Abstract
Background
Coronavirus disease 2019 (COVID-19) remains a threat to vulnerable populations such as long-term care facility (LTCF) residents, who are often older, severely frail, and have multiple comorbidities. Although associations have been investigated between COVID-19 mRNA vaccine immunogenicity, durability, and response to booster vaccination and chronological age, data on the association of clinical factors such as performance status, nutritional status, and underlying comorbidities other than chronological age are limited. Here, we evaluated the anti-spike IgG level and neutralizing activity against the wild-type virus and Delta and Omicron variants in the sera of LTCF residents, outpatients, and healthcare workers before the primary vaccination; at 8, 12, and 24 weeks after the primary vaccination; and approximately 3 months after the booster vaccination. This 48-week prospective longitudinal study was registered in the UMIN Clinical Trials Registry (Trial ID: UMIN000043558).
Results
Of 114 infection-naïve participants (64 LTCF residents, 29 outpatients, and 21 healthcare workers), LTCF residents had substantially lower anti-spike IgG levels and neutralizing activity against the wild-type virus and Delta variant than outpatients and healthcare workers over 24 weeks after the primary vaccination. In LTCF residents, booster vaccination elicited neutralizing activity against the wild-type virus and Delta variant comparable to that in outpatients, whereas neutralizing activity against the Omicron variant was comparable to that in outpatients and healthcare workers. Multiple regression analyses showed that age was negatively correlated with anti-spike IgG levels and neutralizing activity against the wild-type virus and Delta variant after the primary vaccination. However, multivariate regression analysis revealed that poor performance status and hypoalbuminemia were more strongly associated with a lower humoral immune response than age, number of comorbidities, or sex after primary vaccination. Booster vaccination counteracted the negative effects of poor performance status and hypoalbuminemia on the humoral immune response.
Conclusions
LTCF residents exhibited suboptimal immune responses following primary vaccination. Although older age is significantly associated with a lower humoral immune response, poor performance status and hypoalbuminemia are more strongly associated with a lower humoral immune response after primary vaccination. Thus, booster vaccination is beneficial for older adults, especially those with a poor performance status and hypoalbuminemia.
Similar content being viewed by others
Background
In early 2020, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection led to coronavirus disease 2019 (COVID-19), which later disseminated worldwide and claimed numerous lives. With the progress in COVID-19 vaccination and the replacement with the Omicron variant, the mortality and morbidity of COVID-19 have substantially decreased [1]. However, despite the progress in COVID-19 vaccination, older adults and those with multiple comorbidities still have higher levels of mortality and morbidity from COVID-19 than from influenza [2, 3]. Therefore, COVID-19 remains a significant threat to vulnerable populations that are older, severely frail, poorly nourished, and have multiple comorbidities. Although data on immunogenicity suggest that sera from individuals who received booster doses had better neutralizing activity against the Omicron variant [4,5,6,7], data on the extent of improvement in older and more vulnerable individuals following booster vaccination compared to that in the general population remains limited.
To develop strategies to protect older and vulnerable populations against the development of severe COVID-19, there is a need to investigate, especially in individuals with the greatest risk of severe COVID-19, the immunogenicity and durability of COVID-19 vaccines, the degree of immune escape by SARS-CoV-2 variants of concern (VOCs) and the booster vaccination effect against that. Although studies have investigated the association between COVID-19 mRNA vaccine immunogenicity, durability, and chronological age [8,9,10,11,12], data on the association between immunogenicity, durability, and clinical factors such as performance status, nutritional status, and underlying comorbidities other than chronological age are limited. Accordingly, better longitudinal evidence on vaccine immunogenicity, durability of immunity, and degree of immune escape by SARS-CoV-2 VOCs, specifically in older and vulnerable individuals, such as residents of long-term care facilities (LTCFs), is required to strategize best practices for controlling infection, preventing outbreaks, and identifying potential indications for further booster vaccinations.
Immune function generally declines with age [13,14,15,16,17], but how and to what extent the humoral immune response to stimulation in vivo changes with clinical factors, such as performance status and nutritional status, other than chronological age remains unknown. In this study, we evaluated this aspect by taking advantage of this rare opportunity for vaccination, in which humans are exposed to uniform antigenic stimulation. In this rare prospective longitudinal 48-week study, not only the kinetics of anti-spike IgG levels and neutralizing activity against the wild-type virus were evaluated, but also neutralizing activity against the Delta and Omicron VOCs of SARS-CoV-2 were determined before and following COVID-19 mRNA primary and booster vaccination in LTCF residents, outpatients, and healthcare workers. Furthermore, we investigated the association between the changes in neutralizing activity with viral mutations and various clinical factors.
Our results could be useful for the development of robust booster strategies as a control measure for SARS-CoV-2 VOCs in older and more vulnerable individuals such as LTCF residents.
Methods
Study design and population
Written informed consent was obtained from all participants or their legal guardians. The study protocol adhered to the Declaration of Helsinki and was approved by the Institutional Review Board of Yamaguchi University Hospital (Registration No. 2020–214). This prospective longitudinal study was registered in the UMIN Clinical Trials Registry (UMIN Trial ID: UMIN000043558). The detailed protocol of this study is available at https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000049712. Other objectives of this study were to: 1) evaluate cellular immunity after COVID-19 vaccination and 2) investigate the relationship between the microbiomes in the intestinal tract and the immunogenicity and durability of the COVID-19 vaccine. However, it takes some time to obtain these results. Therefore, in this paper, we report the results of the humoral immune responses. This study was conducted from March 5, 2021 to July 6, 2022. LTCF residents, outpatients, and healthcare workers were enrolled in this prospective, longitudinal cohort study. The LTCFs included four nursing homes and one long-term care hospital in Yamaguchi, Japan. The outpatients included individuals who regularly visited Yamaguchi University Hospital or Hofu Rehabilitation Hospital in Yamaguchi, Japan. The participants were recruited before they received the COVID-19 vaccine. The eligibility criteria included the absence of SARS-CoV-2 infection before receiving the first vaccine dose.
All participants were asked to provide peripheral blood samples for serological assays at five time points before and during the 48-week period after receiving the first vaccine dose: during the baseline period (before receiving the first vaccine dose), 8 weeks after the first dose (period 1), 12 weeks after the first dose (period 2), 24 weeks after the first dose (period 3), and 48 weeks after the first dose (period 4).
The end of the study for any participant was defined as 350 days after administration of the first vaccine dose, death, or lack of follow-up. A nucleic acid amplification test for SARS-CoV-2 was performed if any COVID-19–associated symptom or exposure to a SARS-CoV-2–infected person was reported. All participants were tested for antibodies specific to the viral nucleocapsid protein (IgG(N)) to rule out a COVID-19 breakthrough infection during the study period (at the baseline period and periods 1, 2, 3, and 4). Individuals with positive results were excluded from the final analysis.
Serological assays
Serological testing for antibodies to the receptor-binding domain (RBD) of the S1 subunit of the viral spike protein [IgG(S-RBD)] and IgG(N) was performed using the Abbott Architect SARS-CoV-2 IgG II Quant assay and SARS-CoV-2 IgG assay (both Abbott Laboratories, Sligo, Ireland), respectively, according to the manufacturer’s instructions. An IgG(N) S/C ≥ 1.4 denoted seropositive status due to prior infection or SARS-CoV-2 exposure during the observation period, based on a previously established cut-off point [18].
Surrogate virus neutralization test
A commercially available surrogate virus neutralization test (sVNT; cPass SARS-CoV-2 Neutralization Antibody Detection Kit, Genscript Biotech Corporation, Piscataway, NJ, USA) was used. The surrogate virus neutralization assay had high sensitivity and specificity (with a recommended positive threshold of 30%) and showed an excellent correlation with the plaque reduction neutralization test. The assay detects functional antibodies that neutralize the interaction between the spike protein RBD (spike-RBD) and human angiotensin-converting enzyme 2 (ACE2) [19,20,21]. The assay was performed in accordance with the manufacturer's instructions. For the Delta and Omicron variant sVNT, the same protocol was followed by replacing the wild-type horseradish peroxidase-conjugated recombinant spike protein RBD (HRP-RBD) with the commercially available recombinant proteins for the Delta (B.1.617.2) and Omicron variants (B.1.1.529, sublineage BA.1) HRP-RBD from Genscript Biotech Corporation.
Statistical analysis
The data were stratified into three groups: healthcare workers, outpatients, and LTCF residents. Values are summarized as median and interquartile range (IQR) for continuous variables and as frequencies (percentage) for categorical variables. Intergroup differences were tested using Fisher’s exact test for categorical variables and Wilcoxon’s rank-sum test or Kruskal–Wallis test for numerical variables. All pairwise comparisons after the Kruskal–Wallis test were performed using Dunn’s test with Bonferroni correction for multiple testing. Correlations between variables were calculated using Spearman's rank correlation coefficient analysis. Factors of variation of the IgG (S-RBD) level and neutralizing activity against the wild-type virus and the Delta and Omicron variants were analyzed using multiple regression analyses (MRA) that were performed separately for each type of serological assay in periods 1, 3, and 4. As the IgG (S-RBD) levels showed a highly skewed distribution, they were logarithmically transformed before analysis. The optimal regression model was built by a repeated stepwise selection procedure based on the level of adjusted coefficient of determinations. In the selection process, "age" was always included in the model as a control variable to avoid its confounding influence on other parameters. The practical significance of the parameters retained in the regression model was interpreted based on a standardized partial regression coefficient, which corresponds to the partial correlation coefficient (rp) and has values between -1.0 and 1.0. In reference to Cohen's criterion for the effect size of the correlation coefficient [22], we regarded 0.20≦|rp|< 0.3 as "weak", 0.30≦|rp|< 0.5 as "moderate", and 0.5≦|rp| as "strong" correlation. As a sub-analysis, logistic regression analysis was performed to identify potential risk factors for negative neutralizing activity against the Omicron variant observed at 48 weeks. The levels of association were expressed as unadjusted and adjusted odds ratios (ORs) and 95% confidence intervals (CIs). All statistical analyses were performed using StatFlex for Windows Ver. 7 (Artech Inc., Osaka). Scatter plots and box-and-whisker plots were generated using JMP Pro 16.1.0 (SAS Institute Inc., Cary, NC, USA).
Results
Study population and serological assays
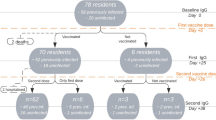
The final study sample comprised 114 infection-naïve participants (64 LTCF residents, 29 outpatients, and 21 healthcare workers) who underwent at least two serological tests from the baseline period. The sample population consisted of 100% Asian individuals, of whom 60% were females. Detailed baseline demographic characteristics for each subpopulation are summarized in Table 1. The number of participants included in the final analysis who underwent IgG (S-RBD) tests and neutralizing antibody tests at each period is presented in Fig. 1. From the baseline to period 1, one participant refused to complete the two vaccination doses and was excluded from the final analysis. The remaining participants completed two vaccination doses with BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine in the primary vaccine series (two intramuscular doses of 30 mcg each given three weeks apart) from baseline to period 1. From periods 3 to 4, two participants failed to receive the booster vaccination and were excluded from the final analysis in period 4. The remaining participants received booster vaccination from periods 3 to 4. Therefore, the assessment at 48 weeks after the first dose constituted an assessment approximately three months after the booster vaccination, wherein all healthcare workers, 14 out of 26 outpatients, and 15 out of 50 LTCF residents received the BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine, and 12 out of 26 outpatients and 35 out of 50 LTCF residents received the mRNA-1273 (Moderna) COVID-19 vaccine. Vaccine types for booster vaccination for healthcare workers and LTCF residents were specified by the local governments. Although a nucleic acid amplification test for SARS-CoV-2 was performed if any COVID-19–associated symptom or exposure to a SARS-CoV-2–infected person was reported, no COVID-19 patients were identified among the participants during the study period. However, five participants showed positive IgG(N) results and were considered to have been infected with SARS-CoV-2 asymptomatically during the study period; they were excluded from the final analysis.
Recruitment of participants, testing, and follow-up. This study included a prospective cohort of residents of long-term care facilities (LTCFs), outpatients, and healthcare workers. During the study period (March 5, 2021, to July 6, 2022), the participants provided peripheral blood samples for serological assays at five time points before and during the 48 weeks after receiving the first vaccine dose: during the baseline period (before the first vaccine dose) and at 8 weeks (period 1), 12 weeks (period 2), 24 weeks (period 3), and 48 weeks (period 4) after the first dose. From the baseline to period 1, one participant refused to complete the two vaccination doses and was excluded from the final analysis. The remaining participants completed two vaccination doses with BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine in the primary vaccine series (two intramuscular doses of 30 mcg each were given three weeks apart) from baseline to period 1. From periods 3 to 4, two participants failed to receive booster vaccination and were excluded from the final analysis in period 4. The remaining participants received booster vaccination from periods 3 to 4. Therefore, the assessment at 48 weeks after the first dose constituted an assessment approximately 3 months after the booster vaccination, wherein all healthcare workers, 14 out of 26 outpatients, and 15 out of 50 LTCF residents received the BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine and 12 out of 26 outpatients and 35 out of 50 LTCF residents received the mRNA-1273 (Moderna) COVID-19 vaccine. Vaccine types for booster vaccination for healthcare workers and LTCF residents were specified by local governments
SARS-CoV-2 IgG (S-RBD) level and neutralizing activity kinetics
The kinetics of the humoral immune response were assessed throughout the 48-week period (Fig. 2). The sera of LTCF residents had significantly lower IgG (S-RBD) levels and neutralizing activity against the wild-type virus and the Delta variant than those of outpatients and healthcare workers in periods 1–3. During period 3, 51% of LTCF residents showed negative neutralizing activity against the wild-type virus in the sera, whereas only 7% of the outpatients and none of the healthcare workers showed negative results; in this period, 67% of LTCF residents showed negative neutralizing activity against the Delta variant, whereas only 5% of healthcare workers and 7% of outpatients showed negative results. Neutralizing activity against the Omicron variant in the sera was below the cut-off level in most participants throughout the 24 weeks after the first dose, including in healthcare workers during period 1.
SARS-CoV-2 IgG (S-RBD) and neutralizing activity kinetics. A Antibodies to the receptor-binding domain of the S1 subunit of the viral spike protein [IgG (S-RBD)] and neutralizing activity against (B) wild-type virus, (C) Delta variant, and (D) Omicron variants in sera were determined in infection-naïve participants who provided peripheral blood samples for serological assays at five time points during 48 weeks after receiving the first vaccine dose: at baseline (before the first vaccine dose) and at 8 weeks (period 1), 12 weeks (period 2), 24 weeks (period 3), and 48 weeks (period 4) after the first dose. Between 24 (period 3) and 48 weeks (period 4) after the first dose, booster vaccination with BNT162b2 or mRNA-1273 was completed. Period 4 was approximately 3 months after the booster vaccination. The participants were stratified into three subgroups: healthcare workers, outpatients, and residents of long-term care facilities. Each dot represents an individual participant. The boxes span the interquartile range; the line within each box denotes the median, and the whiskers are the largest and smallest values within the range of ± 1.5-fold in the interquartile range from the first and third quartile. The dashed line in panel A indicates a cut-off of 50 AU/mL for assay positivity, whereas those in panels B–D indicate the cut-off of 30% for assay positivity, as determined previously. The gray areas in panels A–D represent negative results. The numbers above each column indicate the number of participants with positive/negative assay results and the proportion of participants with positive assay results. Fold-comparison in geometric mean IgG (S-RBD) levels relative to that in healthcare workers in period 1 is shown as a number with the “ × ” symbol in panel A. Fold-comparison in median neutralizing activity relative to that against the wild-type virus in healthcare workers in period 1 is shown as a number with the “ × ” symbol in panels B–D. P-values are indicated above each plot. HW; healthcare workers, OP; outpatients, LTCFs; residents of long-term care facilities
In contrast, booster vaccination elicited IgG (S-RBD) levels in LTCF residents comparable to those in healthcare workers and outpatients. Booster vaccination also elicited neutralizing activity against both wild-type virus and the Delta variant in LTCF residents comparable to that in outpatients. Furthermore, the inter-individual differences in neutralizing activity against the wild-type virus and the Delta variant decreased conspicuously after the booster vaccination in all subgroups, with a few exceptions. Meanwhile, in LTCF residents, the booster vaccination elicited neutralizing activity against the Omicron variant comparable to that in healthcare workers and outpatients. However, only 46% of the participants who received booster vaccines exhibited positive neutralizing activity against the Omicron variant, and the inter-individual differences remained high.
Potential factors responsible for the variation in IgG (S-RBD) levels and neutralizing activity
Factors that are likely involved in the variation in IgG (S-RBD) levels and neutralizing activity against the wild-type virus and the Delta and Omicron variants were analyzed using MRA (Table 2). Age-related changes in IgG (S-RBD) and neutralizing activity were first examined univariately by MRA, and their magnitude was expressed by the standardized partial regression coefficients (rp). By setting |rp|≥ 0.20 as a practical level of importance, age was negatively correlated with IgG (S-RBD) levels, neutralizing activity against the wild-type and Delta variant during periods 1 and 3, and with neutralizing activity against the Delta variant in period 4. However, according to the multivariate analysis, age was not independently associated with the variation of IgG (S-RBD) and neutralizing activity, except for IgG (S-RBD) in period 1 and neutralizing activity against wild-type virus and Delta variant in period 4. Furthermore, serum albumin showed positive correlations with neutralizing activity against the wild-type virus and the Delta variant in period 1 and with the wild-type virus in period 3. Additionally, the Eastern Cooperative Oncology Group Performance Status Scale (ECOG-PS) score showed a negative correlation with IgG (S-RBD) level in periods 1 and 3 and with the neutralizing activity against the wild-type virus and the Delta variant in period 3. MRA was not performed for neutralizing activity against the Omicron variant in periods 1 and 3, because the activity was below the cut-off level in most participants. In period 4, after the booster vaccination, age was negatively correlated with neutralizing activity against the wild-type and Delta variant. Meanwhile, no significant factors were found to be associated with the variation in neutralizing activity against the Omicron variant after booster vaccination, except for minor sex-related differences. Logistic regression analysis was performed as a sub-analysis to identify potential risk factors for negative neutralizing activity against the Omicron variant in period 4 (Table 3). However, no significant factors were identified by either univariate or multivariate analyses.
Correlation between IgG (S-RBD) level and neutralizing activity
We assessed the correlation between the IgG (S-RBD) level and neutralizing activity against the wild-type virus and the Delta and Omicron variants (Fig. 3). IgG (S-RBD) level and the neutralizing activity against the wild-type virus and the Delta variant showed a strong positive correlation in periods 1, 3, and 4 (Spearman’s rank correlation: 0.561–0.932). Meanwhile, no significant correlation was found between the IgG (S-RBD) level and neutralizing activity against the Omicron variant in period 1 (Spearman’s rank correlation: -0.061). Although IgG (S-RBD) level and the neutralizing activity against the Omicron variant appeared to show a weak negative correlation in period 3 (Spearman’s rank correlation: -0.284), this was regarded as irrelevant as the neutralizing activity against the Omicron variant was below the cut-off level in most participants. In contrast, in period 4, IgG (S-RBD) levels and neutralizing activity against the Omicron variant showed an appreciable positive correlation (Spearman’s rank correlation: 0.390).
Correlation between IgG (S-RBD) and neutralizing activity. We assessed the correlation between antibodies to the receptor-binding domain of the S1 subunit of the viral spike protein [IgG (S-RBD)] and neutralizing activity against the wild-type virus and the Delta and Omicron variants. The left, middle, and right columns show correlations between neutralizing activity against the wild-type virus and the Delta and Omicron variants, respectively, and the IgG (S-RBD) levels. The upper, middle, and lower rows show the correlations in periods 1, 3, and 4, respectively. The neutralizing activity was plotted as zero when the recordings were negative
Discussion
The aging immune system undergoes immune-senescence, which can result in impaired vaccine responses [13]. Immune-senescence-related changes include reduced immune function, such as constrained germinal center responses, a reduced naive cell repertoire, accumulation of an expanded memory pool, and an increase in inflammatory subsets of adaptive immune cells [14,15,16,17]. In fact, the negative impact of age on immunogenicity with COVID-19 vaccination was highlighted recently [23,24,25,26,27,28,29,30,31]. In the present study, LTCF residents exhibited suboptimal immune responses following primary vaccination. These results were consistent with previous reports, indicating a lower intensity of humoral immune response and a narrower breadth of cross-neutralization in older and more vulnerable individuals than in the general population.
However, the humoral responses to vaccination showed large inter-individual differences. Results of multivariate regression analysis showed that poor ECOG-PS and hypoalbuminemia were more strongly associated with a lower humoral immune response than age, number of comorbidities, and sex after the primary vaccination series. Although older age was an important factor associated with a lower humoral immune response, vulnerable individuals, particularly those with poor ECOG-PS and hypoalbuminemia, showed a lower humoral immune response. Older adults with frailty show impaired vaccine effectiveness, including influenza, varicella-zoster, and pneumococcal pneumonia vaccine [32,33,34]. Furthermore, frailty is an independent predictor of impaired antibody responses to COVID-19 mRNA vaccines [35, 36]. Physical inactivity has also been shown to be a risk factor for severe COVID-19 [35, 37]. Moreover, there is a dose–response relationship suggesting that the higher the physical activity, the higher the efficacy of vaccines, including the COVID-19 vaccine [38,39,40,41,42]. These findings suggest that immunogenicity with COVID-19 vaccination could be improved by encouraging exercise in frail and older individuals through appropriate rehabilitation programs. Physical inactivity triggers persistent low-grade systemic inflammation, which may cause immune system dysfunction [43]. Inhibitory substances associated with inflammatory states, such as tumor necrosis factor and interleukin-1, impede albumin synthesis [44,45,46]. Among the participants in this study, those with hypoalbuminemia might have had latent chronic inflammation, in addition to malnutrition. This may be one of the mechanisms by which poor ECOG-PS and hypoalbuminemia were associated with a lower humoral immune response. Although this study did not aim to clarify these mechanisms, further studies should investigate the mechanisms underlying the low humoral immune response, which might herald the identification of measures to improve immunogenicity in vulnerable populations.
Compared to antibody levels, which decline over time, memory B cells exhibit a more sustained presence following vaccination and/or SARS-CoV-2 infection [47]. Some studies have demonstrated that after COVID-19 mRNA vaccination, B cells continue to undergo affinity maturation [47,48,49,50], which facilitates improved antibody functionality in neutralizing the virus. Thus, booster doses can increase the levels of antibodies and enhance the breadth of the immune response against SARS-CoV-2 [4,5,6,7]. However, the specific duration of B cell persistence, affinity maturation, and extent of booster effect may vary among individuals and may be influenced by factors such as age, performance status, and nutritional status. In this study, LTCF residents exhibited suboptimal immune responses with primary vaccination series alone. However, booster vaccination elicited an immune response in LTCF residents comparable to that in healthcare workers and outpatients. Of the LTCF residents in the present study, 87.5% had an ECOG-PS score of 3 or higher. Notably, even in LTCF residents with poor ECOG-PS and hypoalbuminemia, booster vaccination elicited humoral immune responses comparable to those in the general population. Furthermore, inter-individual differences in neutralizing activity against the wild-type virus and the Delta variant decreased after the booster vaccination. Notably, after the booster vaccination, the humoral immunity was enhanced relatively uniformly among vulnerable older individuals, outpatients, and healthcare workers. The booster vaccination counteracted the negative effects of poor performance status and hypoalbuminemia on humoral immune responses, as shown in Table 2. Thus, booster vaccination is particularly beneficial for older adults, especially LTCF residents with poor ECOG-PS and hypoalbuminemia.
Although booster vaccination elicited a higher neutralizing activity against the Omicron variant than the primary vaccination series in all subgroups, only 46% participants exhibited positive neutralizing activity against the Omicron variant, even after the booster vaccination, and inter-individual differences remained very high. The risk factors for negative neutralizing activity against the Omicron variant after booster vaccination are unknown. Unlike that observed with the wild-type and Delta variant after the primary vaccination, older vulnerable individuals did not show particularly poor neutralizing activity against the Omicron variant after the booster vaccination, as shown in Tables 2 and 3. There are healthy non-responders to the hepatitis B virus vaccination. The lack of response appears to be genetically determined and related to the human leukocyte antigen haplotypes [51]. Our sample population was limited to a single geographic region in Japan and was 100% Asian. Although a simple comparison is not possible owing to different measurement methods, healthcare workers and outpatients in the present study appeared to show a lower acquisition rate for neutralizing antibodies against the Omicron variant after the booster vaccination in comparison to previous reports from the United States and Israel [4, 5, 7]. However, whether there are any genetic basis or racial differences in the non-development of neutralizing activity against the Omicron variant following the COVID-19 booster vaccination based on the wild-type virus remains unclear and further research is required to clarify this issue.
Herein, IgG (S-RBD) levels and neutralizing activity against the wild-type virus and the Delta variant showed a positive correlation. Since the presence of neutralizing antibodies is indicative of protection [52, 53], our observations suggest that the results of IgG (S-RBD) testing may be used to predict protection from the wild-type virus and Delta variant as an alternative test to VNT. In contrast, no significant correlation was found between the IgG (S-RBD) level and neutralizing activity against the Omicron variant after the primary vaccination. Therefore, when Omicron was the dominant strain worldwide, protection from infection could not have been predicted based on the IgG test results. Nonetheless, booster vaccination elicited a better correlation between IgG (S-RBD) levels and neutralizing activity against the Omicron variant than the primary vaccination series, highlighting the notable advantage of booster vaccination. Moreover, the data in Fig. 3 show that the neutralizing activity was enhanced after the booster dose, even at similar IgG (S-RBD) levels. In particular, the phenomenon was observed at IgG (S-RBD) levels within the range of 500–5000 AU/mL for the wild-type virus and the Delta variant and at IgG (S-RBD) levels > 5000 AU/mL for the Omicron variant. These findings suggest the persistence of SARS-CoV-2-specific B cells that continue to undergo affinity maturation even in older vulnerable individuals. However, even after booster vaccination, the correlation between IgG (S-RBD) levels and neutralizing activity against the Omicron variant was weak. Most previous studies evaluating immunogenicity after COVID-19 vaccination in LTCF residents only evaluated IgG antibody levels. Therefore, the results of this study, which evaluated the neutralization activity against VOCs, including the Omicron variant, are valuable in considering infection control measures for LTCF residents.
The main limitation of our study is the small sample size, owing to which it is difficult to firmly establish the effects of performance status and hypoalbuminemia. Whether other antibody-mediated functions, such as complement deposition, antibody-dependent cellular cytotoxicity, and antibody-dependent cellular phagocytosis, are lower in older vulnerable individuals than in the general population remains an important but unresolved issue. Moreover, it remains unclear whether there are differences in the long-term durability of the humoral immune response after booster vaccination between vulnerable older individuals and the general population. Further research is needed to answer these questions. These findings will enable the development of robust boosting strategies as control measures for SARS-CoV-2 VOCs in older vulnerable individuals.
Availability of data and materials
The data are available to approved individuals upon reasonable request to the Yamaguchi University after fulfilling specific requirements.
References
Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared With Previous Waves. JAMA. 2022;327(6):583–4.
Xie Y, Choi T, Al-Aly Z. Risk of Death in Patients Hospitalized for COVID-19 vs Seasonal Influenza in Fall-Winter 2022–2023. JAMA. 2023;329(19):1697–9.
Portmann L, de Kraker MEA, Frohlich G, Thiabaud A, Roelens M, Schreiber PW, et al. Hospital Outcomes of Community-Acquired SARS-CoV-2 Omicron Variant Infection Compared With Influenza Infection in Switzerland. JAMA Netw Open. 2023;6(2):e2255599.
Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185(3):457-66.e4.
Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C, et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N Engl J Med. 2022;386(5):492–4.
Wu M, Wall EC, Carr EJ, Harvey R, Townsley H, Mears HV, et al. Three-dose vaccination elicits neutralising antibodies against omicron. Lancet. 2022;399(10326):715–7.
Pajon R, Doria-Rose NA, Shen X, Schmidt SD, O’Dell S, McDanal C, et al. SARS-CoV-2 Omicron Variant Neutralization after mRNA-1273 Booster Vaccination. N Engl J Med. 2022;386(11):1088–91.
Blain H, Tuaillon E, Gamon L, Pisoni A, Miot S, Picot MC. Strong Decay of SARS-CoV-2 Spike Antibodies after 2 BNT162b2 Vaccine Doses and High Antibody Response to a Third Dose in Nursing Home Residents. J Am Med Dir Assoc. 2022;23(5):750–3.
Gimenez E, Albert E, Burgos JS, Peiro S, Salas D, Vanaclocha H, et al. SARS-CoV-2 adaptive immunity in nursing home residents up to eight months after two doses of the Comirnaty(R) COVID-19 vaccine. J Infect. 2022;84(6):834–72.
Dyer AH, Noonan C, McElheron M, Batten I, Reddy C, Connolly E, et al. Previous SARS-CoV-2 Infection, Age, and Frailty Are Associated With 6-Month Vaccine-Induced Anti-Spike Antibody Titer in Nursing Home Residents. J Am Med Dir Assoc. 2022;23(3):434–9.
Chong Y, Goto T, Tani N, Yonekawa A, Ikematsu H, Shimono N, et al. Pronounced antibody elevation after SARS-CoV-2 BNT162b2 mRNA booster vaccination in nursing home residents. Influenza Other Respir Viruses. 2022;16(6):1066–71.
Canaday DH, Oyebanji OA, Keresztesy D, Payne M, Wilk D, Carias L, et al. Significant Reduction in Vaccine-Induced Antibody Levels and Neutralization Activity Among Healthcare Workers and Nursing Home Residents 6 Months Following Coronavirus Disease 2019 BNT162b2 mRNA Vaccination. Clin Infect Dis. 2022;75(1):e884–7.
Ford BN, Savitz J. Depression, aging, and immunity: implications for COVID-19 vaccine immunogenicity. Immun Ageing. 2022;19(1):32.
Frasca D, Blomberg BB. Aging induces B cell defects and decreased antibody responses to influenza infection and vaccination. Immun Ageing. 2020;17(1):37.
Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, Medaglini D. Vaccination in the elderly: The challenge of immune changes with aging. Semin Immunol. 2018;40:83–94.
Pereira B, Xu XN, Akbar AN. Targeting Inflammation and Immunosenescence to Improve Vaccine Responses in the Elderly. Front Immunol. 2020;11: 583019.
Fulop T, Pawelec G, Castle S, Loeb M. Immunosenescence and vaccination in nursing home residents. Clin Infect Dis. 2009;48(4):443–8.
Chew KL, Tan SS, Saw S, Pajarillaga A, Zaine S, Khoo C, et al. Clinical evaluation of serological IgG antibody response on the Abbott Architect for established SARS-CoV-2 infection. Clin Microbiol Infect. 2020;26(9):1256.e9-11.
Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9):1073–8.
Taylor SC, Hurst B, Charlton CL, Bailey A, Kanji JN, McCarthy MK, et al. A New SARS-CoV-2 Dual-Purpose Serology Test: Highly Accurate Infection Tracing and Neutralizing Antibody Response Detection. J Clin Microbiol. 2021;59(4):e02438-e2520.
Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, Yuki EFN, Pedrosa T, Fusco SRG, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27(10):1744–51.
Cohen J. A power primer. Psychol Bull. 1992;112(1):155–9.
Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med. 2021;385(24): e84.
Canaday DH, Carias L, Oyebanji OA, Keresztesy D, Wilk D, Payne M, et al. Reduced BNT162b2 mRNA vaccine response in SARS-CoV-2-naive nursing home residents. medRxiv. 2021.
Causa R, Almagro-Nievas D, Rivera-Izquierdo M, Benitez-Munoz N, Lopez-Hernandez B, Garcia-Garcia F, et al. Antibody Response 3 Months after 2 Doses of BNT162b2 mRNA COVID-19 Vaccine in Residents of Long-Term Care Facilities. Gerontology. 2022;68(8):910–6.
Tut G, Lancaster T, Sylla P, Butler MS, Kaur N, Spalkova E, et al. Antibody and cellular immune responses following dual COVID-19 vaccination within infection-naive residents of long-term care facilities: an observational cohort study. Lancet Healthy Longev. 2022;3(7):e461–9.
Tober-Lau P, Schwarz T, Vanshylla K, Hillus D, Gruell H, Group ECS, et al. Long-term immunogenicity of BNT162b2 vaccination in older people and younger health-care workers. Lancet Respir Med. 2021;9(11):e104-5.
Nordstrom P, Ballin M, Nordstrom A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden. Lancet. 2022;399(10327):814–23.
Richards NE, Keshavarz B, Workman LJ, Nelson MR, Platts-Mills TAE, Wilson JM. Comparison of SARS-CoV-2 Antibody Response by Age Among Recipients of the BNT162b2 vs the mRNA-1273 Vaccine. JAMA Netw Open. 2021;4(9): e2124331.
Wright BJ, Tideman S, Diaz GA, French T, Parsons GT, Robicsek A. Comparative vaccine effectiveness against severe COVID-19 over time in US hospital administrative data: a case-control study. Lancet Respir Med. 2022;10(6):557–65.
Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–44.
Andrew MK, Shinde V, Ye L, Hatchette T, Haguinet F, Dos Santos G, et al. The Importance of Frailty in the Assessment of Influenza Vaccine Effectiveness Against Influenza-Related Hospitalization in Elderly People. J Infect Dis. 2017;216(4):405–14.
Curran D, Kim JH, Matthews S, Dessart C, Levin MJ, Oostvogels L, et al. Recombinant Zoster Vaccine Is Efficacious and Safe in Frail Individuals. J Am Geriatr Soc. 2021;69(3):744–52.
Macintyre CR, Ridda I, Gao Z, Moa AM, McIntyre PB, Sullivan JS, et al. A randomized clinical trial of the immunogenicity of 7-valent pneumococcal conjugate vaccine compared to 23-valent polysaccharide vaccine in frail, hospitalized elderly. PLoS ONE. 2014;9(4): e94578.
Semelka CT, DeWitt ME, Callahan KE, Herrington DM, Alexander-Miller MA, Yukich JO, et al. Frailty and COVID-19 mRNA Vaccine Antibody Response in the COVID-19 Community Research Partnership. J Gerontol A Biol Sci Med Sci. 2022;77(7):1366–70.
Semelka CT, DeWitt ME, Blevins MW, Holbrook BC, Sanders JW, Alexander-Miller MA. Frailty impacts immune responses to Moderna COVID-19 mRNA vaccine in older adults. Immun Ageing. 2023;20(1):4.
Sallis R, Young DR, Tartof SY, Sallis JF, Sall J, Li Q, et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br J Sports Med. 2021;55(19):1099–105.
Hallam J, Jones T, Alley J, Kohut ML. Exercise after influenza or COVID-19 vaccination increases serum antibody without an increase in side effects. Brain Behav Immun. 2022;102:1–10.
Edwards KM, Pung MA, Tomfohr LM, Ziegler MG, Campbell JP, Drayson MT, et al. Acute exercise enhancement of pneumococcal vaccination response: a randomised controlled trial of weaker and stronger immune response. Vaccine. 2012;30(45):6389–95.
Edwards KM, Burns VE, Allen LM, McPhee JS, Bosch JA, Carroll D, et al. Eccentric exercise as an adjuvant to influenza vaccination in humans. Brain Behav Immun. 2007;21(2):209–17.
Ranadive SM, Cook M, Kappus RM, Yan H, Lane AD, Woods JA, et al. Effect of acute aerobic exercise on vaccine efficacy in older adults. Med Sci Sports Exerc. 2014;46(3):455–61.
Collie S, Saggers RT, Bandini R, Steenkamp L, Champion J, Gray G, et al. Association between regular physical activity and the protective effect of vaccination against SARS-CoV-2 in a South African case-control study. Br J Sports Med. 2023;57(4):205–11.
Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–9.
Bernardi M, Angeli P, Claria J, Moreau R, Gines P, Jalan R, et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut. 2020;69(6):1127–38.
Perlmutter DH, Dinarello CA, Punsal PI, Colten HR. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986;78(5):1349–54.
Dinarello CA. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984;311(22):1413–8.
Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639–44.
Sokal A, Barba-Spaeth G, Fernandez I, Broketa M, Azzaoui I, de La Selle A, et al. mRNA vaccination of naive and COVID-19-recovered individuals elicits potent memory B cells that recognize SARS-CoV-2 variants. Immunity. 2021;54(12):2893-907.e5.
Cho A, Muecksch F, Schaefer-Babajew D, Wang Z, Finkin S, Gaebler C, et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature. 2021;600(7889):517–22.
Tang J, Grubbs G, Lee Y, Huang C, Ravichandran S, Forgacs D, et al. Antibody affinity maturation and cross-variant activity following SARS-CoV-2 mRNA vaccination: Impact of prior exposure and sex. EBioMedicine. 2021;74: 103748.
Alper CA, Kruskall MS, Marcus-Bagley D, Craven DE, Katz AJ, Brink SJ, et al. Genetic prediction of nonresponse to hepatitis B vaccine. N Engl J Med. 1989;321(11):708–12.
Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11.
Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43–50.
Acknowledgements
The authors thank the staff and residents of the long-term care facilities who participated in this study, Dr. K. Ichihara (Faculty of Health Sciences, Yamaguchi University Graduate School of Medicine, Ube, Japan) for conducting the statistical analysis, and Mr. Y. Yoshida, Ms. T. Okamura, Ms. K. Sakaguchi, Ms. N. Uchida and Ms. S. Azuma for their excellent technical support. We also thank Editage (www.editage.jp) for English language editing.
Funding
No specific funding was received for the study.
Author information
Authors and Affiliations
Contributions
TK conceptualized the study and designed the study protocol. TK, KD, YO, HK, and MK were responsible for the project administration. KD performed the surrogate virus neutralization test. TK, KD, and YO curated and validated the data. TK drafted the original manuscript. All authors have reviewed and edited the manuscript. TK, KD, and YO verified the data. All authors have full access to the data used in this study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol adhered to the Declaration of Helsinki and was approved by the Institutional Review Board of Yamaguchi University Hospital (Registration No. 2020–214). Written informed consent was obtained from all participants or their legal guardians.
Consent for publication
All authors give their consent for publication.
Competing interests
TK and KD are employees of the Department of Pulmonology and Gerontology, Graduate School of Medicine, Yamaguchi University, Ube, Japan, which is funded by the Medical Corporation, WADOKAI. The other authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kakugawa, T., Doi, K., Ohteru, Y. et al. Kinetics of COVID-19 mRNA primary and booster vaccine-associated neutralizing activity against SARS-CoV-2 variants of concern in long-term care facility residents: a prospective longitudinal study in Japan. Immun Ageing 20, 42 (2023). https://doi.org/10.1186/s12979-023-00368-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12979-023-00368-2