Abstract
Sarcopenia is closely associated with gut dysbiosis. Probiotics alleviate gut dysbiosis. Therefore, we selected probiotics Lactobacillus paracasei P62 (Lp) and Bifidobacterium bifidum P61 (Bb), which suppressed muscle RING-finger protein-1 (MuRF1) expression and NF-κB activation in C2C12 cells, and examined their effects on muscle mass loss and dysfunction in aged mice. Oral administration of Lp, Bb, or their mix (LB) increased grip strength and treadmill running distance and time. They significantly increased muscle weight in aged mice. They also increased AKT activation, PGC1α, SIRT1, and myosin heavy chain (MyHC) expression, MyHC-positive cell population, and cell size in the gastrocnemius (GA) muscle, while FOXO3a and NF-κB activation, MuRF1, muscle atrophy F-box, and p16 expression, and NF-κB+CD11c+ cell population decreased. Furthermore, they reduced cognitive impairment-like behavior, IL-6 expression, FOXO3a activation, and NF-κB-positive cell population in the hippocampus, GA, and colon, while hippocampal brain-derived neurotropic factor expression increased. They shifted gut microbiota composition in aged mice: they increased Akkermansiaceae and Bacteroidaceae populations, which were positively correlated with total muscle weight and MyHC expression, and decreased Odoribacteraceae and Deferribacteriaceae populations, which were positively correlated with MuRF1 and IL-6 expression. LB alleviated sarcopenia- and cognitive impairment-like symptoms more potently than Lp or Bb alone. Based on these findings, probiotics, particularly Lp, Bb, and LB, can alleviate aging-dependent sarcopenia and cognitive impairment by regulating gut microbiota-mediated AKT, NF-κB, and/or FOXO3a signaling pathways.
Similar content being viewed by others
Introduction
Sarcopenia is a progressive disorder characterized by significant loss of skeletal muscle mass and strength, and is observed in various diseases such as cancer, cirrhosis, and chronic obstructive pulmonary disease [1, 2]. Aging is the main risk factor for sarcopenia with Alzheimer’s disease [3]. Inflammation, oxidative stress, and mitochondrial dysfunction induced by stressors such as aging and pathogens cause an imbalance of protein synthesis and protein degradation in skeletal muscles, which prevents myoblast activation, proliferation, and differentiation, resulting in sarcopenia [4, 5]. Skeletal muscle mass and function in mice are reported to be regulated by gut microbiota and their metabolites, which cause muscle atrophy in germ-free mice [6]. Fecal microbiota transplantation from young mice rejuvenates physical fitness with muscle thickness in the transplanted aged mice [7]. Fecal microbiota transplantation from pigs with myostatin deletion alleviates muscle atrophy in pigs [8]. Therefore, sarcopenia may be closely associated with gut microbiota.
Probiotics exhibit anti-inflammatory, anti-depressive, and cognitive impairment-ameliorating effects [9, 10]. In addition, Chen et al. reported that Lactobacillus casei Shirota, which was isolated from a fermented food, had significant anti-inflammatory effects in mice and human studies and induced muscles in aged mice [11]. Lee et al. reported that Lactobacillus plantarum HY7715 isolated from kimchi increased skeletal muscle mass and function in aged mice, resulting in the amelioration of sarcopenia [12]. Chen et al. reported that Lactobacillus paracasei PS23 isolated from human feces attenuated muscle loss in senescence-accelerated mouse prone-8 (SAMP8) mice by ensuring mitochondrial function [13]. Munukka et al. reported that Faecalibacterium prausnitzii reduced systemic inflammation and increased muscle mass in high‐fat fed mice [14]. A mixed supplement of multiple probiotics also reduces inflammation and muscle atrophy markers [15]. Nevertheless, the action mechanism of sarcopenia-ameliorating probiotics remains elusive.
Therefore, to confirm whether probiotics could alleviate sarcopenia, we first screened muscle RING-finger protein-1 (MuRF1, a muscle atrophy factor) expression-suppressing probiotics Lactobacillus paracasei P62 (Lp) and Bifidobacterium bifidum P61 (Bb) in dexamethasone- or LPS-treated C2C12 cells and examined their effects in aged mice.
Methods
Culture of probiotics
Probiotics including L. paracasei P62 (KCCM 13368P) and B. bifidum P61 (KCCM 13367P), selected from human gut microbiota collection, were cultured in general media for probiotics such as MRS broth (BD, Sparks, MD), collected by centrifugation, and freeze-dried. For the in vitro study, cells were washed with saline twice and suspended in saline. For the in vivo study, cells were suspended in 1% maltose solution.
Culture of C2C12 cells
C2C12 cells (Korean Type Culture Collection, Seoul, Korea) were cultured in DMEM medium containing 10% fetal bovine serum, 1% antibiotic–antimycotic solution, and 3.7 g/L NaHCO3 at 37oC in a 5% CO2/95% air humidified incubator [16]. Confluent myoblasts (80%) were differentiated in DMEM medium containing 2% horse serum for 4 days. Probiotics (1 × 104 cells/mL) or creatine (Cr, 5 mM, C0780, Sigma) were treated 10 h after treatment with dexamethasone (10 µM, D4902, Sigma) or lipopolysaccharide (LPS, 100 ng/mL) in differentiated C2C12 cells (1 × 105 cells/mL).
Animals
Aged C57BL/6 mice (male, 18 months old) and young C57BL/6 mice (male, 12-weeks old) were purchased from Orientbio Co. ltd., maintained in a controlled room with water and food ad libitum, and acclimatized for 7 days before the experimental use. All animal experiments were approved by the Committee for the Care and Use of Laboratory Animals in Kyung Hee University (IACUC No, KHUASP(SE)-22-567) and were ethically carried in accordance of the Guideline of the University for Laboratory Animals Care and Use.
We randomly divided mice into six groups (Yg, Vh, Lp, Bb, LB, and Cr). Each group consisted of 6 mice. Test agents (Yg, vehicle in young mice; Vh, vehicle in aged mice; Lp, 1 × 109 CFU/mouse of Lactobacillus paracasei P62 in aged mice; Bb, 1 × 109 CFU/mouse of Bifidobacterium bifidum P61 in aged mice; LB, 1 × 109 CFU/mouse of Bifidobacterium bifidum P61 and Lactobacillus paracasei P62 (1:4) mix in aged mice; Cr, 75 mg/kg of creatine in aged mice) were orally gavaged once a day for 8 weeks (6 days per week). Physical performance was measured using grip strength and treadmill exercise tests 20 h after the final treatment with test agents. Cognitive function-like behaviors were measured 20 h after the physical performance tests. Mice were euthanized by the exposure to CO2 in the chamber and then sacrificed by cervical dislocation. Sera, brains, colons, and feces were then collected and stored at – 80 °C for biomarker assays.
Physical performance test
For the measurement of grip strength, mice were placed with their all limbs on the grid of a grip strength meter and grip strength was measured immediately before mice fell from the bar [17].
For the measurement of treadmill running time and distance, mice were first adapted for 1 week to become familiar with treadmill before treadmill exercise. Total running time and distance were measured at speed of 23 m/min for 30 min, as previously reported [17].
Cognitive function-like behaviors
The cognitive behavior (Y-maze) task was measured in a three-arm (120o) horizontal maze consisted of 40-cm-long and 3-cm-wide with 12-cm-high walls, as previously reported [18].
Enzyme-linked immunosorbent assay (ELISA) and immunoblotting
Muscle, colon, and brain tissues and cells were homogenized in the RIPA lysis buffer containing 1% phosphatase inhibitor cocktail and 1% protease inhibitor cocktail (RPP) on ice and centrifuged at 15,000 g at 4 °C for 15 min. The biomarker levels were assayed, as previously reported [19].
For the assay of cytokines, the homogenate supernatants were transferred in 96-well plates and assayed using ELISA kits (R&D system, Minneapolis, MN).
For the immunoblotting analysis, the homogenate supernatants of tissues and cells were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride. Proteins were probed with antibodies, detected with horseradish peroxidase-conjugated secondary antibodies, and visualized with enhanced chemiluminescence detection kit.
The used antibodies are follows: phospho-Akt (Ser473) (193H12, Cell Signaling, Danvers, MA), Akt (11E7) (4685, Cell Signaling), muscle atrophy F-box gene (MAFbx, F-9, sc-166,806, Santa Cruz Biotechnology, Santa Cruz, CA), MuRF1(C-11) (sc-398,608, Santa Cruz Biotechnology), phospho-mTOR (ser2448) (2971, Cell Signaling), mammalian target of rapamycin (mTOR, 7C10, 2983,Cell Signaling), p-NF-κB-p65 (S536) (93H1, Cell Signaling), NF-κB-p65 (D14E12) (8242, Cell Signaling), myosin heavy chain (MyHc, B-5, sc-376,157, Santa Cruz Biotechnology), p16INK4A (E5F3Y, Cell signaling), p-FOXO3a (ser253, Cell Signaling), FOXO3a (75D8, Cell Signaling), β-actin (sc-47,778, Santa Cruz Biotechnology), and peroxisome proliferator-activated receptor gamma coactivator (PGC)1α (ab191838, Abcam).
Quantitative real-time polymerase chain reaction (qPCR) analysis
Total RNA was purified from C2C12 cells and GA muscles using Qiagen RNeasy mini kit and Qiagen RNeasy Fibrous Tissue mini kit, respectively. The isolated RNA (2 µg) was reverse-transcribed using a PrimeScript cDNA synthesis kit (Takara, Shiga, Japan). qPCR was performed using the Rotor-Gene Q 5plex Platform (Qiagen) with TB Green Premix Ex Taq II (Takara). Primer sequences were described in Supplement (Table S1).
Mitochondrial DNA (mtDNA) analysis
Total DNA was extracted from C2C12 cells and GA muscle using Genomic DNeasy kit (Qiagen). The mtDNA copy number was measured via qRT-PCR. Primer sequences were described in Supplement (Table S1).
Hematoxylin & eosin staining
Mice were transcardiacally perfused with paraformaldehyde. Gastrocnemius (GA) muscles were cut into 5 μm sections and stained with hematoxylin and eosin (H&E) [12].
Immunofluorescence staining
Hypothalamus, GA muscle, and colon tissues were collected from mice transcardiacally perfused with paraformaldehyde, sectioned, incubated with primary antibodies (for brain-derived neurotropic factor [BDNF], NeuN, NF-κB, Iba1, and/or CD11c) for 12 h, then treated with secondary antibodies conjugated with Alexa Fluor 594 or Alexa Fluor 488, and observed using a confocal microscope, as previously reported [20].
Microbiota analysis
Fecal microbiota genomic DNAs were extracted using a QIAamp DNA stool mini kit. 16 S rRNA genes were amplified and sequenced, as previously reported [21]. Sequenced data were deposited in the NCBI (PRJNA1011499).
Statistics
Data are expressed as mean ± SD using GraphPad Prism. The significance (p < 0.05) for all data except muscle weights was analyzed using one-way ANOVA followed by Dunnett’s multiple range test. The significance for muscle weights was analyzed using one-way ANOVA followed by Fisher’s LSD test. The correlation between gut microbiota and total muscle weight, GA weight, tumor necrosis factor (TNF)-α/interleukin (IL)-10, IL-6/IL-10, MuRF1, or MyHC expression level was analyzed using Spearman correlation coefficient.
Results
Lp and Bb suppressed dexamethasone-induced MuRF1 expression in C2C12 cells
First, we screened MuRF1 expression-suppressing probiotics in the lactic acid bacteria collection isolated from human fecal bacteria using dexamethasone-treated C2C12 cells (Fig. 1). Dexamethasone treatment significantly induced MuRF1 expression. However, of tested bifidobacteria and lactobacilli, P61 and P62 significantly suppressed dexamethasone-induced MuRF1 expression. They also suppressed dexamethasone-induced MAFbx/atrogin-1 expression. They furthermore significantly suppressed LPS-induced tumor necrosis factor (TNF)-α and interleukin (IL)-6 expression and NF-κB activation in C2C12 cells (Fig. 1, Supplement Figure S1). When P61 and P62 were mixed (4:1, 1:1, and 1:4), the [1:4] mix most potently suppressed IL-6 expression in LPS-stimulated C2C12 cells (Supplement Figure S2). P61 and P62 were Bifidobacterium bifidum and Lactobacillus paracasei, respectively, based on the results of Gram staining, API kit assay, and whole genome analyses. The genome sequences of P61 and P62 showed the highest phylogenetic similarity to B. bifidum JCM1255 (98.8%) and L. paracasei subsp. paracasei JCM 8130 (98.6%), respectively, using OrthoANI (Supplement Tables S2).
Effects of Bb and Lp on dexamethasone-induced MuRF1 and Atrogin-1/MAFbx expression and LPS-induced TNF-α and IL-6 expression and NF-κB activation in C2C12 cells. Effects on MuRF1 (a) and Atrogin-1/MAFbx expression (b). Effects on TNF-α (c) and IL-6 expression (d) and NF-κB activation (e). C2C12 cells (1 × 105 cells/mL) were treated with dexamethasone (10 µM) or LPS (100 ng/mL) in the absence or presence of Bb, Lp, or LB (1 × 104 colon-forming units [CFUs] /mL and Cr [5 mM]). Data are indicated as mean ± SD (n = 4). #p < 0.05 vs. NC. *p < 0.05 vs. group treated with vehicle with dexamethasone or LPS
Effects of Lp and Bb on the skeletal muscle strength and mass in aged mice
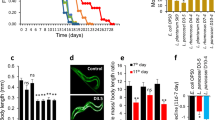
To confirm whether Lp and Bb could alleviate sarcopenia, we examined their effects on the skeletal muscle mass and strength in aged mice (Fig. 2). The bodyweight of aged mice slowly increased for 8 weeks (Supplement Figure S3). There was no significant difference in food intake and bodyweight gain between aged mice treated with Lp, Bb, LB (their [4:1] mix), creatine (Cr), and vehicle (Vh). First, we examined their effects on the skeletal muscle strength in a treadmill exhaustion test and grip strength tests. The treadmill distance was significantly shorter in aged mice than that in young mice. However, oral administration of LB most potently increased the treadmill distance and running time in aged mice, followed by Lp, Bb, and Cr. In an all-limb test, Lp, Bb, and LB increased grip strength 1.14-, 1.15-. and 1.16-fold, respectively, compared with those treated with vehicle. Lp, Bb, and LB increased grip strength and treadmill distance and running time more potently than Cr.
Effects of Bb, Lp, and LB on the skeletal muscle strength and mass in aged mice. Effects on treadmill running distance (a) and time (b) and grip strength (c). Effects on the weights of gastrocnemius (GA, d), soleus (SOL, e), quadriceps femoris (QD, f), extensor digitorum longus (EDL, g), tibialis anterior (TA, h), and total muscles (i). Bb, Lp, and LB (1 × 109 CFU/mouse/day) and Cr (75 mg/kg) were orally gavaged once a day (6 days in one week) for 8 weeks. Data are indicated as mean ± SD (n = 6). #p < 0.05 vs. Yg. *p < 0.05 vs. Vh/Ag
Next, we investigated the effects of Lp, Bb, and LB on muscle weight. The gastrocnemius (GA), soleus (SOL), quadriceps femoris (QD), extensor digitorum longus (EDL), and tibialis anterior (TA) muscle weight of aged mice were lower than those of young mice. However, oral administration of Lp, Bb, or LB significantly increased the weight of GA, SOL, QD, EDL, and TA muscles. Of these, LB most potently increased their weight: it increased the weight of GA, SOL, QD, EDL, and TA to 109.1%, 132.4%, 107.3%, 115.4%, and 106.1% of aged mice.
Effects of Lp and Bb on the AKT signal activation in the skeletal muscle of aged mice
To understand the action mechanism of probiotics on muscle weight and strength, we examined the effects of Lp, Bb, and LB on protein synthesis-involved AKT and mTOR activation in the GA muscle in aged mice (Fig. 3, Supplement Figure S4). The activation of AKT and mTOR was lower in the GA of aged mice than in those of young mice. Oral administration of Lp, Bb, or LB increased AKT and mTOR activation in aged mice.
Effects of Lp and Bb on the myogenesis-related gene expression in the skeletal muscle of aged mice
FOXO3a and NF-κB are transcription factors that regulates the transcription of mitogenesis-related genes in the muscle [22, 23]. The activation of these factors suppresses myoblast-differentiating myogenesis genes including MyHC and myogenin (MyoG) and induces muscle-degrading atrogenes including MuRF1 and MAFbx. We also found that FOXO3a and NF-κB were potently activated in the GA muscle of aged mice compared to that of young mice (Fig. 4). Therefore, we examined the effects of Lp, Bb, and LB on the myogenesis gene expression in the GA muscle of aged mice (Fig. 4, Supplement Figure S5). Oral administration of Lp, Bb, or LB significantly suppressed FOXO3a and NF-κB activation in aged mice. Furthermore, they decreased MuRF1, MAFbx, and p16 expression, assessed by immunoblotting. In the immunofluorescence staining, they also decreased MuRF+ and NF-κB+CD11c+ cell populations. Furthermore, they suppressed TNF-α and IL-6 expression. However, Cr did not affect their expression.
Effects of Bb, Lp, and LB on the myogenesis gene expression in the GA muscle of aged mice. a Effects on MuRF-1, MAFbx, p16, p-FOXO3a, FOXO3a, p-p65, p65, and β-actin expression, assessed by immunoblotting. b Effects on NF-κB+/CD11c+ and MuRF1+ cell population, assessed by immunofluorescence staining. Effects on MuRF-1 (c), MAFbx/Atrogin-1 (d), TNF-α (e), and IL-6 expression (f), assessed by qPCR. Effects on PGC1α, MyHC, and β-action expression (g, immunoblotted), GA muscle cell size (h, H&E-stained), and MyHC-positive cell population (i, stained with immunofluorescence-stained). Effects on MyHC (j), MyHC 2 A (k), MyHC 2X (l), MyHC 2B (m), MyoG (n), PGC1a (o), SIRT1 (p), and mtDNA (q) expression (n), assessed by qPCR. Bb, Lp, and LB (1 × 109 CFU/mouse/day) and Cr (75 mg/kg) were orally gavaged once a day (6 days in one week) for 8 weeks. Data are indicated as mean ± SD (n = 6). #p < 0.05 vs. Yg. *p < 0.05 vs. Vh/Ag
Oral administration of Lp, Bb, or LB increased MyHC and PGC1α expression, assessed by immunoblotting. They also increased muscle cell size and MyHC-positive cell population. In a qPCR analysis, they decreased MuRF1 and MAFbx expression, while MyHC and MyoG expression increased. Of MyHCs, MyHC2A expression was significantly increased by treatment with Lp or LB, while Bb did not affect its expression. However, Lp, Bb, and LB all increased the expression of MyHC2X, MyH2B, and MyHC more potently than Cr.
Next, we investigated the effects of Lp, Bb, and LB on mitochondrial gene expression-involved PGC1α, SIRT1, and mtDNA expression levels in the GA muscle. PGC1α and SIRT1 expression was lower in aged mice than in young mice. However, oral administration of Lp, Bb, or LB significantly increased PGC1α and SIRT1 expression in aged mice, assessed by qPCR analysis.
Effects of Lp and Bb on the cognitive function in aged mice
Aging induces cognitive decline with systemic inflammation including neuroinflammation [24]. We found that cognitive impairment-like behaviors and TNF-α and IL-6 expression were higher in the hippocampus of aged mice than in those of young mice (Fig. 5, Supplement Figure S6). However, oral administration of Lp, Bb, or LB alleviated cognitive impairment-like behavior in the Y-maze test. They also decreased TNF-α and IL-6 expression, FOXO3a activation, and NF-κB+Iba1+ cell population, while IL-10 and BDNF expression and BDNF+NeuN+ cell population increased in the hippocampus. Ageing increases LPS and corticosterone (cortisol) in the blood and LPS levels in the feces [25]. The bacterial endotoxin induces cognitive impairment with systemic inflammation [26]. We also found that LPS and corticosterone levels were higher in the blood of aged mice than in that of young mice. However, oral administration of Lp, Bb, or LB decreased blood LPS and corticosterone levels in aged mice.
Effects of Bb, Lp, and LB on the cognitive function in aged mice. a Effects on spontaneous alternation in the Y-maze task. Effects on hippocampal BDNF (b), TNF-α (c), IL-6 (d), IL-1β (e), and IL-10 (f) expression, TNF-α to IL-10 expression ratio (g), and IL-6 to IL-10 expression ratio (h), assessed by qPCR. Effects on BDNF+NeuN+ (i) and NF-κB+Iba1+ cell populations (j). (k) Effects on p-FOXO3a and FOXO3a expression, assessed by immunoblotting. Effects on blood endotoxin (l) and corticosterone levels (m). Bb, Lp, and LB (1 × 109 CFU/mouse/day) and Cr (75 mg/kg) were orally gavaged once a day (6 days in one week) for 8 weeks. Data are indicated as mean ± SD (n = 6). #p < 0.05 vs. Yg. *p < 0.05 vs. Vh/Ag
Effects of Bb, Lp, and LB on the gut inflammation and microbiota composition in aged mice
Ageing increases systemic inflammation including gut inflammation, which induces gut dysbiosis [27]. We found that the expression of inflammatory markers TNF-α, IL-1β, IL-6, and myeloperoxidase was higher in the colon of aged mice than in that of young mice (Fig. 6). Oral administration of Lp, Bb, or LB decreased TNF-α, IL-1β, and IL-6 expression, TNF-α to IL-10 expression ratio, and NF-κB+CD11c+ cell population in aged mice, while IL-10 expression increased. However, Cr did not affect the expression of inflammatory markers in aged mice.
Effects of Bb, Lp, and LB on the gut inflammation in aged mice. Effects on TNF-α (a), IL-6 (b), IL-10 (c), IL-1β (d), and myeloperoxidase (MPO) expression (e), and TNF-α to IL-10 expression ratio (f), and IL-6 to IL-10 expression ratio (g), assessed by qPCR. h Effects on NF-κB+CD11c+ cell population. Bb, Lp, and LB (1 × 109 CFU/mouse/day) and Cr (75 mg/kg) were orally gavaged once a day (6 days in one week) for 8 weeks. Data are indicated as mean ± SD (n = 6). #p < 0.05 vs. Yg. *p < 0.05 vs. Vh/Ag
The gut microbiota composition of aged mice was significantly different to that of young mice (Fig. 7). Although the α-diversity was not significantly different between aged and young mice, the β-diversity significantly different. Oral administration of Lp, Bb, or LB decreased Deferribacteres population in aged mice. They increased Prevotellaceae, Akkermansiaceae, and Bacteroidaceae populations and decreased Odoribacteraceae, Deferribacteraceae, Coriobacteriaceae, and Acholeplasmataceae populations at the family level.
Effects of Bb, Lp, and LB on the gut microbiota composition in aged mice. Effect on the microbiota composition at the phylum (a) or family levels (b). Effects on OTUs (α-diversity, c) and β-diversity (principal coordinate analysis [PCoA] plot based on BrayCurtis) (d). The relationship between gut microbiota and total muscle weight (e), GA muscle weight (f), TNF-α to IL-10 expression ration (g), IL-6 to IL-10 expression ratio (h), MuRF1 expression (i), or MyHC expression (j) in the GA, assessed by Spearman coefficient test. Bb, Lp, and LB (1 × 109 CFU/mouse/day) and Cr (75 mg/kg) were orally gavaged once a day (6 days in one week) for 8 weeks. Data are indicated as mean ± SD (n = 6). *p < 0.05 vs. Vh/Ag
Of these bacteria, Akkermansiaceae and Bacteroidaceae populations, which were increased in aged mice, were positively correlated with total muscle weight, while Lachnospiraceae, Deferribacteraceae, and Selenomonadaceae populations were negatively correlated. GA muscle weight was positively correlated with the populations of Akkermansiaceae and Bacteroidaceae, while Odoribacteraceae, Deferribacteraceae, and Acholeplasmataceae populations were negatively correlated. MuRF1 expression was positively correlated with Odoribacteraceae, AC160630_f, and Acholeplasmataceae populations, while Akkermansiaceae and Bacteroidaceae populations were negatively correlated.
MyHC expression was positively correlated with Saccharimonas_f, SC160630_f, and Lactobacillaceae populations, while Deferribacteriaceae and Veillonellaceae populations were negatively correlated. TNF-α to IL-10 expression ratio was positively correlated with Streptococcaceae, Coriobacteriaceae, Veillonellaceae, and Muribacteriaceae populations, while Saccharimonas_f and Lactobacillaceae populations were negatively correlated. IL-6 to IL-10 expression ratio was positively correlated with Deferribacteraceae and Acholeplasmataceae populations, while FR888536_f, Clostridiales_us, Bacteroidaceae, and Akkermansiaceae populations were negatively correlated.
Discussion
The muscle mass, quality, and strength are regulated by the orchestrated activation, proliferation, and differentiation of myoblast in the muscle, which are regulated by muscle-specific myogenic regulatory factors such as MyoD, MyoG, and MyHC [21, 28, 29]. MyoD and MyoG are transcription factors involved in regulating the proliferation and differentiation of muscles. MyHC is a major contractile myosin protein expressed in the differentiated muscle. MuRF1 and MAFbx/Atrogin-1 are E3 ubiquitin ligases that regulate ubiquitin-mediated protein degradation in skeletal muscle [30]. In addition, myogenic gene expression (protein synthesis) is increased by the activation of AKT and mTOR, but decreased by the activation of NF-κB [31, 32]. The activation of NF-κB and FOXO3 causes muscle atrophy by inducing MuRF1 and MAFbx and suppressing MyHC expression [33, 34]. Mitochondria-related genes, such as PGC1α and SIRT1, regulate energy metabolism and prevent physiological fatigue in the muscles [35]. Therefore, to maintain skeletal muscle mass, quality, and strength, these myogenic gene expression and signals must be harmoniously regulated in myoblasts. However, their imbalanced regulation can cause sarcopenia.
In the present study, we selected MuRF1 expression-suppressing and AKT activation-inducing Lp and Bb, which belonged to L. paracasei and Bifidobacterium bifidum, respectively, in dexamethasone-stimulated C2C12 cells from human fecal lactic acid bacteria collection. They significantly increased muscle weight, including GA muscle, and physical strength and endurance in aged mice. They also increased AKT and mTOR activation and MyHC expression in GA muscle, while FOXO3a and NF-κB activation decreased. The activation of FOXO3a and NF-κB increases MuRF1 and MAFbx/atrogin-1 expression and decreases MyHC expression [33, 34]. These results suggest that these probiotics may decrease muscle protein degradation-inducing MuRF1 and MAFbx/atrogin-1 expression and increase differentiation/proliferation-inducing MyHC expression through the suppression of FOXO3a and NF-κB activation, resulting in the alleviation of ageing-dependent muscle weight loss and strength and endurance weakness.
Aging causes myofiber death from the accumulation of damaged mitochondria and weakens the intensity of physical exercise [36]. Oral administration of Lp, Bb, or LB increased PGC1α and SIRT1 expression in aged mice, while mtDNA expression increased in mice treated with LB alone. They increased running time and distance in the treadmill test. In addition, Chen et al. reported that Lactobacillus casei-contained probiotic supplementation attenuated age-related inflammation and reactive oxygen species production in SAMP8 mice by regulating gut microbiota and mitochondrial function [13]. These suggest that Lp and Bb, in particular, LB can alleviate ageing-dependent muscle fatigue by regulating mitochondrial function.
Aging is closely associated with chronic, low-grade inflammation [37, 38]. This inflammation increases the expression of proinflammatory cytokines such as TNF-α and IL-6 in muscle, brain, and intestine. These cytokines suppress protein synthesis and muscle proliferation and differentiation in the myoblast and BDNF expression in the neuronal cells [37, 39]. However, oral administration of Lp, Bb, or LB alleviated ageing-dependent expression of inflammatory markers TNF-α and IL-6 in the hippocampus, while IL-10 and BDNF expression increased. Furthermore, they suppressed FOXO3a activation and NF-κB-positive immune cell populations in hippocampus, while the BDNF-positive neuron cell population increased. In particular, Lp and LB alleviated ageing-dependent cognitive impairment-like behaviors in mice. They suppressed ageing-dependent LPS and corticosterone expression in the blood. LPS and corticosterone down-regulate BDNF expression [18, 40]. BDNF suppresses FOXO3 activation and neuronal differentiation [41, 42]. Kim et al. also reported that Bifidobacterium longum-contained probiotic supplementation alleviated mental flexibility with gut microbiota modulation in healthy older adults [43]. These results suggest that Lp and Bb, in particular LB, can alleviate ageing-dependent cognitive decline with neuroinflammation by increasing NF-κB-suppressed BDNF expression and decreasing BDNF-suppressed FOXO3a activation.
Oral administration of Lp, Bb, or LB shifted the β-diversity (PCoA analysis) of gut microbiota composition in aged mice, while the α-diversity (Shannon index) was not affected. At the family level, they also increased Prevotellaceae, Akkermandiaceae, and Bacteroidaceae populations, which were positively correlated with muscle weight and/or MyHC expression, and decreased Odoribacteraceae, Deferribacteraceae, Coriobacteriaceae, and Acholeplasmataceae populations, which were positively correlated with MuRF1 expression and/or IL-6 or TNF-α to IL-10 expression ratio. These results suggest that Odoribacteriaceae, Deferribacteraceae and Acholeplasmataceae may be inflammation-inducible and Akkermansiaceae and Bacteroidaceae may be inflammation-suppressible. These gut bacteria may control muscle protein synthesis and degradation-related transcription factors by regulating inflammation-related cytokine expression.
Conclusions
Gut dysbiosis inducers such as ageing can cause systemic inflammation including colitis. Gut bacteria including inflammation-inducible Odoribacteriaceae, Deferribacteraceae, and Acholeplasmataceae and inflammation-suppressible Akkermansiaceae and Bacteroidaceae may be closely connected with muscle weight and strength by regulating muscle protein biosynthesis-related MyHC/MyoG and muscle degradation-related MuRF1/MAFbx expression and cognitive impairment by regulating hippocampal BDNF expression. Lp, Bb, and LB, can alleviate muscle weight loss and atrophy (strength and endurance) cognitive impairment by regulating gut microbiota-mediated AKT, NF-κB, and/or FOXO3a signaling pathways.
Availability of data and materials
Gut microbiota sequence data were deposited in the NCBI (PRJNA1011499). Other datasets used and/or analyzed during the current study are available from the corresponding author on request.
Abbreviations
- Bb:
-
Bifidobacterium bifidum P61
- BDNF:
-
Brain-derived neurotropic factor
- EDL:
-
Extensor digitorum longus
- ELISA:
-
Enzyme-linked immunosorbent assay
- GA:
-
Gastrocnemius
- H&E:
-
Hematoxylin and eosin
- IL:
-
Interleukin
- Lp:
-
Lactobacillus paracasei P62
- LPS:
-
Lipopolysaccharide
- MAFbx:
-
Muscle atrophy F-box gene
- MuRF1:
-
Muscle RING-finger protein-1
- mTor:
-
Mammalian target of rapamycin
- MyHC:
-
Myosin heavy chain
- PGC:
-
Peroxisome proliferator-activated receptor gamma coactivator
- QD:
-
Quadriceps femoris
- qPCR:
-
Quantitative real-time polymerase chain reaction
- SOL:
-
Soleus
- TNF:
-
Tumor necrosis factor
- TA:
-
Tibialis anterior
References
Sayer AA, Cruz-Jentoft A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing. 2022;51:51. https://doi.org/10.1093/ageing/afac220.
Smith C, Woessner MN, Sim M, Levinger I. Sarcopenia definition: does it really matter? Implications for resistance training. Ageing Res Rev. 2022;78: 101617. https://doi.org/10.1016/j.arr.2022.101617.
Beeri MS, Leugrans SE, Delbono O, Bennett DA, Buchman AS. Sarcopenia is associated with incident Alzheimer’s dementia, mild cognitive impairment, and cognitive decline. J Am Geriatr Soc. 2021;69:1826–35. https://doi.org/10.1111/jgs.17206.
Meng SJ, Yu LJ. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11:1509–26. https://doi.org/10.3390/ijms11041509.
Ji Y, Li M, Chang M, Liu R, Qiu J, Wang K, et al. Inflammation: roles in skeletal muscle atrophy. Antioxid (Basel). 2022;11. https://doi.org/10.3390/antiox11091686.
Lahiri S, Kim H, Garcia-Perez I, Reza MM, Martin KA, Kundu P, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. 2019;11:11. https://doi.org/10.1126/scitranslmed.aan5662.
Kim KH, Chung Y, Huh JW, Park DJ, Cho Y, Oh Y, et al. Gut microbiota of the young ameliorates physical fitness of the aged in mice. Microbiome. 2022;10:238. https://doi.org/10.1186/s40168-022-01386-w.
Luo ZB, Han S, Yin XJ, Liu H, Wang J, Xuan M, et al. Fecal transplant from myostatin deletion pigs positively impacts the gut-muscle axis. Elife. 2023;12:12. https://doi.org/10.7554/eLife.81858.
Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. Probiotics and immunity. J Gastroenterol. 2009;44:26–46. https://doi.org/10.1007/s00535-008-2296-0.
Kim SK, Guevarra RB, Kim YT, Kwon J, Kim H, Cho JH, et al. Role of probiotics in human gut microbiome-associated diseases. J Microbiol Biotechnol. 2019;29:1335–40. https://doi.org/10.4014/jmb.1906.06064.
Chen LH, Huang SY, Huang KC, Hsu CC, Yang KC, Li LA, et al. Lactobacillus paracasei PS23 decelerated age-related muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging. 2019;11:756–70. https://doi.org/10.18632/aging.101782.
Lee K, Kim J, Park SD, Shim JJ, Lee JL. Lactobacillus plantarum HY7715 ameliorates Sarcopenia by improving skeletal muscle Mass and function in aged Balb/c mice. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms221810023.
Chen LH, Chang SS, Chang HY, Wu CH, Pan CH, Chang CC, et al. Probiotic supplementation attenuates age-related sarcopenia via the gut-muscle axis in SAMP8 mice. J Cachexia Sarcopenia Muscle. 2022;13:515–31. https://doi.org/10.1002/jcsm.12849.
Munukka E, Rintala A, Toivonen R, Nylund M, Yang B, Takanen A, et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. Isme J. 2017;11:1667–79. https://doi.org/10.1038/ismej.2017.24.
Prokopidis K, Giannos P, Kirwan R, Ispoglou T, Galli F, Witard OC, et al. Impact of probiotics on muscle mass, muscle strength and lean mass: a systematic review and meta-analysis of randomized controlled trials. J Cachexia Sarcopenia Muscle. 2023;14:30–44. https://doi.org/10.1002/jcsm.13132.
Yoon JH, Lee SM, Lee Y, Kim MJ, Yang JW, Choi JY, et al. Alverine citrate promotes myogenic differentiation and ameliorates muscle atrophy. Biochem Biophys Res Commun. 2022;586:157–62. https://doi.org/10.1016/j.bbrc.2021.11.076.
Kim C, Hwang JK. The 5,7-Dimethoxyflavone suppresses Sarcopenia by regulating protein turnover and Mitochondria Biogenesis-Related pathways. Nutrients. 2020;12. https://doi.org/10.3390/nu12041079.
Kim JK, Lee KE, Lee SA, Jang HM, Kim DH. Interplay between human gut bacteria escherichia coli and lactobacillus mucosae in the occurrence of neuropsychiatric disorders in mice. Front Immunol. 2020;11: 273. https://doi.org/10.3389/fimmu.2020.00273.
Jang SE, Lim SM, Jeong JJ, Jang HM, Lee HJ, Han MJ, et al. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunol. 2018;11:369–79. https://doi.org/10.1038/mi.2017.49.
Lee KE, Kim JK, Han SK, Lee DY, Lee HJ, Yim SV, et al. The extracellular vesicle of gut microbial paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome. 2020;8:107. https://doi.org/10.1186/s40168-020-00881-2.
Rescan PY. Regulation and functions of myogenic regulatory factors in lower vertebrates. Comp Biochem Physiol B Biochem Mol Biol. 2001;130:1–12. https://doi.org/10.1016/s1096-4959(01)00412-2.
Chen K, Gao P, Li Z, Dai A, Yang M, Chen S, et al. Forkhead box O signaling pathway in skeletal muscle atrophy. Am J Pathol. 2022;192:1648–57. https://doi.org/10.1016/j.ajpath.2022.09.003.
Thoma A, Lightfoot AP. NF-kB and inflammatory cytokine signalling: role in skeletal muscle atrophy. Adv Exp Med Biol. 2018;1088:267–79. https://doi.org/10.1007/978-981-13-1435-3_12.
Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC. Cognitive dysfunction with aging and the role of inflammation. Ther Adv Chronic Dis. 2011;2:175–95. https://doi.org/10.1177/2040622311399145.
Kim KA, Jeong JJ, Yoo SY, Kim DH. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016;16:9. https://doi.org/10.1186/s12866-016-0625-7.
Inaba T, Yamashiro K, Kurita N, Ueno Y, Miyamoto N, Hira K, et al. Microbial lipopolysaccharide-induced inflammation contributes to cognitive impairment and white matter lesion progression in diet-induced obese mice with chronic cerebral hypoperfusion. CNS Neurosci Ther. 2023;29(Suppl 1):200–12. https://doi.org/10.1111/cns.14301.
Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21:455–66e4. https://doi.org/10.1016/j.chom.2017.03.002.
Funk WD, Ouellette M, Wright WE. Molecular biology of myogenic regulatory factors. Mol Biol Med. 1991;8:185–95.
Zanou N, Gailly P. Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol Life Sci. 2013;70:4117–30. https://doi.org/10.1007/s00018-013-1330-4.
Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307:E469–484. https://doi.org/10.1152/ajpendo.00204.2014.
Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–500. https://doi.org/10.1101/gad.1662308.
Zhong Q, Zheng K, Li W, An K, Liu Y, Xiao X, et al. Post-translational regulation of muscle growth, muscle aging and sarcopenia. J Cachexia Sarcopenia Muscle. 2023. https://doi.org/10.1002/jcsm.13241.
Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–84. https://doi.org/10.1016/j.biocel.2005.04.018.
Sartori R, Romanello V, Sandri M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun. 2021;12:330. https://doi.org/10.1038/s41467-020-20123-1.
Tang BL. Sirt1 and the mitochondria. Mol Cells. 2016;39:87–95. https://doi.org/10.14348/molcells.2016.2318.
Chen M, Wang Y, Deng S, Lian Z, Yu K. Skeletal muscle oxidative stress and inflammation in aging: focus on antioxidant and anti-inflammatory therapy. Front Cell Dev Biol. 2022;10: 964130. https://doi.org/10.3389/fcell.2022.964130.
Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):4–9. https://doi.org/10.1093/gerona/glu057.
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–90. https://doi.org/10.1038/s41574-018-0059-4.
Shirakawa T, Rojasawasthien T, Inoue A, Matsubara T, Kawamoto T, Kokabu S. Tumor necrosis factor alpha regulates myogenesis to inhibit differentiation and promote proliferation in satellite cells. Biochem Biophys Res Commun. 2021;580:35–40. https://doi.org/10.1016/j.bbrc.2021.09.067.
Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress. 2000;3:201–8. https://doi.org/10.3109/10253890009001124.
Wang H, Duan X, Ren Y, Liu Y, Huang M, Liu P, et al. FoxO3a negatively regulates nerve growth factor-induced neuronal differentiation through inhibiting the expression of neurochondrin in PC12 cells. Mol Neurobiol. 2013;47:24–36. https://doi.org/10.1007/s12035-012-8357-7.
Polter A, Yang S, Zmijewska AA, van Groen T, Paik JH, Depinho RA, et al. Forkhead box, class O transcription factors in brain: regulation and behavioral manifestation. Biol Psychiatry. 2009;65:150–9. https://doi.org/10.1016/j.biopsych.2008.08.005.
Kim CS, Cha L, Sim M, Jung S, Chun WY, Baik HW, et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci. 2021;76:32–40. https://doi.org/10.1093/gerona/glaa090.
Acknowledgements
This work was supported by the Medical Research Program (2017R1A5A2014768) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT and the Ministry of Food and Drug Safety (22203MFDS539) funded by the Korea Healthy Industry Development Institute, Republic of Korea.
Author information
Authors and Affiliations
Contributions
Ji-Su Baek, Yoon-Jung Shin, Yun-Ha Hwang, and Dong-Hyun Kim wrote the main manuscript text. Ji-Su Baek prepared Fig. 1. Ji-Su Baek, Yoon-Jung Shin, Xiaoyang Ma, and Hee-Seo Park prepared Figs. 2, 3 and 4. Ji-Su Baek, Yoon-Jung Shin, and Xiaoyang Ma prepared figures Figs. 5 and 6. Ji-Su Baek and Yoon-Jung Shin prepared Fig. 7. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were approved by the Committee for the Care and Use of Laboratory Animals in Kyung Hee University (IACUC No, KHUASP(SE)-22-567) and were ethically carried in accordance of the Guideline of the University for Laboratory Animals Care and Use.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
The present manuscript contains supplementary materials. Table S1. Primers for qPCR. Table S2. Whole genome properties of P61 and P62. Table S3. Effects of Bb, Lp, and LB on the gut microbiota composition at the phylum level. Table S4. Effects of Bb, Lp, and LB on the gut microbiota composition at the family level. Table S5. Effects of Bb, Lp, and LB on the gut microbiota composition at the genus level. Table S5. Effects of Bb, Lp, and LB on the gut microbiota composition at the species level. Figure S1. Effects of Bb, Lp, and LB on LPS-induced NF-κB activation in C2C12 cells. Figure S2. Effects of Bb, Lp, and their (4:1, 1:1, and 1:4) mix LB on LPS-induced IL-6 expression in C2C12 cells. Figure S3. Effects of Bb, Lp, and LB on bodyweights in aged mice. Figure S4. Effects of Bb, Lp, and LB on pAKT (a), AKT (b), p-mTOR (c), mTOR (d), and β-actin (e) in the GA muscle of aged mice. Figure S5. Effects of Bb, Lp, and LB on p-p65 (a), p65 (b), p16 (c), p-Foxo3a (d), Foxo3a (e), MuRF1 (f), MAFbx (g), PGC1a (h), MyHC (i), and β-actin (j) in the GA muscle of aged mice.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Baek, JS., Shin, YJ., Ma, X. et al. Bifidobacterium bifidum and Lactobacillus paracasei alleviate sarcopenia and cognitive impairment in aged mice by regulating gut microbiota-mediated AKT, NF-κB, and FOXO3a signaling pathways. Immun Ageing 20, 56 (2023). https://doi.org/10.1186/s12979-023-00381-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12979-023-00381-5