Abstract
Background
Older people achieve lower levels of antibody titers than younger populations after Covid-19 vaccination and show a marked waning humoral immunity over time, likely due to the senescence of the immune system. Nevertheless, age-related predictive factors of the waning humoral immune response to the vaccine have been scarcely explored. In a cohort of residents and healthcare workers from a nursing home that had received two doses of the BNT162b2 vaccine, we measured specific anti-S antibodies one (T1), four (T4), and eight (T8) months after receiving the second dose. Thymic-related functional markers, including thymic output, relative telomere length, and plasma thymosin-α1 levels, as well as immune cellular subsets, and biochemical and inflammatory biomarkers, were determined at T1, and tested for their associations with the magnitude of the vaccine response (T1) and the durability of such response both, at the short- (T1-T4) and the long-term (T1-T8). We aimed to identify age-related factors potentially associated with the magnitude and persistence of specific anti-S immunoglobulin G (IgG)-antibodies after COVID-19 vaccination in older people.
Results
Participants (100% men, n = 98), were subdivided into three groups: young (< 50 years-old), middle-age (50–65 years-old), and older (≥65 years-old). Older participants achieved lower antibody titers at T1 and experienced higher decreases in both the short- and long-term. In the entire cohort, while the magnitude of the initial response was mainly associated with the levels of homocysteine [β (95% CI); − 0.155 (− 0.241 to − 0.068); p = 0.001], the durability of such response at both, the short-term and the long-term were predicted by the levels of thymosin-α1 [− 0.168 (− 0.305 to − 0.031); p = 0.017, and − 0.123 (− 0.212 to − 0.034); p = 0.008, respectively].
Conclusions
Higher plasma levels of thymosin-α1 were associated with a lower waning of anti-S IgG antibodies along the time. Our results suggest that plasma levels of thymosin-α1 could be used as a biomarker for predicting the durability of the responses after COVID-19 vaccination, possibly allowing to personalize the administration of vaccine boosters.
Similar content being viewed by others
Background
Although Coronavirus disease 2019 (COVID-19) vaccines are highly effective in preventing severe disease and death, a major clinical problem remains regarding the weaker humoral response and the rapid waning in protection early after vaccination in older people [1]. Consequently, vaccinated older people still show higher mortality rates due to COVID-19 than younger populations [2, 3]. This raises a question about the optimal COVID-19 vaccination strategy among older subjects, which may differ from the general population, as it has been shown in other contexts, such as hepatitis B virus or Influenza vaccination [4,5,6].
There is evidence that higher antibody levels following COVID-19 vaccination are associated with better protection against severe outcomes [7,8,9]. In accordance, COVID-19 breakthrough infections occur mainly among individuals with lower antibody titers [10]. Therefore, understanding the factors associated with the persistence of protective antibody levels seems crucial to guide the optimal design of additional vaccine boosters. However, most studies evaluate COVID-19 vaccine responses focusing on clinical efficacy, without considering the humoral response in terms of antibody titers [2, 11], and only a few studies have addressed the problem of waning immunity by longitudinal measures of humoral responses [1].
Immunosenescence is a dysregulation of the immune system characteristic of aging linked to the involution of the thymus [12]. With aging, the thymus atrophies and declines in function, reducing the output of naïve T-cells to the periphery, which leads to clonal expansions and accumulations of exhausted/senescent memory T-cells with altered functionality [13, 14]. These alterations contribute to a chronic low-grade inflammatory state or inflammaging characteristic of advanced age [15]. Thymic involution also affects the production of thymic hormones, such as thymosin-α1, a peptide hormone with immunomodulatory properties, which induces the maturation of B and T-lymphocytes and increases the efficiency of antigen presentation, enhancing and restoring immune functions [16]. Nevertheless, the potential role of thymic function in the magnitude and long-term persistence of anti-Spike (anti-S) antibody titers along the time in COVID-19 vaccination has not yet been explored.
Our aim was to analyze potential predictive factors of the magnitude and durability of the response to COVID-19 vaccination in older people. We explored thymus-related parameters, as well as other relevant immunological factors that are also known to be altered along immunosenescence, such as the distribution of immunological subsets and levels of inflammatory and biochemical biomarkers.
Results
Demographical, anthropometrical and clinical characteristics of the study subjects
Ninety-eight subjects from the nursing home accepted to participate in the study, of which 88 did it from the beginning (Supplementary Fig. 1). All participants were men (100%) with a median age of 73 [64–80] years-old. Participants were categorized according to their ages, and the baseline characteristics of groups are summarized in Supplementary Table 1. The groups were: i) young (less than 50 years-old), including ten health care workers; ii) middle-age (between 50 and 65 years-old), including nine health care workers and eight residents; and iii) older (over 65 years-old), comprising 14 health care workers and 57 residents. Comorbidities were recorded from 56 out of 65 residents, being the most frequent hypertension (83.7%), diabetes (42.9%), and obesity (31.9%) (Supplementary Table 2). The comparison of thymic activity-related parameters, the distribution of immunological subsets, and soluble biochemical and inflammatory markers in the three age-based groups are summarized in Supplementary Table 3. Summing up, older participants showed a typical immunosenescent profile, characterized by lower thymic output with a trend to shortened telomeres and a generalized higher inflammatory profile compared with younger participants. They also exhibited the lowest levels of all immune subsets analyzed, except for natural killer (NK) cells that were expanded in this group.
Age influences the magnitude and durability of the response to SARS-CoV-2 antigen
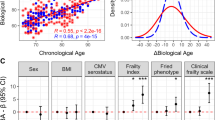
The magnitude of the initial immune response achieved 1 month after the second dose of the BNT162b2 vaccine (T1) is represented in Fig. 1A. The analysis of anti-S Immunoglobulin G (IgG) antibodies at T1 revealed a strong induction of humoral immunity in response to vaccination in all study participants, with a 100% of seroconversion rate in the entire cohort, although we found that anti-S antibody titers achieved at T1 were significantly lower in older adults compared to younger participants. However, it is remarkable that participants of the older group showed a mixed phenotype since a substantial number of participants of the older group had T1 responses as good as the young group. Interestingly, these two subgroups of older participants differed in thymosin-α1 levels as well as in other studied variables (Supplementary Fig. 2). Moreover, we observed a negative correlation between the magnitude of the initial response and age (r = − 0.291; p = 0.007) (Fig. 1B).
Age-related differences in the humoral response to the BNT162b2 vaccine. A Comparison of anti-S antibody titers between study groups at T1. Differences in anti-S IgG antibodies were maintained even when the two outliers from the young group (**) were excluded (p = 0.048). B Correlation between age and the log10 of anti-S antibody titers at T1. p values < 0.05 were considered statistically significant
The dynamics of anti-S antibodies over time are represented in Fig. 2. In the entire population, a decline in antibody titers over time was observed (Friedman test p < 0.001). Anti-S IgG titers rapidly decreased for the first 3 months (74% between T1 and T4), followed by a relatively slow decrease thereafter (92.0% between T1 and T8). By groups, median anti-S IgG titers decreased between T1 and T4 by approximately 2.6-folds in young participants, compared with a 4.7 and 4-folds decrease in participants of the middle-age and older groups (p = 0.010). In the entire population, the rate of seropositivity also declined from 100% at T1 to 95.5% at T4, and 88.8% at T8. At T4, four participants from the old-group switched to seronegative, whereas at T8, two participants from the middle-age-group and 12 from the old-group became seronegative. No correlation was found between the loss of anti-S IgG titers and the age of participants, neither in the short-term nor in long-term.
Longitudinal dynamics of Anti-S antibody titers by age-groups. Follow-up of anti-S antibody titers between T1 (one month after the second dose), T4 (four months after the second dose) and T8 (eight months after the second dose) in A) Young, B) Middle-Age and C) Older groups. For all comparisons, p values 0.05 were considered statistically significant. Friedman test p < 0.001 for all comparisons
Interestingly, we observed that anti-S antibody titers achieved at T1, T4, and T8 strongly correlated with each other (Supplementary Fig. 3). No correlations between the anti-S antibody titers achieved at T1 and the loss of anti-S antibody levels at the short-term (r = − 0.163, p = 0.159) or the long-term (r = 0.017, p = 0.891) were observed.
Age-related factors associated to the magnitude of the initial response to two doses of BNT162b2 vaccine
To address the potential effects of thymic activity-related biomarkers, the distribution of the immune cell subsets, biochemical and inflammatory markers on the magnitude of the initial response to the vaccine, we analyzed the potential relationships between these parameters and the anti-S titers achieved at T1 (Fig. 3). Regarding the potential role of the thymic activity parameters in the initial response to the vaccine, we only found a trend to a positive correlation between the ratio of signal-joint to beta T-cell receptor excision circles (sj/β-TRECs) and anti-S IgG titers at T1. Interestingly, although the levels of thymosin-α1 at T1 did not correlate with anti-S IgG titers at T1, they did at T8 (r = 0.334, p = 0.01). The magnitude of the response also correlated positively to total lymphocytes, CD3, CD4, CD8 T, and B cell counts, but was not associated with NK cells. Attending to the biochemical markers analyzed, we observed that, except for immunoglobulin A (IgA), the anti-S IgG titers achieved at T1 were positively associated with the level of all blood proteins analyzed, as well as with the levels of vitamin B12, folate, and calcium. Examining the potential effect of inflammatory markers, we found that homocysteine, β2-microglobulin, and high sensitivity C reactive protein (hsCRP) were negatively associated with the magnitude of the initial response to the vaccine. As can be seen in Supplementary Fig. 4, all these correlations depended on the study group.
Factors associated with the magnitude and loss of anti-S titers after BNT162b2 vaccination. Spearman’s correlation analysis between potential age-related factors associated to the magnitude of the initial response to the vaccine (T1, one month after the second dose of the vaccine), as well as to the short-term loss (between T1 and T4) and to the long-term loss (between T1 and T8). Color intensity of boxes represents Spearman’s rank correlation coefficient value as indicated in the color legend. All colored boxes represent statistically significant correlations (p values < 0.05). * p values between 0.1 and 0.05
We next performed a multivariable analysis in which all variables that significantly correlated in the bivariate analysis with the anti-S antibody titers achieved at T1, were included. Linear multivariable analysis showed that homocysteine was the unique variable independently associated with the magnitude of the initial response to the vaccine [β coefficient; (95% confidence interval (CI), − 0.155 (− 0.241 to − 0.068); p = 0.001] (Table 1).
Age-related factors as predictors of the loss of anti-S antibody titers along the time
We analyzed the relationships between the percentages of loss of anti-S antibody titers at both, the short- (T1-T4) and the long-term (T1-T8), and thymus activity-related parameters, the distribution of immune cell subsets, levels of biochemical markers and inflammatory-related biomarkers (Fig. 3). Attending to thymus activity-related parameters in the entire population, we observed a negative correlation between the sj/β-TRECs ratio and the short-term loss of antibodies. Interestingly, the levels of thymosin-α1 were also inversely correlated with the short-term loss of antibodies (r = − 0.251, p = 0.046). This negative correlation was maintained in the long-term (r = − 0.279, p = 0.034). Regarding the biochemical markers analyzed, we found that the short-term loss of anti-S antibodies correlated positively with total IgA, but was negatively associated with total immunoglobulin M (IgM). We also observed that both transferrin and transferrin saturation index (TfSI) tended to correlate with the short-term loss of anti-S titers. Respect the inflammatory status, only hsCRP levels were associated with the short-term loss of anti-S titers, but this correlation was not maintained in the long-term.
We next performed a multivariable analysis to determine independent factors affecting the loss of anti-S IgG titers in both the short- and long-term. We included all variables significantly associated in the bivariate analysis with the short-term loss of antibodies. For the long-term multivariate analysis, in addition to thymosin-α1, which was the unique variable that significantly correlated with the long-term loss of antibodies in the bivariate analysis, total IgM was also included due to its high tendency to correlate (r = − 0.235, p = 0.055). Linear regression analysis revealed that thymosin-α1 at T1 was the unique variable independently associated with the loss of anti-S antibody titers at both, the short-term [β coefficient − 0.168; 95% confidence interval [CI], (− 0.305 to − 0.031); p = 0.017] and at long-term [β coefficient − 0.123; 95% confidence interval [CI], (− 0.212 to − 0.034); p = 0.008] (Table 1).
Discussion
In this study, carried out in a nursing home, the humoral immunogenicity elicited by the BNT162b2 vaccine and the durability of such response along the time were conditioned by age and age-related factors. While the magnitude of the initial response to the vaccine was independently associated with the inflammatory status of the participants, the durability of such response in the long-term (up to 8 months), was only predicted by the previous levels of thymosin-α1, a thymic-derived immunomodulatory hormone.
Immunity against emerging viruses and de novo vaccine responses depend on the efficacy of mounting primary responses, which is known to be impaired in older adults, mainly due to immunosenescence [12]. Thus, the benefits of vaccination in the prevention of infectious diseases are limited in older people, who normally show defective humoral responses to several vaccines [4], such as Influenza [5, 6], Hepatitis B virus [17], and SARS-CoV2 [1, 18,19,20,21]. In fact, despite the considerable high efficacy of the available SARS-CoV-2 mRNA vaccines, aged people, even vaccinated, maintain increased mortality rates due to COVID-19 than younger subjects [22].
Preclinical studies in animal models, as well as vaccine efficacy studies and the early use of convalescent plasma, suggest that neutralizing antibodies are the best correlators of protection against the more severe outcomes of COVID-19 [1, 4, 8, 9, 23,24,25,26]. In our study, although the seroconversion rate was 100% for all vaccinees, the magnitude of the initial humoral response was significantly lower in the oldest participants, which was in accordance with previous reports [1, 18]. We also observed a negative association between anti-S IgG titers 1 month after vaccination with the age of the participants, a correlation that was maintained 4 months later (data not shown). In addition to the diminished magnitude of the initial response among older adults, current evidence indicates the increasing risk of SARS-CoV-2 symptomatic infection with time due to the waning of humoral immunity [1, 10]. We found that anti-S IgG titers rapidly decayed early after vaccination in all participants without exception, but especially among aged vaccinees, in accordance with previous reports [19]. On the other hand, the loss of anti-S IgG titers did not correlate with the age of the participants. Consequently, the identification of reliable age-related factors associated with the immunogenicity and durability of the response over time is mandatory and could help to improve personalized vaccination/boosting strategies.
It is known that the distribution of immune cell subsets affects the immune responses against vaccine antigens [27,28,29]. Moreover, age-dependent differences in T and B lymphocytes, as well as in NK populations distribution and functions have been well-defined [30]. Thus, their relationship with antibody responses following vaccination and the durability of the humoral response over time could provide valuable information for understanding protective long-term immunity against COVID-19. As expected, in our cohort, the distribution of the peripheral immune cell subset depended on age. Thus, older participants showed lower levels of total lymphocytes, CD3, CD4, CD8, and B lymphocytes, but higher levels of NK cells, an immune profile tightly related to immunosenescence [30,31,32,33]. Interestingly, all immune subsets analyzed correlated positively to the magnitude of the humoral response to the vaccine, except NK cells, which were not associated with such a response.
Another age-related immune defect is inflammaging, a low grade chronic persistent inflammatory phenotype found in older populations [34], that contributes to the decline in the immune response and has been shown to inhibit immune responses to vaccination [17, 35, 36]. Accordingly, older participants of our study showed significantly higher levels of inflammatory markers, including homocysteine, β2-microglobulin, and D-dimers, and a trend to higher levels of hsCRP. All of these markers correlated negatively with the magnitude of the response, but only the levels of hsCRP correlated with the short-term loss of anti-S antibody titers. These results point out that pharmacological strategies aiming at blocking baseline inflammation can be potentially used to boost COVID-19 vaccine responses, but would not be probably enough to avoid the intense waning of humoral protection.
The aging of the immune system is proposed to impact COVID-19 vaccination efficiency [37]. One of the main factors underlying immunosenescence is age-related thymic involution [15]. Hence, we hypothesized that deficient thymic function related to aging might impact COVID-19 vaccination efficiency and should also be considered a key player in aged populations. In our study, older participants showed a reduced thymic output, determined by a lower sj/β-TRECs ratio in the periphery. Interestingly, the RTL of immune cells from the older group tended to be shorter than that from the other groups, suggesting an age-dependent shift from naïve to memory phenotype induced by homeostatic T-cell proliferation to compensate for the diminished T-cell thymic output in older participants. Interestingly, the sj/β-TRECs ratio tended to correlate positively with the magnitude of the response to the vaccine, also being associated with the short-term loss of anti-S IgG titers. In accordance, diminished responsiveness to vaccination against other viruses as yellow fever virus has been previously associated with lower thymus activity [38]. Remarkably, thymus activity does not rely only on cellular output, but also on its production of immunomodulatory hormones. We found that although the levels of thymosin-α1 at T1 did not correlate with anti-S IgG titers at T1, they did at T8. Moreover, and despite there being no differences in the levels of thymosin-α1 between age groups, we found that older people with a higher magnitude of response at T1 had higher levels of thymosin- α1. Furthermore, levels of thymosin- α1 predicted the short- and long-term loss of anti-S IgG titers, being the unique variable independently associated in the multivariable analysis with the loss of antibodies at both the short- and the long-term. Our results are of relevance since thymosin-α1, a peptide with immunomodulatory properties that induces differentiation of B and T lymphocytes has already been used in several clinical trials (phase I/II/III/IV) and research settings, such as different types of cancer, sepsis, and infectious diseases, including COVID-19 [39,40,41,42,43,44]. Notably, its use as an adjuvant to the Influenza and hepatitis B vaccines improved the immunogenicity and durability of the humoral responses, showing to be safe and well tolerated [39, 40, 42]. Future research addressing the potential benefit of using this immunomodulator as an adjuvant of COVID-19 vaccination would be desirable since it could also improve the durability of responses. Also remarkable, the potential role of the thymus in the severity of COVID-19 has been discussed [45,46,47,48,49], and thymosin-α1 has also been used as a treatment for severe COVID-19, although with controversial results [44, 50, 51].
Our study has several limitations. We present an exploratory study with a relatively small sample size but fully representative of a nursing home, allowing us a close monitor follow-up. On the other hand, all participants from the study were men, and consequently, our results might not be extrapolated to cohorts including women. However, this approximation allowed us to avoid the potential influence of gender in age-related factors measured and its relation with the magnitude and loss of antibodies over time. Finally, the vaccination of residents of nursing homes was considered a priority, and the urgent vaccination of this especially vulnerable population did not allow us to collect samples before vaccinations. Nevertheless, our results support and extend previous observations from COVID-19 vaccine responses following vaccination in older people and raise novel potential approaches to optimize COVID vaccination/boosting protocols in this vulnerable population.
Conclusion
In conclusion, while the magnitude of the initial response to the vaccine was associated with the inflammatory status of the participants, the durability of such response in the short- and long-term was predicted by the previous levels of thymosin-α1. Our results suggest that thymosin-α1 could be used as a biomarker for the durability of the responses after COVID-19 vaccination, possibly allowing to personalize the administration of vaccine boosters.
Methods
Study design and participants
This study was performed at the “Santa Caridad elderly home” (Seville, Spain), a nursing home where only men are admitted as residents. To avoid gender differences due to the different distribution of sexes along age, only men health workers were included in this study. Residents and health workers, neither previously diagnosed with COVID-19 (determined by medical history) nor under immunosuppressive therapy that had received two doses of the BNT162b2 vaccine, were invited to participate in the study. Participants who acquired SAR-COV-2 infection during the follow-up were not considered thereafter. A schematic representation of the vaccine administration protocol and sample collection is shown in Supplementary Fig. 1. Participants received the first and second doses of the BNT162b2 vaccine on January 4th and 25th 2021 respectively. Blood samples started to be collected one-month after the second dose (T1) to assess factors potentially associated with the magnitude of the initial response and also four (T4) and eight (T8) months later to evaluate the potential predictive factors of the durability of such response at both, short-term (T1-T4) and long-term (T1-T8). Specific antibody titers were determined at all time-points, whereas the rest of the parameters were analyzed only at T1. The study was performed according to the Helsinki Declaration of the World Medical Association and approved by the Research Ethics Committee of the Virgen del Rocío and Virgen Macarena University Hospitals (PEIBA Acta CEI_02/2021). Written informed consent was obtained from all participants.
Quantification of IgG anti-SARS-CoV-2 antibodies
IgG antibodies against the trimeric SARS-CoV-2 Spike protein were quantified in serum samples by using chemiluminescence assay (LIAISON® SARS-CoV-2 TrimericS IgG, Diasorin S.p.A, Saluggia, Italy) and run on a DiaSorin LIAISON XL platform (DiaSorin, Stillwater, USA). According to the manufacturer’s data, the sensitivity and specificity of this test were 98.7 and 99.5%, showing a good correlation with the microneutralization test (Positive percent agreement (PPA): 100%, Negative percent agreement (NPA): 96.9%) [22]. Antibody concentration, expressed as Binding Arbitrary Units (BAU)/mL, was automatically calculated by the analyzer from Arbitrary Units (AU/mL) by the following conversion formula: AU/mL*2.6 = BAU/mL, and a positive result was considered as ≥33.8 BAU/mL.
Immune subsets
Absolute numbers of lymphocytes, CD3, CD4, and CD8 T-cells, B-cells and NK-cells were routinely determined in fresh blood samples collected at T1, with a Navious EX flow cytometer (Beckman-Coulter, Brea, California), by standard procedures at the Immunology Service of our hospital.
Biochemical soluble markers
Biochemical soluble markers, including blood proteins, micronutrients, soluble iron metabolism-related markers, and inflammatory biomarkers, were routinely determined by standard procedures at the Biochemistry Service of our hospital. Briefly, total iron, transferrin, and soluble transferrin receptor (sTfR) were measured by photometry and ferritin by particle enhanced immunoturbidimetric assay in a Hitachi Cobas C702 modular analyzer (Roche Diagnostics, Rotkreuz, Switzerland). hsCRP and β2-microglobulin levels were determined in frozen serum samples with an immunoturbidimetric assay using Cobas 701 (Roche Diagnostics, Mannheim, Germany). Measurements of the levels of homocysteine were performed by photometry according to the manufacturer’s instructions, and D-dimer levels were quantified by using an automated latex enhanced immunoassay using frozen plasma samples (HemosIL D-Dimer HS 500, Instrumentation Laboratory, Bedford, Massachusetts).
Thymic function-related determinations
DNA extraction
DNA was extracted from cryopreserved peripheral blood mononuclear cells (PBMCs) by using Omega BIO-TEK, E.Z.N.A blood DNA Mini Kit. Extracted DNA was used for both, quantification of the relative telomere length (RTL) and the thymic output (sj/β-TRECs ratio).
RTL quantification
For RTL quantification, copy number quantifications were performed by quantitative polymerase chain reaction (qPCR). We determined the ratio between the number of copies of the telomere sequence and the single copy gene Beta-globin. The fluorescent reading for the copy number quantification was performed in a Light-cycler 480 (Roche).
Thymic output quantification (sj/β-TRECs ratio)
The thymic output was calculated as the sj/β-TRECs ratio by droplet digital PCR (ddPCR) in a single reaction using a QX200 system (BIORAD). The results were analyzed by using the Quantasoft 1.7.1 Software.
Thymosin-α1 quantification
Thymosin-α1 was quantified in plasma samples using the Human Thymosin-α1 competitive ELISA kit (MyBiosurce®) following the manufacturer’s instructions.
More detail on the methods can be found in Supplementary Methodology.
Statistical analysis
Continuous variables were recorded as median values and interquartile ranges [IQR], and categorical variables were recorded as numbers and percentages n (%). For continuous variables, multiple comparisons between groups were made using the non-parametric Kruskal-Wallis test, and comparisons between two groups were performed using the Mann-Whitney U-test. The Friedman test and Bonferroni adjustment to a series of post hoc Wilcoxon matched pairs tests were applied for multiple longitudinal comparisons. Comparisons between categorical variables were performed by using the χ2 or the Fisher exact test as appropriate. Correlations between quantitative variables were explored by using the Spearman correlation coefficient test. Linear regression multivariable analyses were performed to determine predicting factors of the magnitude of the initial response and loss of antibody titers at both short- and long-term. Except for the Bonferroni corrections (p < 0.017), p values p < 0.05 were considered statistically significant. Statistical analyses were performed using the SPSS software (SPSS 21.0, Chicago, IL), and the graphics were generated with the Prism program (GraphPad Software v8.0).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- Anti-S:
-
Anti-Spike
- AU:
-
Arbitrary units
- BAU:
-
Binding arbitrary units
- COVID-19:
-
Coronavirus disease 2019
- ddPCR:
-
Droplet digital polymerase chain reaction
- hsCRP:
-
High sensitivity C reactive protein
- IgA:
-
Immunoglobulin A
- IgG:
-
Immunoglobulin G
- IgM:
-
Immunoglobulin M
- NK:
-
Natural killer
- NPA:
-
Negative percent agreement
- PBMCs:
-
Peripheral blood mononuclear cells
- PPA:
-
Positive percent agreement
- qPCR:
-
Quantitative polymerase chain reaction
- RTL:
-
Relative telomere length
- sj/β-TRECs ratio:
-
Ratio of signal-joint to beta T-cell receptor excision circles
- sTfR:
-
Soluble transferrin receptor
- TfSI:
-
Transferrin saturation index
References
Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84.
Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalizations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–29.
Cabezas C, Coma E, Mora-Fernandez N, et al. Associations of BNT162b2 vaccination with SARS-CoV-2 infection and hospital admission and death with covid-19 in nursing homes and healthcare workers in Catalonia: prospective cohort study. BMJ. 2021;374:n1868.
Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25.
Nakaya H, Hagan T, Duraisingham SS, et al. Systems analysis of immunity to influenza vaccination across multiple years and in diverse populations reveals shared molecular signatures. Immunity. 2015;43:1186–98.
Herrero-Fernández I, Rosado-Sánchez I, Álvarez-Ríos AI, et al. Effect of homeostatic T-cell proliferation in the vaccine responsiveness against influenza in elderly people. Immun Ageing. 2019;16(1):1–12.
Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50.
Israelow B, Mao T, Klein J, et al. Adaptive immune determinants of viral clearance and protection in mouse models of SARS-CoV-2. Sci Immunol. 2021;6:eabl4509.
McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590:630–4.
Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–84.
Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83.
Appay V, Sauce D. Naive T cells: the crux of cellular immune aging? Exp Gerontol. 2014;54:90–3.
Li G, Yu M, Lee W, et al. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18:1518–24.
Briceño O, Lissina A, Wanke K, et al. Reduced naïve CD8+ T-cell priming efficacy in elderly adults. Aging Cell. 2016;15:14–21.
Thomas R, Wang W, Su DM. Contributions of age-related Thymic involution to Immunosenescence and Inflammaging. Immun Ageing. 2020;17:1–17.
Dominari A, Hathaway Iii D, Pandav K, et al. Thymosin alpha 1: a comprehensive review of the literature. World J Virol. 2020;9:67–78.
Fourati S, Cristescu R, Loboda A, et al. Pre-vaccination inflammation and B-cell signalling predict age-related hyporesponse to hepatitis B vaccination. Nat Commun. 2016;7:1–12.
Collier DA, Ferreira IATM, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–22.
Tober-Lau P, Schwarz T, Vanshylla K, et al. Long-term immunogenicity of BNT162b2 vaccination in older people and younger health-care workers. Lancet Respir Med. 2021;9:e104–5.
Müller L, Andrée M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021;73:2065–72.
Demaret J, Corroyer-Simovic B, Alidjinou EK, et al. Impaired functional T-cell response to SARS-CoV-2 after two doses of BNT162b2 mRNA vaccine in older people. Front Immunol. 2021;12:778679.
Wong MK, Brooks DJ, Ikejezie J, et al. COVID-19 mortality and Progress toward vaccinating older adults — World Health Organization, worldwide, 2020–2022. MMWR Morb Mortal Wkly Rep. 2023;72:113–8.
Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11.
Mercado NB, Zahn R, Wegmann F, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–8.
Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384:610–8.
Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med. 2021;384:1885–98.
Duni A, Markopoulos GS, Mallioras I, et al. The humoral immune response to BNT162b2 vaccine is associated with circulating CD19+ B lymphocytes and the Naïve CD45RA to memory CD45RO CD4+ T helper cells ratio in hemodialysis patients and kidney transplant recipients. Front Immunol. 2021;12:1–11.
Rydyznski C, Daniels KA, Karmele EP, et al. Generation of cellular immune memory and B-cell immunity are impaired by natural killer cells. Nat Commun. 2015;27:6375.
Jedicke N, Stankov MV, Cossmann A, et al. Humoral immune response following prime and boost BNT162b2 vaccination in people living with HIV on antiretroviral therapy. HIV Med. 2022;23:558–63.
Sansoni P, Cossarizza A, Brianti V, et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82:2767–73.
Kokuina E, Breff-Fonseca MC, Villegas-Valverde CA, Mora-Díaz I. Normal values of T, B and NK lymphocyte subpopulations in peripheral blood of healthy Cuban adults. MEDICC Rev. 2019;21:16–21.
Lin Y, Kim J, Metter EJ, et al. Changes in blood lymphocyte numbers with age in vivo and their association with the levels of cytokines/cytokine receptors. Immun Ageing. 2016;13:1–10.
Le Garff‐Tavernier M, Béziat V, Decocq J, et al. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell. 2010;9:527–35.
Fulop T, Larbi A, Dupuis G, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2018;8:1960.
Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and ‘Garb-aging’. Trends Endocrinol Metab. 2017;28:199–212.
Pereira B, Xu XN, Akbar AN. Targeting inflammation and Immunosenescence to improve vaccine responses in the elderly. Front Immunol. 2020;11:583019.
Connors J, Bell MR, Marcy J, Kutzler M, Haddad EK. The impact of immuno-aging on SARS-CoV-2 vaccine development. Geroscience. 2021;43:31–51.
Schulz AR, Mälzer JN, Domingo C, et al. Low Thymic activity and dendritic cell numbers are associated with the immune response to primary viral infection in elderly humans. J Immunol. 2015;195:4699–711.
Carraro G, Naso A, Montomoli E, et al. Thymosin-alpha 1 (Zadaxin™) enhances the immunogenicity of an adjuvated pandemic H1N1v influenza vaccine (Focetria™) in hemodialyzed patients: a pilot study. Vaccine. 2012;30:1170–80.
Shen S, Josselson J, McRoy C, Sadler J, Chretien P. Effect of thymosin alpha 1 on Heptavax-B vaccination among hemodialysis patients. Kidney Int. 1987;31:217.
Robert S, King CWT. Thymosin Apha 1–a peptide immune modulator with a broad range of clinical applications. Clin Exp Pharmacol. 2013;03:1–17.
Panatto D, Amicizia D, Lai PL, Camerini R, de Rosa A, Gasparini R. Utility of thymosin α-1 (Zadaxin™) as a co-adjuvant in influenza vaccines: a review. J Prev Med Hyg. 2011;52:111–5.
Bersanelli M, Giannarelli D, Leonetti A, et al. The right immune-modulation at the right time: Thymosin α1 for prevention of severe COVID-19 in cancer patients. Future Oncol. 2021;17:1097–104.
Liu Y, Pang Y, Hu Z, et al. Thymosin alpha 1 (Tα1) reduces the mortality of severe COVID-19 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin Infect Dis. 2020;71:2150–7.
Kellogg C, Equils O. The role of the thymus in COVID-19 disease severity: implications for antibody treatment and immunization. Hum Vaccin Immunother. 2021;17:638–43.
Genebat M, Calderón A, Tarancón-Díez L, Muñoz-Fernández MA, Leal M. Enhanced Thymopoiesis as an alternative therapeutic option for COVID-19. Gerontol Geriatr Res. 2021;7:id1054.
Genebat M, Tarancón-Díez L, de Pablo-Bernal R, Calderón A, Muñoz-Fernández MÁ, Leal M. Coronavirus disease (COVID-19): a perspective from immunosenescence. Aging Dis. 2021;12:3–6.
Hazeldine J, Lord JM. Immunesenescence: a predisposing risk factor for the development of COVID-19? Front Immunol. 2020;11:1–20.
Pietrobon AJ, Teixeira FME, Sato MN. Immunosenescence and Inflammaging: risk factors of severe COVID-19 in older people. Front Immunol. 2020;11:1–18.
Liu J, Shen Y, Wen Z, et al. Efficacy of Thymosin alpha 1 in the treatment of COVID-19: a multicenter cohort study. Front Immunol. 2021;12:1–10 54. Huang CL, Fei L, Xu W, et al. efficacy evaluation of Thymosin alpha 1 in non-severe patients with COVID-19: a retrospective cohort study based on propensity score matching. Front Med. 2021; 8:1–8.
Huang CL, Fei L, Xu W, et al. Efficacy evaluation of Thymosin alpha 1 in non-severe patients with COVID-19: a retrospective cohort study based on propensity score matching. Front Med. 2021;8:1–8.
Acknowledgments
We thank all residents for their collaboration and Rafael Martínez Alba, director of the “Residencia Hogar de la Santa Caridad”, for allowing us to perform the study in this institution. We also thank the nurses at the “Residencia Hogar de la Santa Caridad” for their clinical assistance and blood sampling.
Funding
This work was supported by the Instituto de Salud Carlos III through the project “PI21/00357” (Co-funded by European Regional Development Fund/European Social Fund “A way to make Europe”/“Investing in your future”). MMPB was supported by a postdoctoral contract from Consejería de Transformación Económica, Industria, Conocimiento y Universidades, Junta de Andalucía (DOC_01646). ABR, IOM and VGR were supported by Instituto de Salud Carlos III (Sara Borrell program CD19/00143, Río Hortega program CM19/00051, and PFIS program FI19/00298, respectively). YMP was supported by the Consejería de Salud y Familias of Junta de Andalucía through the “Nicolás Monardes” program (RC-0006-2021).
Author information
Authors and Affiliations
Contributions
Concept and design: MMPB, ML and YMP. Subject recruitment and clinical support: ML. Sample processing and preservation: MMPB, ABR, IOM and VGR. Thymic function-related determinations: ABR. Antibody quantification: CL. Biochemical analyses: AIAR. Immune subset analyses: IMC, JMML and BSS. Blood sampling: JSC, RB and EBL. MMPB and YMP had full access to all data and take responsibility for the integrity of the data and the accuracy of the data analysis, but all authors contributed with partial analyses and to the global interpretation of data. Drafting of the manuscript: MMPB and ABR. Critical revision of the manuscript for important intellectual content: all authors. Administrative, technical and material support: MFGE, ML and YMP. Funding: YMP. Supervision and coordination: YMP. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed according to the Helsinki Declaration of the World Medical Association and was approved by the Research Ethics Committee of the Virgen del Rocío and Virgen Macarena University Hospitals (PEIBA Acta CEI_02/2021). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Fig. 1.
Study design and Flow-chart.
Additional file 2: Supplementary Table 1.
Demographical and anthropometrics characteristics of the study population.
Additional file 3: Supplementary Table 2.
Comorbidities and behavioral factors of residents from the Santa Caridad Nursing Home.
Additional file 4: Supplementary Table 3.
Thymic activity, Biochemical, Inflammatory and Immunological profiles of the study populations one month after the second dose (T1).
Additional file 5: Supplementary Fig. 2.
Levels of Thymosin-α1 in higher vs. lower responders of the older group.
Additional file 6: Supplementary Fig. 3.
Correlations among Log of anti-S antibody titers at all different study points.
Additional file 7: Supplementary Fig. 4.
Factors associated with the magnitude of the initial response to the BNT162B2 vaccine at T1 by age-groups.
Additional file 8.
Detailed methodology.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pozo-Balado, M.d.M., Bulnes-Ramos, Á., Olivas-Martínez, I. et al. Higher plasma levels of thymosin-α1 are associated with a lower waning of humoral response after COVID-19 vaccination: an eight months follow-up study in a nursing home. Immun Ageing 20, 9 (2023). https://doi.org/10.1186/s12979-023-00334-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12979-023-00334-y