at ART start was not fitted in the multivariable model due to multicollinearity with duration of ART by week 96; and duration of viral load suppression by week 248 was not fitted in the multivariable model due to multicollinearity with duration of ART by week 248.
Effect of time-off ART on HIV-1 proviral DNA
The effect of time off ART in the first (ART-Def, n = 70), second (ART-40W, n = 56) or third (ART-96W, n = 43) year of life was assessed by comparing HIV-1 proviral DNA between the treatment strategies at 248 weeks. In the children examined, the total time spent on ART from enrolment until 248 weeks was higher in ART-Def [median 228 (IQR 211, 240) weeks, 220 (196, 235) for ART-40W, 216 (168, 247) for ART-96W; p = 0.0001], as was the duration of viral load suppression [median 200 (IQR 163, 214) weeks for ART-Def, 163 (110, 192) for ART-40W, 164 (108, 200) for ART-96W; p = 0.0001]. There was no significant difference at 248 weeks between the 3 arms in HIV-1 proviral DNA [median 1165 (IQR 167; 10,900) HIV-1 proviral DNA/106 PBMCs for ART-Def, 4165 (294; 26,150) for ART-40W, 915 (172; 15,400) for ART-96W; p = 0.2553, Fig. 2C].
Figure 2D, E illustrate the spread of the data and how some individuals have high HIV-1 proviral DNA despite long duration of ART or viral load suppression. Of the 229 children studied, only 2 children had HIV-1 proviral DNA measurements ≤ 50 copies/106 PBMCs: 1 child from ART-Def with undetectable proviral DNA at 252 weeks and 1 child from ART-96W with HIV-1 proviral DNA 6 copies/106 PBMCs at 96 weeks.
Discussion
Our study is currently the largest analysis of HIV-1 proviral DNA data from a randomized controlled trial in children and demonstrated that HIV-1 proviral DNA was significantly lower after 96 weeks of ART in children who started early ART than those with ART deferred until clinically or immunologically indicated. In multivariable analyses, longer duration of ART was significantly associated with lower levels of HIV-1 proviral DNA at both weeks 96 and 248, thus supporting the results from similar but smaller studies [34,35,36,37,38,39,40,41].
The effect of duration of HIV infection and age of ART initiation on HIV-1 proviral DNA levels cannot be distinguished, since these two variables are intrinsically related. Also, it is not possible to determine the exact timing of infection, which can be either in utero, intrapartum or after birth. There is an inherent selection bias as more children died in ART-Def than early treated arms, none of whom had samples available for evaluation. Clinically unwell children may also have been less likely to have an aliquot of PBMCs or plasma stored. However, these biases most likely underestimate the associations seen as unwell children are more likely to have uncontrolled HIV-1 with potentially higher proviral DNA levels. This is reflected by the fact that the greater reduction in HIV-1 proviral DNA in children with CDC Stage B at ART initiation may be due to having higher levels of integrated HIV-1 DNA before starting ART due to more advanced disease. The assay used measured total HIV-1 proviral DNA, i.e. both integrated and episomal LTR, therefore it was essential that children were virally suppressed for at least 12 weeks and it was not possible to measure baseline proviral DNA in these children.
There was no significant difference in HIV-1 proviral DNA at 248 between the 3 trial arms, suggesting the beneficial effect on reservoir reduction through early ART may be lost with treatment interruption, as reflected by other smaller studies [42, 43]; yet equally how continuous ART may counteract the detrimental effect of delayed ART initiation compared to early-ART followed by interruption. Subsequent analysis from a small group of children who did not interrupt early ART in the CHER trial demonstrated lower cell-associated HIV-1 DNA and RNA at 7–8 years of age in those that started ART before 2 months of age compared to after 2 months of age [41]. Furthermore, initiation of continuous ART within the first week of life in another group of HIV-infected infants [43, 44] has been associated with more rapid HIV-1 DNA decay compared to ART-initiation at median of 7 weeks of age followed by ART-interruption (ART-96W) and ART-initiation at median 22 weeks followed by continuous ART (ART-Def). These data, and other recent studies support earlier initiation and sustained virological suppression within the first 2 years of life to most effectively reduce reservoir size [27, 28, 45, 46].
HIV-1 seropositivity at 84 weeks was associated with higher proviral DNA at 248 weeks, however quantitative HIV-1 anti-gp120 antibody levels did not show this relationship [31], although when applying a wider range of HIV-specific antibodies has since demonstrated to estimate reservoir size [47]. While duration of ART is clearly a key determinant of the viral reservoir, we observed individual children with high HIV-1 proviral DNA levels despite long-term ART and RNA suppression. This has been described previously [48, 49] and might be explained by longer periods of intra-uterine infection, and possibly interval viral load testing not capturing “viral blips”. It may also be due to homeostatic proliferation of HIV-infected cells in the absence of viral reactivation [50, 51], although this has not been demonstrated in peripheral blood mononuclear cells [52]. Despite virological control with ART, persistent inflammation and immune activation is recognized in perinatally-acquired HIV [53, 54], potentially driving proliferation of cells that harbor latent integrated HIV [12] such as within follicular B-cells [55, 56].
Higher CD8 count at ART initiation was associated with a greater reduction in HIV-1 proviral DNA. This may be due to the proportion of CD8 T-cells in the PBMCs from which DNA was extracted; or reflect a functional immune response to HIV-infection, implying that the dynamics of reservoir decline may not be solely reliant on adequate viral suppression [57]. In response to HIV, total levels of CD8 increase from a combination of thymic output of naïve CD8 T-cells and proliferation via clonal expansion. This may be regarded as “immune-attenuation” and suggests that alongside ART, CD8 T-cells may play an important role in controlling HIV infection and potentially mediate eradication of viral reservoirs of infection through interaction with various HLA types such as HLA-B*27:02 [58]. The vast majority of children had viral loads from enrolment reported as > 7,50,000 copies/ml therefore we are unable to determine whether a relationship exists between higher viral loads and higher CD8 counts.
A site effect was observed as children at KIDCRU had significantly lower levels of HIV-1 proviral DNA at week 248 than at PHRU. This may reflect variation in clinician approach to starting or re-starting ART, as reflected by increased time on ART in KIDCRU participants compared to PHRU.
Conclusions
Our study affirms the association between earlier initiation and longer duration of ART and reduced levels of HIV-1 proviral DNA in children. We also demonstrated associations between higher CD8 count at ART initiation with greater reduction of HIV-1 proviral DNA, inferring a possible beneficial effect of early HIV-1 viral exposure and “immune-attenuation” alongside ART. While we have analysed multiple factors, there are other factors that have not been examined that may plausibly influence levels of HIV-1 proviral DNA: timing of HIV-transmission and initial maternal viral burden, ART adherence, co-infections, thymic output and immune activation. Further work is required to better understand these dynamics and identify potential targets for adjunctive HIV-1 reduction strategies, now a major approach for reducing HIV reservoirs as children become adults.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
UNAIDS. UNAIDS report on the global AIDS epidemic. UNAIDS/10.11E | JC1958E. Globalreport. Documents. 2010. http://www.unaids.org. Accessed 8 July 2016.
Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384(9939):258–71.
Cotton MF, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet. 2013;382(9904):1555–63.
Violari A, Cotton M, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, McIntyre JA. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):11.
Lewis J, et al. Age and CD4 count at initiation of antiretroviral therapy in HIV-infected children: effects on long-term T-cell reconstitution. J Infect Dis. 2012;205(4):548–56.
Suspene R, Meyerhans A. Quantification of unintegrated HIV-1 DNA at the single cell level in vivo. PloS ONE. 2012;7(5):e36246.
De Rossi A, et al. Quantitative HIV-1 proviral DNA detection: a multicentre analysis. New Microbiol. 2010;33(4):293–302.
Zhao Y, et al. Quantification of human immunodeficiency virus type 1 proviral DNA by using TaqMan technology. J Clin Microbiol. 2002;40(2):675–8.
Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–300.
Sleasman JW, et al. CD4+ memory T cells are the predominant population of HIV-1-infected lymphocytes in neonates and children. AIDS. 1996;10(13):1477–84.
Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6(5):388–400.
Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15(8):893–900.
Saez-Cirion A, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211.
Luzuriaga K, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med. 2015;372(8):786–8.
Persaud D, Luzuriaga K. Absence of HIV-1 after treatment cessation in an infant. N Engl J Med. 2014;370(7):678.
Violari A, et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat Commun. 2019;10(1):412.
Frange P, et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV. 2016;3(1):e49-54.
Battistini A, Sgarbanti M. HIV-1 latency: an update of molecular mechanisms and therapeutic strategies. Viruses. 2014;6(4):1715–58.
Archin NM, et al. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009;25(2):207–12.
Chun TW, et al. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: implications for eradication. AIDS. 2010;24(18):2803–8.
Chun TW, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–7.
Palmer S, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105:3879–84.
Natarajan V, et al. HIV-1 replication in patients with undetectable plasma virus receiving HAART. Highly active antiretroviral therapy. Lancet. 1999;353(9147):119–20.
Ramratnam B, et al. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6(1):82–5.
Hatano H, et al. Increase in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection: a randomized, placebo-controlled trial. J Infect Dis. 2013;208(9):1436–42.
Persaud D, et al. Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr. 2014. https://doi.org/10.1001/jamapediatrics.2014.1560.
Bitnun A, et al. Clinical correlates of human immunodeficiency virus-1 (HIV-1) DNA and inducible HIV-1 RNA reservoirs in peripheral blood in children with perinatally acquired HIV-1 infection with sustained virologic suppression for at least 5 years. Clin Infect Dis. 2020;70(5):859–66.
Moragas M, et al. Impact of the time to achieve viral control on the dynamics of circulating HIV-1 reservoir in vertically infected children with long-term sustained virological suppression: a longitudinal study. PLoS ONE. 2018;13(10):e0205579.
Zanchetta M, et al. Early therapy in HIV-1-infected children: effect on HIV-1 dynamics and HIV-1-specific immune response. Antivir Ther. 2008;13(1):47–55.
Murray JM, et al. Integrated HIV DNA accumulates prior to treatment while episomal HIV DNA records ongoing transmission afterwards. AIDS. 2012;26(5):543–50.
Payne H, et al. Reactivity of routine HIV antibody tests in children who initiated antiretroviral therapy in early infancy as part of the children with HIV early antiretroviral therapy (CHER) trial: a retrospective analysis. Lancet Infect Dis. 2015. https://doi.org/10.1016/S1473-3099(15)00087-0.
Smith NM, et al. Proof-of-principle for immune control of global HIV-1 reactivation in vivo. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015;61(1):120–8.
Folks TM, et al. Biological and biochemical characterization of a cloned Leu-3-cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986;164(1):280–90.
Ananworanich J, et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS. 2014;28(7):1015–20.
Luzuriaga K, et al. Reduced HIV reservoirs after early treatment HIV-1 proviral reservoirs decay continously under sustained virologic control in early-treated HIV-1-infected children. J Infect Dis. 2014. https://doi.org/10.1093/infdis/jiu297.
Persaud D, et al. Dynamics of the resting CD4(+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS. 2012;26(12):1483–90.
Martinez-Bonet M, et al. Establishment and replenishment of the viral reservoir in perinatally HIV-1-infected children initiating very early antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015;61(7):1169–78.
Tagarro A, Chan M, Zangari P, Ferns B, Foster C, Rossi A, Nastouli E, Muñoz-Fernández M, Gibb D, Rossi P, Giaquinto C, Babiker A, Fortuny C, Freguja R, Cotugno N, Judd A, Noguera-Julian A, Navarro ML, Mellado MJ, Klein N, Palma P, Rojo P. Early and highly suppressive antiretroviral therapy are main factors associated with low viral reservoir in European perinatally HIV-infected children. J Acquir Immune Defic Syndr. 2018;79(2):269–76. https://doi.org/10.1097/QAI.0000000000001789.
Kuhn L, Paximadis M, Da Costa-Dias B, Loubser S, Strehlau R, Patel F, Shiau S, Coovadia A, Abrams EJ, Tiemessen CT. Age at antiretroviral therapy initiation and cell-associated HIV-1 DNA levels in HIV-1-infected children. Plos ONE. 2018;13(4):e0195514.
Foster C, Pace M, Kaye S, Hopkins E, Jones M, Robinson N, Mant C, Cason J, Fidler S, Frater J, CHERUB Investigators. Early antiretroviral therapy reduces HIV DNA following perinatal HIV infection. AIDS. 2017;31(13):1847–51.
van Zyl GU, et al. Early antiretroviral therapy in South African children reduces HIV-1-infected cells and cell-associated HIV-1 RNA in blood mononuclear cells. J Infect Dis. 2015;212(1):39–43.
Pankau MD, Wamalwa D, Benki-Nugent S, Tapia K, Ngugi E, Langat A, Otieno V, Moraa H, Maleche-Obimbo E, Overbaugh J, John-Stewart GC, Lehman DA. Decay of HIV DNA in the reservoir and the impact of short treatment interruption in Kenyan infants. Open Forum Infect Dis. 2017;5(1):268.
Veldsman KA, et al. HIV-1 DNA decay is faster in children who initiate ART shortly after birth than later. J Int AIDS Soc. 2019;22(8):e25368.
Veldsman KA, et al. Rapid decline of HIV-1 DNA and RNA in infants starting very early antiretroviral therapy may pose a diagnostic challenge. AIDS. 2018;32(5):629–34.
Massanella M, et al. Continuous prophylactic antiretrovirals/antiretroviral therapy since birth reduces seeding and persistence of the viral reservoir in children vertically infected with human immunodeficiency virus. Clin Infect Dis. 2021;73(3):427–38.
McManus M, et al. Early combination antiretroviral therapy limits exposure to HIV-1 replication and cell-associated HIV-1 DNA levels in infants. PLoS ONE. 2016;11(4):e0154391.
Rocca S, et al. Human immunodeficiency virus (HIV)-antibody repertoire estimates reservoir size and time of antiretroviral therapy initiation in virally suppressed perinatally HIV-infected children. J Pediatr Infect Dis Soc. 2019;8(5):433–8.
Zanchetta M, et al. Long-term decay of the HIV-1 reservoir in HIV-1-infected children treated with highly active antiretroviral therapy. J Infect Dis. 2006;193(12):1718–27.
Palma P, et al. The HIV-1 antibody response: a footprint of the viral reservoir in children vertically infected with HIV. Lancet HIV. 2020;7(5):e359–65.
Bosque A, et al. Homeostatic proliferation fails to efficiently reactivate HIV-1 latently infected central memory CD4+ T cells. PLoS Pathog. 2011;7(10):e1002288.
Reeves DB, Duke ER, Wagner TA, Palmer SE, Spivak AM, Schiffer JT. A majority of HIV persistence during antiretroviral therapy is due to infected cell proliferation. Nature Commun. 2018;9(1):4811.
Van Zyl GU, et al. No evidence of HIV replication in children on antiretroviral therapy. J Clin Invest. 2017;127(10):3827–34.
Eckard AR, et al. Neurocognitive dysfunction in HIV-infected youth: investigating the relationship with immune activation. Antivir Ther. 2017;22(8):669–80.
Eckard AR, et al. Increased immune activation and exhaustion in HIV-infected youth. Pediatr Infect Dis J. 2016;35(12):e370–7.
Sung JM, David M. HIV persistence on antiretroviral therapy and barriers to a cure. Adv Exp Med Biol. 2018;1075:165–85.
Bronnimann MP, Skinner PJ, Connick E. The B-cell follicle in HIV infection: barrier to a cure. Front Immunol. 2018;9:20.
Burbelo PD, et al. HIV antibody characterization as a method to quantify reservoir size during curative interventions. J Infect Dis. 2014;209(10):1613–7.
Adland E, Hill M, Lavandier N, Csala A, Edwards A, Chen F, Radkowski M, Kowalska JD, Paraskevis D, Hatzakis A, Valenzuela-Ponce H, Pfafferott K, Williams I, Pellegrino P, Borrow P, Mori M, Rockstroh J, Prado JG, Mothe B, Dalmau J, Martinez-Picado J, Tudor-Williams G, Frater J, Stryhn A, Buus S, Teran GR, Mallal S, John M, Buchbinder S, Kirk G, Martin J, Michael N, Fellay J, Deeks S, Walker B, Avila-Rios S, Cole D, Brander C, Carrington M, Goulder P. Differential immunodominance hierarchy of CD8+ T-cell responses in HLA-B*27:05- and -B*27:02-mediated control of HIV-1 infection. J Virol. 2018;30:92.
Acknowledgements
We are grateful to all the children and their families who participated in the CHER trial, the staff at the Perinatal Research Unit and the Children’s Infectious Disease Clinical Research Unit for performing the trial, Wendy Stevens at Clinical Laboratory Services for sample storage, and Lynn Morris at the National Institute for Communicable Diseases for her support in the quantitative HIV-antibody work. We also thank Gerhard Walzl and the Stellenbosch Immunology Group for laboratory infrastructure and support.
Funding
This study was funded by The Wellcome Trust, and The CHER trial, collection and storage of samples and data was funded by The US National Institute of Allergy and Infectious Diseases (NIAID).
Author information
Authors and Affiliations
Contributions
HP, NJK and DMG conceived the study. AV and MFC were the protocol co-chairs for each site of the CHER trial. KO provided the specimens and necessary data from the CHER trial. SW established and taught the assay to HP. HP processed the specimens. MC performed the statistical analysis with assistance from AB. NYH processed the specimens for CMV DNA. NJK, DMG, AB, AV, MFC and RC contributed to the study design and data interpretation. HP prepared the manuscript. Professor Robin Callard (RC) sadly passed away before completion of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The use of stored samples was approved by the Human Research Ethics Committees of Stellenbosch and Witwatersrand Universities (M12/01/005 and 040703) where the CHER trial was conducted at their respective trial sites: Family Center for Research with Ubuntu (FAMCRU) and The Perinatal HIV Research Unit (PHRU).
Consent for publication
This manuscript does not contain any individual person’s data in any form (including any individual details, images or videos), therefore consent for publication is not applicable.
Competing interests
There are no financial and non-financial competing interests to be declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Payne, H., Chan, M.K., Watters, S.A. et al. Early ART-initiation and longer ART duration reduces HIV-1 proviral DNA levels in children from the CHER trial. AIDS Res Ther 18, 63 (2021). https://doi.org/10.1186/s12981-021-00389-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12981-021-00389-1